Abstract
Nobiletin (NOB) possesses multiple pharmacological effects, but its anti-apoptotic property has acquired a great deal of attention. Endoplasmic reticulum (ER) stress (ERS)-induced apoptosis acts as the pivotal aetiology in neuronal oxygen-glucose deprivation and reoxygenation (OGD/R) injury. The aim of this study focused on whether NOB exerts neuro-protective effects on OGD/R injury by repressing ERS-induced apoptosis. The PC12 neuronal cell line was subjected to 4 h OGD and 24 h reoxygenation following NOB treatment. A PI3K/AKT inhibitor (LY294002) was added during the mechanistic experiments. Cell viability, lactate dehydrogenase (LDH) release and apoptosis were determined. Western blotting was used to measure protein expression levels. The results showed that OGD/R caused neuronal damageas exhibited by the increase in LDH release and the reduction of cellular viability. Moreover, ERS-induced apoptosis was markedly stimulated by OGD/R in PC12 cells, as evidenced by the elevation in the apoptotic rate and protein levels of C/EBP homologous protein/glucose-regulated protein-78. However, NOB administration significantly reversed neuronal damage and the ERS-induced apoptosis in response to OGD/R injury. Mechanistic detections showed that the neuron-favorable and ERS-repressing contributions of NOB were, in part, a result of the activation of the PI3K/AKT pathway, which was validated by a specific PI3K/AKT inhibitor (LY294002). Therefore, NOB protects PC12 cells from ERS-induced apoptosis in OGD/R injury mainly through enhancement of the PI3K/AKT pathway, which may provide a novel therapeutic avenue for the prevention of cerebral ischemia/reperfusion injury.
Keywords: nobiletin, OGD/R, endoplasmic reticulum stress, apoptosis, PI3K/AKT
Introduction
Cerebral ischemia/reperfusion (I/R) injury is an irreversible event in the resistance of neuron-favorable contributions generated by the revascularization therapeutic strategy for ischemic stroke (1–4). Lowering I/R-led detrimental outcomes is of clinical importance because of the influences of these outcomes on hemorrhagic transformation and blood-brain barrier destruction (1–5). Although pathophysiological mechanisms, such as apoptosis, have been proposed to be involved in cerebral I/R damage, to date, few evidence-based strategies for the reduction of cerebral I/R injury have been explored (1–6). Thus, expanded investigations of effective drugs or interventions to protect neurons from I/R injury are required to achieve greater clinical benefits.
Recently, a broader concept highlighted that neuronal apoptosis induced by endoplasmic reticulum (ER) stress (ERS) serves as the pivotal pathogenesis of I/R injury, which is characterized by elevations in C/EBP homologous protein (CHOP) and glucose-regulated protein-78 (GRP78), in concert with upregulated apoptotic caspase cascades (1–7). Once ER homeostasis is disturbed and sustained in response to I/R injury, the inaccurate synthesis and assemblage of proteins are drastically initiated (1–8). This change leads to the substantial accumulation of mis-folded proteins and causes unfolded protein response (UPR) within the ER, collectively referred to as ERS (6–8). Despite the affirmed benefits against neuronal I/R injury that developed from ERS inhibition, more precise molecular mechanisms and available therapeutic avenues for the dynamic regulation of ERS-associated apoptosis remain largely obscure in cerebral I/R injury.
Nobiletin (NOB) is a ubiquitous bioflavonoid and polyphenolic compound isolated from citrus fruits peels that has been verified to participate in a diverse array of pathological events such as apoptosis, inflammation and angiogenesis (9–12). For instance, Wu et al indicated that NOB exerts protective effects against I/R injury after liver transplantation mainly in an inflammation-diminished manner (10). Yasuda et al previously provided clues suggesting that the anti-apoptotic action is regarded as a neuroprotective mechanism of NOB in transient middle cerebral artery-occluded rats (12). As such, the I/R-protective roles of NOB have raised a great deal of attention. However, the exact contributions of NOB in cerebral I/R injury are far from clear, and the potential pathogenesis implicated in ERS-related apoptosis remains completely unknown. An additional study found that NOB could lower myocardial apoptosis in pressure overload-induced cardiac hypertrophy, in which the mechanisms are implied with NOB's ERS-repressing action (13). Intriguingly, a number of investigations have shown the crucial involvement of the phosphoinositide 3-kinase (PI3K) and serine/threonine kinase (AKT) pathway in the multiple bioactivities of NOB as a compelling approach to anticancer effects (14,15). According to the above evidence with respect to ERS-repressing and I/R-reducing activities, the aim of the present study was to detect the potential protective effects of NOB in response to I/R injury in a PI3K/AKT-dependent manner, and its interventions in ERS-induced apoptosis, by using a model of OGD/R injury in neuronal PC12 cell in vitro.
