Abstract
The treatment of chronic hepatitis C has radically changed due to the development of direct-acting antiviral agents (DAAs). Twelve-week treatment with ledipasvir and sofosbuvir (LDV/SOF), a combination of DAAs, is highly effective in patients with hepatitis C virus (HCV) genotype 1 infection. However, the overall sustained virological response rate 12 weeks after the end of treatment (SVR12) is not 100%. Elbasvir (EBR) combined with grazoprevir (GZR) is the latest approved therapy for patients with genotype 1 or 4 chronic hepatitis C. However, to the best of our knowledge no case reports have described retreatment with GZR/EBR in patients with a history of failed LDV/SOF treatment. The present case report indicated a case in which GZR/EBR was effective for the retreatment of a patient with a history of failed LDV/SOF treatment and chronic hepatitis C. The present study indicated a 55-year-old Japanese male with a history of chronic hepatitis C and compensated liver cirrhosis. The patient exhibited the amino acid mutation Y93H in NS5A. Therefore, treatment with LDV/SOF was initiated, which was effective and suppressed the virus during oral administration. However, 4 weeks after treatment, the patient's viral load relapsed and returned to its original level. After the patient provided informed consent, treatment with GZR/EBR was initiated. No problems related to GZR/EBR were observed during treatment and the patient's SVR12 was evaluated at 12 weeks posttreatment. In conclusion, GZR/EBR treatment was useful for treating a relapse of HCV genotype 1b infection in the present case after LDV/SOF treatment, despite liver fibrosis, in the presence of the high-frequency amino acid mutation Y93H in NS5A. Although it will be necessary to examine a large number of cases, the present findings suggest that GZR/EBR may be a potential treatment option for relapse of HCV genotype 1b infection after LDV/SOF treatment.
Keywords: hepatitis C, direct-acting antiviral agent, ledipasvir, sofosbuvir, grazoprevir, elbasvir
Introduction
Hepatitis C virus (HCV) remains the main cause of severe liver disease. Due to medical progress, the number of new HCV infections has been decreasing. However, HCV-related morbidity and mortality are expected to continue rising, and approximately 399,000 people die each year from hepatitis C, mostly due to cirrhosis and hepatocellular carcinoma (http://www.who.int/mediacentre/factsheets/fs164/en).
To date, several direct-acting antiviral agents (DAAs) have been designed and developed to target individual portions of the viral proteins, including NS3/4A, NS5B, and NS5A (1). A new treatment method using DAAs is highly active against HCV and has few side effects. In particular, twelve-week combination treatment with the DAAs ledipasvir (LDV), an NS5A inhibitor, and sofosbuvir (SOF), an NS5B polymerase inhibitor, is highly effective in patients with HCV genotype 1 infection (2). However, the treatment is not perfect, as the overall SVR at 12 weeks after the end of treatment is 98.8%, not 100% (3). In addition, the next treatment regimen for LDV/SOF failure cases has not been established.
Elbasvir (EBR), an NS5A inhibitor, combined with grazoprevir (GZR), an NS3/4A protease inhibitor, is the latest approved therapy for patients with genotype 1 or 4 chronic hepatitis C (4). Compared to LDV/SOF, EBR/GZR has few side effects and limited drug interactions and can be given to patients with illnesses such as heart failure. In the 2016 AASLD/IDSA guidelines, 12 weeks of EBR/GZR treatment was recommended as level A therapy for treatment-experienced patients (prior exposure to peginterferon/ribavirin) with genotype 1a or 1b infection. However, clinical experience and trial data on the retreatment of any patient that had previously received treatment with LDV/SOF are very limited (http://www.hcvguidelines.org.). Therefore, the present study reported a case in which GZR/EBR was effective for the retreatment of a patient with a history of failed LDV/SOF treatment for chronic hepatitis C.
Case report
A 55-year-old Japanese male had a history of chronic hepatitis C with compensated liver cirrhosis; his viral load was 6.0 log IU/ml, and his viral genotype was 1b. In addition, he exhibited the high-frequency amino acid mutation (>99%) Y93H in NS5A. Although his medical history included three instances of gastroesophageal varix rupture due to excessive drinking in addition to HCV infection, suggesting uncompensated liver cirrhosis, the patient later stopped drinking, and the liver function reflected compensated cirrhosis. Therefore, we started treatment for the patient's chronic hepatitis C infection using LDV/SOF.