Materials and methods
Chemicals and reagents
NOB was purchased from Shanghai Winherb Medical Science Co., Ltd. (Shanghai, China), and the purity of NOB higher than 98% was detected by high-performance liquid chromatography (HPLC) analysis as previous demonstrations (11,13). PC12 cells were obtained from the American Type Cell Culture Collection (ATCC; Manassas, VA) (16). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 0.25% trypsin, and PBS were obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). A Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). A lactate dehydrogenase (LDH) commercial assay kit was purchased from Shanghai Enzyme-Linked Biotechnology (Shanghai, China). Annexin V-APC/7-AAD reagents were purchased from Beijing Biosea Biotechnology (Beijing, China). LY294002 (a PI3K inhibitor) was obtained from Selleck Chemicals (Houston, TX, USA). Primary antibodies against the following proteins were from Cell Signaling Technology, Inc. (Danvers, MA, USA): phosphorylated (p)-PI3K (no. 4228, 1:600 dilution), AKT (no. 9272, 1:600 dilution) and CHOP (no. 2895, 1:600 dilution). Antibodies against GRP78 (no. ab21685, 1:500 dilution), p-AKT (ab32509, 1:1,000 dilution) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (no. ab181602, 1:1,000 dilution) were purchased from Abcam (Cambridge, UK). The horseradish peroxidase (HRP)-conjugated rabbit anti-rat IgG secondary antibodies were from BIOSS (Beijing, China). The BCA protein assay kit was obtained from Pierce; Thermo Fisher Scientific, Inc.
Cell culture
The PC12 cells were routinely cultured in DMEM/F12 medium supplemented with 10% FBS, 100 U/ml of penicillin, and 100 mg/ml of streptomycin in a humidified atmosphere of 5% CO2 and 95% O2 at 37°C (16–18). The cells were fed every 2-3 days and sub-cultured once they reached 70-80% confluent. The PC12 cells were incubated on 96-well plates or 6-well plates at a density appropriate to the experimental requirements.
OGD/R injury and experimental designation
To mimic the I/R conditions in vitro, the PC12 cells were subjected to OGD/R treatment according to a prior demonstrations with minor modifications (16–18). In detail, the cells were washed two times with glucose-free Earle's balanced salt solution, and were maintained in glucose-free DMEM without FBS. Then, the cells were moved into an anaerobic chamber containing a gas mixture composed of 5% CO2 and 95% N2 for 4 h at 37°C. Following the OGD treatment, the cells were subsequently returned to normal media and were incubated in a normal incubator for an additional 24 h. Control cells were not exposed to OGD/R and were incubated under normal conditions continuously.
To determine the possible functions of NOB in PC12 cells during OGD/R injury, four concentrations of NOB (1, 10, 20 and 50 µM) were performed preliminarily to detect the most effective dosage against OGD/R-caused damages of PC12 cells. In the following study, PC12 cells were pretreated with NOB (50 µM) for 24 h and then subjected to OGD/R injury. The cells were randomly divided into four groups: i) Control+vehicle; ii) Control+NOB; iii) OGD/R+vehicle; and iv) OGD/R+NOB. Moreover, in the mechanistic determinations, a PI3K/AKT inhibitor (LY294002) (10 µM) was added 1 h prior to NOB treatment (19). Each group included at least 3 samples.
Assessment of cell viability
Cell viability was determined using the CCK-8 assay as a previous method (16–18). The PC12 cells were seeded into 96-well plates at a density of 6×103 cells/well for 24 h of incubation. Following the indicated procedures, 20 µl of CCK-8 reagent was added into each well followed by continuous incubation at 37°C for 4 h. The optical density values were measured with a microplate spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a wavelength of 450 nm. Cell viabilities were determined as the percentages relative to the control group.
Cellular injury assay
When cells are damaged by the OGD/R injury, intracellular LDH is rapidly released into the culture supernatant. Cellular injury was measured using LDH assay (16–18). Briefly, after the indicated treatments, the supernatant of the cultured PC12 cells was harvested, centrifuged at 3,000 × g for 10 min and then subjected to a commercial kit using standard protocols according to the manufacturer's protocols. The absorbance was detected at 450 nm, and the data were determined in international units per litre.
Cell apoptosis
Apoptosis of the PC12 cell was determined using a flow cytometric assay with a commercial apoptosis detection kit (16–18). According to the manufacturer's instruction, the cells were harvested after treatment and washed twice with cold PBS. Then, the cells were incubated in 100 µl of binding buffer containing 5 µl of Annexin V-APC and 5 µl of 7-AAD at room temperature in the dark for 10 min. Then, the stained cells were analyzed using a flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) within 1 h. Cells that stained positively for Annexin V-APC or 7-AAD were considered to be undergoing apoptosis.
Western blotting assay
Western blotting was performed as previously described (16–19). PC12 cells were solubilized in lysing buffer. Protein samples (30 µg) were separated by electrophoresis on 12 and 10% SDS-PAGE gels using a slab gel apparatus, and were then transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked in 5% skim milk for 1 h at room temperature, and were incubated with the primary antibodies CHOP, GRP78, p-PI3K, PI3K AKT and p-AKT overnight at 4°C. Next, the membranes were incubated with the corresponding secondary antibodies at room temperature for 1 h and were rinsed three times (5 min/time) with tris buffered saline tween (TBST). GAPDH was used as an internal reference. Proteins were visualized using ECL reagent, and the blots were quantified using BandScan 5.0 software (Glyko, Inc., Novato, CA, USA).
Statistical analysis
All the data are presented as means ± standard deviation (SD). The statistical comparisons among multiple groups were performed using analysis of variance (ANOVA) followed by a post hoc Tukey's test. A Student's t-test was performed for comparisons between two groups. P<0.05 was considered to indicate a statistically significant difference. The data were analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
NOB alleviated the OGD/R injury in PC12 cells
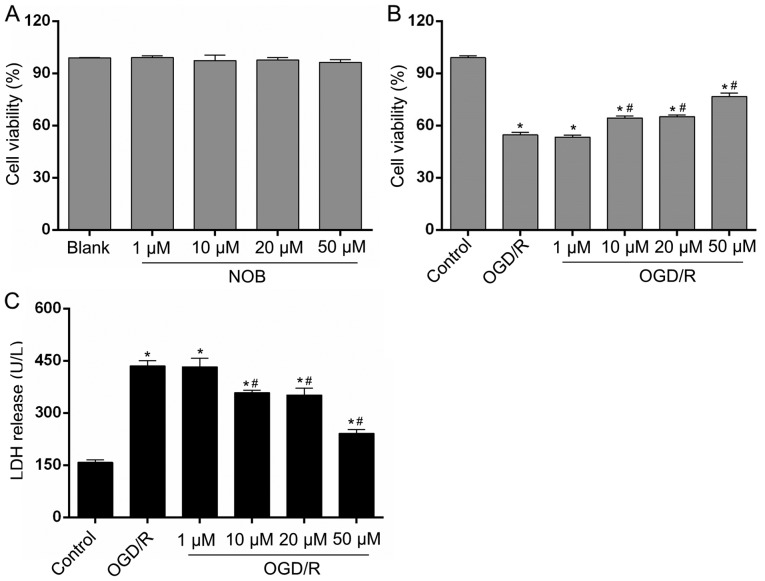
We detected cell viability in each group via a CCK-8 assay. None of the four concentrations of NOB (1, 10, 20 or 50 µM) affected the viability of PC12 cells and showed much lower cytotoxicity on normal PC12 cells (Fig. 1A). To explore the potential contributions of NOB on neuronal OGD/R injury, the PC12 cells were subjected to 4 h of OGD and 24 h of reoxygenation. As shown in Fig. 1B, in comparison with the control group, the cell viability after OGD/R was decreased by 44.78%. Moreover, the gradient doses of NOB (1, 10, 20 and 50 µM) increased cell viability in a concentration-dependent way. Cell viability in the 10 and 20-µM groups was increased by 15.84 and 18.90%, respectively, and the best result was achieved at 50 µM showing a 40.24% increase, relative to the OGD/R group. In addition, LDH release in the cellular supernatant, as a marker of cellular damage, was determined to estimate PC12 cells death. As demonstrated in Fig. 1C, the LDH release induced by OGD/R was repressed by NOB at concentrations starting at 10 µM, with the minimum result at 50 µM, relative to the OGD/R group. In addition, there exhibited no significant difference in the cell viability and LDH release between the 1-µM group and the OGD/R group. Thus, 50 µM of NOB was selected for the subsequent study. These results indicate that NOB could enhance cell survival and limit cell damage in PC12 cells in response to OGD/R injury.
Figure 1.