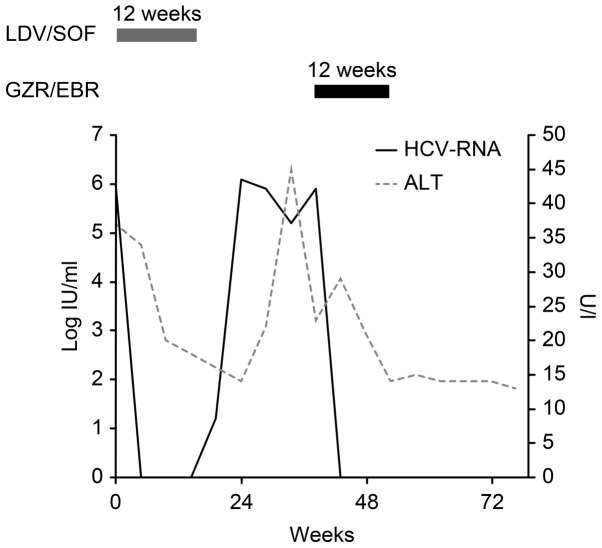
Two weeks after commencing LDV/SOF treatment, his viral load decreased to below the detection sensitivity. However, 4 weeks after treatment, his viral load increased and returned to its original level. No change was observed in the amino acid mutation after LDV/SOF treatment; the high-frequency Y93H mutation (>99%) in NS5A remained stable (Fig 1; Table I).
Figure 1.
The patient's HCV course. The patient had a history of chronic hepatitis C, with a viral load of 6.0 log IU/ml. Before treatment, the patient's ALT levels were slightly elevated; however, these metrics returned to normal immediately after LDV/SOF treatment commenced, with virus antibody titers continuing to decrease. However, 4 weeks after treatment, the patient's viral load began to increase and returned to its original level. Approximately 6 months after LDV/SOF treatment, we started the patient on GZR/EBR for a total of 12 weeks. The patient achieved an SVR at 12 weeks after GZR/EBR treatment.
Table I.
RAV alignment of NS3 and NS5A in patient before and after the treatment of LDV/SOF.
| RAVs | Before LDV/SOF | After LDV/SOF |
|---|---|---|
| NS3/V36A | – | – |
| NS3/T54A | – | – |
| NS3/T54S | – | – |
| NS3/Q80L | – | – |
| NS3/Q80R | – | – |
| NS3/A156S | – | – |
| NS3/A156T | – | – |
| NS3/A156V | – | – |
| NS3/D168A | – | – |
| NS3/D168E | – | – |
| NS3/D168H | – | – |
| NS3/D168T | – | – |
| NS3/D168V | – | – |
| NS5A/L31F | – | – |
| NS5A/L31M | – | – |
| NS5A/L31V | – | – |
| NS5A/Y93H | H>99% | H>99% |
LDV, ledipasvir; SOF, sofosbuvir; RAV, resistance-associated variant; Y93H H>99%, substitution rate of tyrosine to histidine at amino acid 93 is >99%.
There was no evidence for subsequent treatments after failure on LDV/SOF. His liver function reflected the compensated cirrhoss as before treatment with LDV/SOF (Table II). Therefore, after the patient gave informed consent, we started treatment with GZR/EBR, including an NS3/4A protease inhibitor, which was not used in the previous treatment. No side effects of GZR/EBR, such as headache, nausea, fatigue, decreased appetite, anemia, pyrexia, or ALT elevations (4), were observed during the treatment, and the patient achieved an SVR at 12 weeks posttreatment.
Table II.
Laboratory patient data before treatment with grazoprevir/elbasvir.
| Variables | Value | Units | Reference range |
|---|---|---|---|
| Total protein | 8.3 | g/dl | 6.5–8.2 |
| Albumin | 4 | g/dl | 3.5–5.5 |
| Albumin/globulin ratio | 0.93 | 1–1.8 | |
| Blood urea nitrogen | 19.8 | mg/dl | 8.2–4.3 |
| Creatinine | 0.97 | mg/dl | 0.5–1 |
| Total bilirubin | 1.2 | mg/dl | 0.1–1.2 |
| Direct bilirubin | 0.5 | mg/dl | 0.1–0.6 |
| Aspartate transaminase | 32 | U/l | 10–35 |
| Alanine transaminase | 29 | U/l | 5–40 |
| Alkaline phosphatase; | 509 | U/l | 100–340 |
| Lactate dehydrogenase | 113 | U/l | 110–220 |
| γ-glutamyl transpeptidase | 18 | U/l | 0–30 |
| Total cholesterol | 99 | mg/dl | 130–219 |
| Triglyceride | 47 | mg/dl | 30–149 |
| Na | 138 | mmol/l | 135–146 |
| K | 4.2 | mmol/l | 3.5–4.6 |
| Cl | 104 | mmol/l | 96–110 |
| White blood cells | 1,410 | /µl | 4,700–8,700 |
| Red blood cells | 375×104 | /µl | 370–490 |
| Hemoglobin | 10 | g/dl | 13–17 |
| Hematocrit | 31.3 | % | 35–45 |
| Platelets | 2.9×104 | /µl | 15–35 |
| Neutrophils | 78.5 | % | 38–71.9 |
| Eosinophils | 1 | % | 0.2–6.8 |
| Basophils | 0 | % | 0–1 |
| Lymphocytes | 11.5 | % | 26–46.6 |
| Monocytes | 9.5 | % | 2.3–7.7 |
| Prothrombin time | 49 | % | 80–100 |
Laboratory data revealed severe pancytopenia due to increased splenic function. The patient's FIB4 index was 11.27.