NOB alleviated the OGD/R injury in PC12 cells. (A) The cytotoxic effects of NOB (1, 10, 20, and 50 µM) on PC12 cells were evaluated using a CCK-8 assay. The four concentration of NOB displayed no cell toxicity on PC12 cells. The effects of NOB (1, 10, 20, and 50 µM) on the cell viability (B) and the LDH release (C) after OGD/R insult. Data are expressed as the mean ± standard deviation (n=3). *P<0.05, compared with the control; #P<0.05, compared with the OGD/R. NOB, nobiletin; OGD/R, oxygen-glucose deprivation and reoxygenation; LDH, lactate dehydrogenase; CCK-8, Cell Counting Kit-8.
NOB treatment repressed ERS-caused apoptosis in OGD/R injury
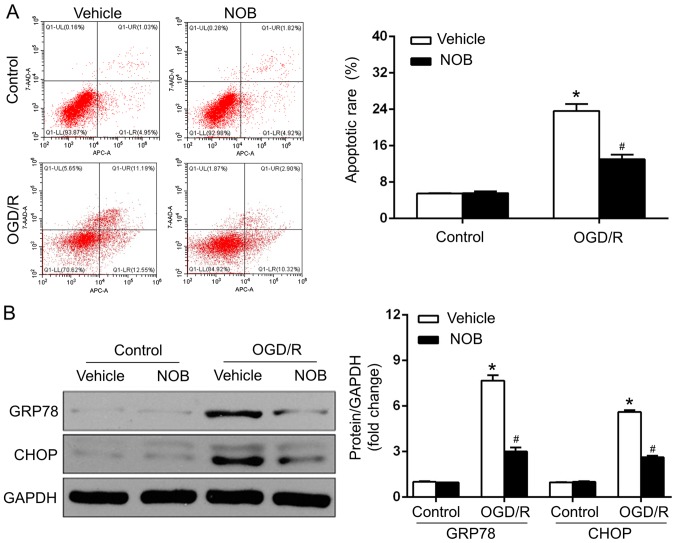
OGD/R injury is closely associated with the stimulation of ERS-caused apoptosis, and NOB has been reported to mediate the process of ERS (1–5,13). GRP78, as an ER chaperone, is pivotal in cell apoptosis in cerebral OGD/R, and ERS drives apoptosis, in particular, through the CHOP-dependent pathway (1–5). To estimate whether NOB (50 µM) affected the neuronal OGD/R injury in an ERS-associated manner, the apoptosis of PC12 cells was measured by a flow cytometric assay, and the protein levels of ERS markers, such asGRP78 and CHOP. were determined by western blotting. The results demonstrated that the apoptotic rate (Fig. 2A) and GRP78/CHOP levels (Fig. 2B) did not differ between the vehicle-control and NOB-control groups. In addition, OGD/R-suffered PC12 cells with vehicle treatment exhibited marked increases in apoptosis and in the GRP78/CHOP expression, compared with those in the control group. In contrast, NOB treatment led to the mitigation of ERS, as reflected by the dramatic decreases in apoptosis and in the GRP78/CHOP levels, compared with that in the vehicle-treated cells after OGD/R (Fig. 2A and B). Therefore, NOB treatment represses OGD/R-induced ERS and the associated apoptosis in PC12 cells.
Figure 2.
NOB repressed ERS-caused apoptosis in OGD/R injury. (A) Apoptotic cells were detected by flow cytometry and apoptotic rates included early and late apoptotic events, in OGD/R-suffered PC12 cells following NOB (50 µM) treatment. (B) Representative blots (left panel) and quantitative results (right panel) regarding the protein levels of GRP78 and CHOP detected by Western blotting assay. GAPDH was used as an internal control. Data are expressed as the mean ± standard deviation (n=3). *P<0.05, compared with the vehicle-control; #P<0.05, compared with the vehicle-OGD/R. NOB, nobiletin; ERS, endoplasmic reticulum stress; OGD/R, oxygen-glucose deprivation and reoxygenation; GRP78, glucose-regulated protein-78; CHOP, C/EBP homologous protein.
NOB activated the PI3K/AKT signaling pathways in OGD/R injury
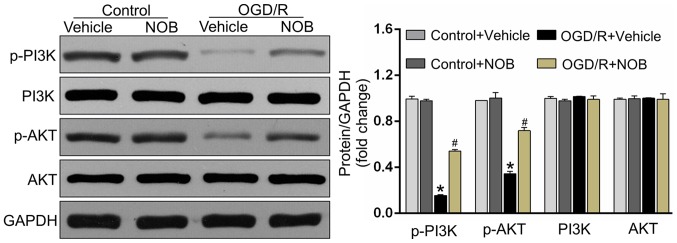
The PI3K/AKT pathway acts as one of the pivotal pro-survival mediators and is fundamental in reducing apoptosis in OGD/R-treated PC12 cells (16,20). To detect whether the PI3K/AKT pathway was involved in the OGD/R-reducing effects of NOB, the following study detected the expressions of the PI3K/AKT pathway by Western blotting. As demonstrated in Fig. 3, OGD/R significantly repressed the activations of the phosphorylated PI3K and AKT in PC12 cells relative to the control cells. Importantly, the drastic increases of the level of p-PI3K/AKT were detected in NOB-treated cells but not in vehicle-operated cells undergoing OGD/R stimulation. Meanwhile, there displayed no significant difference in the p-PI3K/AKT levels between the NOB-treated control and the vehicle-treated control.
Figure 3.
NOB activated the PI3K/AKT signaling pathways in OGD/R injury. The original immunoblots of p-PI3K, PI3K, p-AKT and AKT (left panel), and the corresponding bar graphs of densitometry (right panel) in PC12 cells measured by western blot analysis. GAPDH was used as an internal control. Data are expressed as the mean ± standard deviation (n=3). *P<0.05, compared with the vehicle-control; #P<0.05, compared with the vehicle-OGD/R. NOB, nobiletin; OGD/R, oxygen-glucose deprivation and reoxygenation; PI3K, phosphatidylinositol 3-kinase; AKT, serine/threonine kinase.
The PI3K/AKT inhibition blunted the OGD/R-repressing effects of NOB
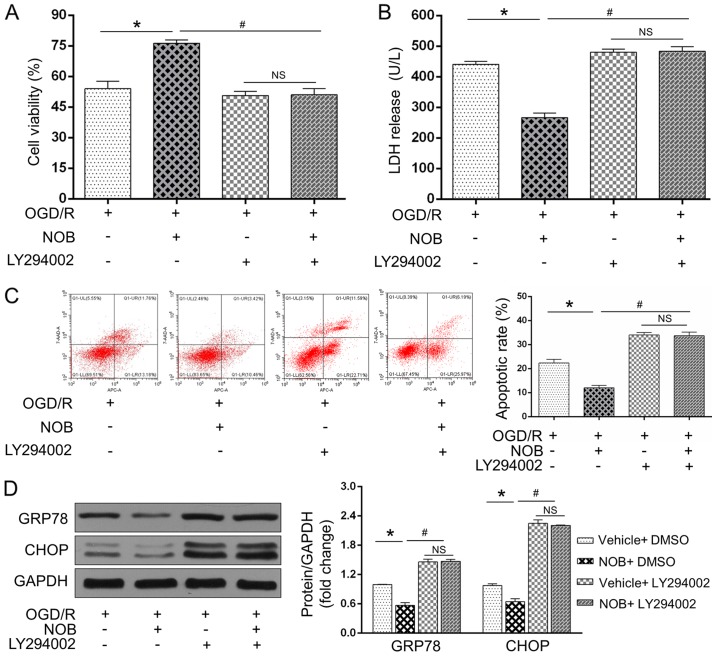
To further examine if the PI3K/AKT signaling acted as a causative roles in the anti-OGD/R effect of NOB, the PC12 cells were subjected to a specific PI3K/AKT inhibitor (LY294002) before the OGD/R insult. As expected, OGD/R-induced cell damages and ERS-induced apoptosis were restored by LY294002, as reflected by the reinforced LDH activity (Fig. 4B), apoptosis (Fig. 4C) and GRP78/CHOP levels (Fig. 4D) and the repressed cell viability (Fig. 4A). Importantly, NOB could not further reverse these alternations following treatment with LY294002 during OGD/R insult (Fig. 4A-D). Of note, no significant difference were observed in these changes in the presence of LY294002 with or without NOB treatment. Together, these findings demonstrated that NOB ameliorates ERS-caused apoptosis in PC12 cells following OGD/R injury through activating the PI3K/AKT pathway.
Figure 4.
The PI3K/AKT inhibition blunted the OGD/R-repressed effects of NOB. (A) Cell viability and (B) LDH release were detected using a CCK-8 assay and ELISA, respectively, in the PC12 cells with or without LY294002 during OGD/R injury. The apoptotic cells were examined by (C) flow cytometry, and GRP78/CHOP expression levels were detected by (D) western blotting. Data are expressed as the mean ± standard deviation (n=3). *P<0.05, compared with the OGD/R; #P<0.05, compared with the NOB-OGD/R. NS, not significant; NOB, nobiletin; OGD/R, oxygen-glucose deprivation and reoxygenation; PI3K, phosphatidylinositol 3-kinase; AKT, serine/threonine kinasel; LDH, lactate dehydrogenase.