Discussion
This case report provides two important clinical suggestions regarding retreatment after failure of LDV/SOF therapy for patients with chronic hepatitis C. First, GZR/EBR treatment might be useful for treating relapsed HCV genotype 1b infection after LDV/SOF treatment. Second, GZR/EBR was effective in a patient with advanced cirrhosis who exhibited the high-frequency amino acid mutation Y93H in NS5A.
Some reports have related a poor response to LDV/SOF with resistance-associated variant (RAV)-positivity and high values of the FIB4 index (5). On the other hand, RAVs in the NS5B region, such as S282T, have not been confirmed in Japan (6). In particular, because a mutant form of NS5A (Y93H) shows diminished binding to LDV, HCVs expressing this mutant are resistant to LDV (7). However, RAV-positivity is also related to a poor response to GZR/EBR (8). To elucidate why GZR/EBR was effective in this case, it will be necessary to examine a large number of cases, and obtaining appropriate informed consent is necessary before initiating this treatment. Despite these facts, GZR/EBR was useful in this case and may provide an option for patients who undergo ineffective LDV/SOF treatment.
In the near future, it will be necessary to examine a large number of cases to determine whether the administration of GZR/EBR may serve as a potential treatment option for relapsed cases of HCV genotype 1b infection after LDV/SOF treatment.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DAA
direct-acting antiviral agent
- LDV
ledipasvir
- SOF
sofosbuvir
- HCV
hepatitis C virus
- SVR
sustained virological response
- EBR
elbasvir
- GZR
grazoprevir
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Authors' contributions
TT, SM and TM designed the case report. AM, KF, TS, TN, JT, HY and TM acquired and interpreted the patient's data. TT and KO collected the patient clinical data and TT was a major contributor in writing the manuscript.
Ethics approval and consent to participate
In accordance with the Declaration of Helsinki, informed consent was obtained from the patient.
Consent for publication
Consent for publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zopf S, Kremer AE, Neurath MF, Siebler J. Advances in hepatitis C therapy: What is the current state-what come's next? World J Hepatol. 2016;8:139–147. doi: 10.4254/wjh.v8.i3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, et al. NS5A resistance-associated variants undermine the effectiveness of ledipasvir and sofosbuvir for cirrhotic patients infected with HCV genotype 1b. J Gastroenterol. 2017;52:845–854. doi: 10.1007/s00535-016-1290-1. [DOI] [PubMed] [Google Scholar]
- 4.Bell AM, Wagner JL, Barber KE, Stover KR. Elbasvir/grazoprevir: A review of the latest agent in the fight against hepatitis C. Int J Hepatol. 2016;2016:3852126. doi: 10.1155/2016/3852126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y, et al. Retreatment efficacy and predictors of ledipasvir plus sofosbuvir to HCV genotype 1 in Japan. J Med Virol. 2017;89:284–290. doi: 10.1002/jmv.24617. [DOI] [PubMed] [Google Scholar]
- 6.Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Ooka Y, Ogasawara S, Chiba T, Saito T, Haga Y, et al. Real-world experiences with the combination treatment of ledipasvir plus sofosbuvir for 12 weeks in hcv genotype 1-infected Japanese patients: Achievement of a sustained virological response in previous users of peginterferon plus ribavirin with HCV NS3/4A inhibitors. Int J Mol Sci. 2017;18(pii):E906. doi: 10.3390/ijms18050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon HJ, Xing W, Chan K, Niedziela-Majka A, Brendza KM, Kirschberg T, Kato D, Link JO, Cheng G, Liu X, Sakowicz R. Direct binding of ledipasvir to HCV NS5A: Mechanism of resistance to an HCV antiviral agent. PLoS One. 2015;10:e0122844. doi: 10.1371/journal.pone.0122844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komatsu TE, Boyd S, Sherwat A, Tracy L, Naeger LK, O'Rear JJ, Harrington PR. Regulatory analysis of effects of hepatitis C virus NS5A polymorphisms on efficacy of elbasvir and grazoprevir. Gastroenterology. 2017;152:586–597. doi: 10.1053/j.gastro.2016.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.