Discussion
The pathophysiology of neuronal I/R injury is intimately implicated in the stimulation of ERS and subsequent apoptosis (1–5). A flavonoid, NOB, possesses anti-ERS and anti-apoptotic properties (11,13). However, the knowledge of the beneficial contributions of NOB on neuronal I/R injury and the underlying mechanisms are still lacking. Our studies herein proved that pretreatment with NOB significantly reduced neuronal damages and impacted ERS-caused apoptosis, thus lowering the susceptibility of PC12 cells to OGD/R injury in vitro. This appealing concept, largely developed from a series of novel evidences, was exhibited by the following: i) First, NOB administrations followed by OGD/R injury rescued cell viability and depressed LDH release in a dose-dependent manner in PC12 cells; ii) next, NOB treatment prior to OGD/R injury reversed apoptosis in concert with reductions of ERS-related markers (CHOP/GRP78); iii) then, NOB pretreatment potentiated the PI3K/AKT pathway during OGD/R, while blockage of the PI3K/AKT axis offset the neuro-protective and ERS-repressing effects of NOB, in particular. To the best of our knowledge, this is the first data that has linked the anti-I/R roles of NOB to a PI3K/AKT-associated and ERS-dependent approach, which supports that NOB is a promising therapeutic option to mitigate cerebral I/R injury.
NOB is a ubiquitous bioflavonoid and polyphenolic ingredient isolated from the peels of citrus fruit (9–12). It has been emphasized that NOB exerts pleiotropic biological activities, including the anti-inflammation, anti-tumor and cardiovascular-protections (9–13). Of note, a previous study revealed the ability of NOB to repress the apoptotic process in I/R-exposed Kupffer cells after liver transplantation (10). Recently, Zhang et al provided evidence that NOB protects the myocardium against pressure overload-caused hypertrophy and fibrosis, in which ERS-initiated apoptosis has been substantially implicated (13). However, there is a paucity of data regarding whether NOB slows the progression of cerebral I/R injury, particularly in an apoptosis-limited manner. Based on prior investigations, we thus hypothesized that NOB may act on neuronal PC12 cells and render anti-apoptotic effect in response to OGD/R injury. Accordingly, our study found that NOB exerts beneficial contributions on injured PC12 cells, which expanded the potential activity of NOB in protecting against cerebral I/R injury.
Timely and successful revascularization serves as the cornerstone for the treatment of ischemic stroke (1–5). Nevertheless, the ischemic brain is susceptible to secondary reperfusion injury, which minimizes the benefits of blood re-establishment itself (1–5). In spite of considerable advances in the elucidation of the molecular mechanisms of cerebral I/R injury, there is still a lack of efficient methods to eliminate the detrimental outcomes of this injury. The investigations of more effective drugs or interventions to protect neurons from I/R injury are required to generate greater clinical benefits (1–5). Plenty of studies have verified that the ERS-regulated apoptotic cascade acts as the central etiology in the pathogenesis of cerebral I/R injury, in which this cascade triggers cell death irrespective of mitochondria-dependent and death receptor-relevant apoptosis (1). The ER is an endogenous self-protective organelle responsible for cellular protein biosynthesis, folding and transportation (1–5). Adaptive ER stress helps in the obliteration of mis-folded proteins, namely, via the UPR. However, sustained over-activation of ERS induced by OGD/R provokes the above process upon adaption failing and drives ERS-related apoptotic cascade events subsequently. In this way, ERS substantially switches from a protective response to a pro-apoptotic process with cerebral I/R progression (1–5). There exist numerous ERS-related mediators that have been implicated in cerebral I/R injury, and GRP78/CHOP essentially determine the initiation and extension of ERS-caused apoptosis (1–5). As was set forth, punctual interventions that focuse on prolonged ERS appear to be a promising way to blunt cerebral I/R lesions, despite the current limitations in lowering I/R impairments. It has been reported that the alleviation of ERS and apoptosis yielded by NOB results from the diminishments of CHOP and GRP78 (13). In line with previous studies, our findings provided evidences that NOB supplement at the beginning of OGD/R was available to reduce GRP78 and CHOP expressions, with improvement of cell viability and the repression of both LDH release and neuronal apoptosis in PC12 cells. NOB has thus garnered attention to further examine the precise molecular mechanisms in the context of cerebral I/R injury.
It is well known that the PI3K and AKT pathway is the important survival mediator in cerebral I/R injury, and PI3K/AKT deactivation seems to be able to explain neuronal vulnerability to I/R insult (19–21). Indeed, the activation of the PI3K/AKT pathway participates in multiple pathologic processes such as the myocardial and choriocarcinoma cell I/R injury, in which the compromised ERS-induced apoptosis has been found to be a predominant factor underlying these protections (22,23). However, there is still a lack of clear evidence derived from ERS-induced apoptosis during cerebral I/R injury via the PI3K/AKT-dependent pathway. A previous study confirmed the neuroprotective effect of PI3K/AKT activation against brain I/R injury, and its anti-apoptotic contribution was greatly highlighted (19–21). Intriguingly, Yuan et al produced clues indicating that the activities of the PI3K/AKT pathway are causatively involved in the anti-I/R effects generated by cerebral ischemic postconditioning in an ERS-related way, thus leading to GRP78/CHOP repression and apoptosis reduction (24). In the current study, we suggested that NOB treatment antagonized the apoptotic process that was observed in OGD/R-suffered PC12 cells and promoted protein expression of p-PI3K/AKT while simultaneously suppressing the ERS markers CHOP and GRP78. Additionally, by applying an PI3K/AKT inhibitor, it was found that ERS-limited and OGD/R-attenuated outcomes were significantly blocked in PC12 cells. Therefore, these data suggested the presence of anti-ERS-induced apoptosis in response to the PI3K/AKT stimulation during cerebral I/R, and more importantly, NOB lower ERS-caused neuronal damages in PC12 cells via the PI3K/AKT-dependent way, as expected.
In conclusion, the present study demonstrated, for the first time, that NOB possesses a strong effect against ERS and subsequent apoptosis in neuronal PC12 cells through the activation of the PI3K/AKT pathway under OGD/R conditions. Thus, these results provided novel insights into the molecular mechanisms underpinning cerebral I/R injury. Although the protective effects of NOB were merely measured in the in vitro model, our findings raised the appealing possibility that NOB may represent a novel therapeutic strategy for cerebral I/R injury. More investigations will be conducted to evaluate the role of NOB in other pathogenic mechanism of cerebral I/R injury, such as autophagy, inflammation and mitochondrial-dependent apoptosis, irrespective of ERS-associated apoptosis.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NOB
nobiletin
- ERS
endoplasmic reticulum stress
- OGD/R
oxygen-glucose deprivation and reoxygenation
- GRP78
glucose-regulated protein-78
- CHOP
C/EBP homologous protein
- PI3K
phosphatidylinositol 3-kinase
- AKT
serine/threonine kinase
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
ZRL made substantial contributions to the conception and design of the present study. LY, JZ, YZ and ZNL performed the experiments including cell culture, OGD/R establishment, apoptotic detection and western blotting assay, and were involved in data interpretation manuscript revision.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080. doi: 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai XH, Li XC, Jin SW, Liang DS, Wen ZW, Cao HC, Mei HF, Wu Y, Lin ZD, Wang LX. Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp Neurol. 2014;257:148–156. doi: 10.1016/j.expneurol.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B. Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int. 2014;68:18–27. doi: 10.1016/j.neuint.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Qiu B, Hu S, Liu L, Chen M, Wang L, Zeng X, Zhu S. CART attenuates endoplasmic reticulum stress response induced by cerebral ischemia and reperfusion through upregulating BDNF synthesis and secretion. Biochem Biophys Res Commun. 2013;436:655–659. doi: 10.1016/j.bbrc.2013.05.142. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Park JH, Maharjan S, Park JA, Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, et al. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J Neuroinflammation. 2017;14:122. doi: 10.1186/s12974-017-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon SK, Ahn M, Song HJ, Kang SK, Jung SB, Harsha N, Jee S, Moon JY, Suh KS, Lee SD, et al. Nafamostat mesilate attenuates transient focal ischemia/reperfusion-induced brain injury via the inhibition of endoplasmic reticulum stress. Brain Res. 2015;1627:12–20. doi: 10.1016/j.brainres.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Min HM, Wang Y, Ren DY, Cheng X, Li J, Jiang XQ, Min LQ, Bao CF. Protective effect of 2-deoxy-D-glucose on the brain tissue in rat cerebral ischemia-reperfusion models by inhibiting Caspase-apoptotic pathway. Histol Histopathol. 2017;32:57–67. doi: 10.14670/HH-11-770. [DOI] [PubMed] [Google Scholar]
- 8.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 9.Sp N, Kang DY, Joung YH, Park JH, Kim WS, Lee HK, Song KD, Park YM, Yang YM. Nobiletin inhibits angiogenesis by regulating Src/FAK/STAT3-mediated signaling through PXN in ER+ breast cancer cells. Int J Mol Sci. 2017;18:E935. doi: 10.3390/ijms18050935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Zhang W, Li M, Cao D, Yang X, Gong J. Nobiletin ameliorates ischemia-reperfusion injury by suppressing the function of Kupffer cells after liver transplantation in rats. Biomed Pharmacother. 2017;89:732–741. doi: 10.1016/j.biopha.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, Yang Z, Xiang SZ, Jin YG, Wei WY, Bian ZY, Deng W, Tang QZ. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: Induced diabetic cardiomyopathy. Mol Cell Biochem. 2016;417:87–96. doi: 10.1007/s11010-016-2716-z. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda N, Ishii T, Oyama D, Fukuta T, Agato Y, Sato A, Shimizu K, Asai T, Asakawa T, Kan T, et al. Neuroprotective effect of nobiletin on cerebral ischemia-reperfusion injury in transient middle cerebral artery-occluded rats. Brain Res. 2014;1559:46–54. doi: 10.1016/j.brainres.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Wei WY, Yang Z, Che Y, Jin YG, Liao HH, Wang SS, Deng W, Tang QZ. Nobiletin, a polymethoxy flavonoid, protects against cardiac hypertrophy induced by pressure-overload via inhibition of NAPDH oxidases and endoplasmic reticulum stress. Cell Physiol Biochem. 2017;42:1313–1325. doi: 10.1159/000478960. [DOI] [PubMed] [Google Scholar]
- 14.Shi MD, Liao YC, Shih YW, Tsai LY. Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways in HGF-treated liver cancer HepG2 cells. Phytomedicine. 2013;20:743–752. doi: 10.1016/j.phymed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee YC, Cheng TH, Lee JS, Chen JH, Liao YC, Fong Y, Wu CH, Shih YW. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011;347:103–115. doi: 10.1007/s11010-010-0618-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JF, Zhang L, Shi LL, Zhao ZH, Xu H, Liang F, Li HB, Zhao Y, Xu X, Yang K, Tian YF. Parthenolide attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β pathway in PC12 cells. Biomed Pharmacother. 2017;89:1159–1165. doi: 10.1016/j.biopha.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Mo ZT, Li WN, Zhai YR, Gao SY. The effects of icariin on the expression of HIF-1α, HSP-60 and HSP-70 in PC12 cells suffered from oxygen-glucose deprivation-induced injury. Pharm Biol. 2017;55:848–852. doi: 10.1080/13880209.2017.1281968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Zhu X, Chen M, Ge Q, Shen Y, Pan S. Resveratrol protects PC12 cells against OGD/R-induced apoptosis via the mitochondrial-mediated signaling pathway. Acta Biochim Biophys Sin (Shanghai) 2016;48:342–353. doi: 10.1093/abbs/gmw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH, Johnson NR, Wei X, Chen DQ, Cao G, Fu XB, et al. bFGF protects against blood-brain barrier damage through junction protein regulation via PI3K-Akt-Rac1 pathway following traumatic brain injury. Mol Neurobiol. 2016;53:7298–7311. doi: 10.1007/s12035-015-9583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Ren Z, Wei X, Wang S, Wang Y, Cheng Y, Gao H, Liu H. Losartan protects against cerebral ischemia/reperfusion-induced apoptosis through β-arrestin1-mediated phosphorylation of Akt. Eur J Pharmacol. 2017;815:98–108. doi: 10.1016/j.ejphar.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, An R, Tian X, Yang M, Li M, Lou J, Xu L, Dong Z. β-caryophyllene pretreatment alleviates focal cerebral ischemia-reperfusion injury by activating PI3K/Akt signaling pathway. Neurochem Res. 2017;42:1459–1469. doi: 10.1007/s11064-017-2202-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang L, Chen L, Feng W, Shi H, Yu X, et al. bFGF attenuates endoplasmic reticulum stress and mitochondrial injury on myocardial ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J Cell Mol Med. 2015;19:595–607. doi: 10.1111/jcmm.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yung HW, Korolchuk S, Tolkovsky AM, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007;21:872–884. doi: 10.1096/fj.06-6054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan Y, Guo Q, Ye Z, Pingping X, Wang N, Song Z. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.