Abstract
The aim of the present study was to explore the effect of baicalin on liver hypoxia/reoxygenation (H/R) injury and the possible mechanism involved. A cellular H/R model was established and cells were treated with 50, 100 and 200 µmol/l baicalin. Following reoxygenation for 6 h, cell viability, lactate dehydrogenase (LDH), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), caspase 3 and cleaved caspase 3 were assessed. Furthermore, levels of endoplasmic reticulum stress markers binding of immunoglobulin protein (BIP) and CCAAT/enhancer-binding protein homologous protein (CHOP) and autophagy markers microtubule-associated proteins 1A/1B light chain 3B (LC3) and beclin 1 were measured. To confirm the involvement of autophagy in baicalin-mediated attenuation of H/R injury, the autophagy inhibitor 3-methyladenine (3-MA) was administered. The results revealed that baicalin administration increased cell viability and decreased LDH levels, most notably at a dosage of 100 µmol/l. Baicalin pretreatment also downregulated the expression of caspase 3, cleaved caspase 3 and Bax, while upregulating the expression of Bcl-2. Furthermore, BIP and CHOP were decreased while LC3 and beclin-1 were significantly increased by baicalin pretreatment. Inhibiting autophagy using 3-MA, resulted in a significant decrease in LC3-II, beclin-1 and LDH, as well as increase in the expression of BIP, CHOP, caspase 3, cleaved caspase 3 and Bax. Bcl-2 and cell viability were also decreased. In conclusion, the results of the present study indicate that baicalin exerts a protective effect on liver H/R injury and this may be achieved via the induction of autophagy.
Keywords: baicalin, liver hypoxia/reoxygenation injury, autophagy
Introduction
Liver ischemia reperfusion (IR) injury is a process initiated by hypoxia, which causes cellular damage, followed by the return of blood flow and oxygen delivery, which exacerbates cellular damage (1,2). Overproduction of reactive oxygen species (ROS) and superoxide free radicals in IR causes the destruction of the hepatic cellular membrane and increases their permeability, leading to hepatocyte death and hepatic injury (3). Liver IR occurs in a number of clinical settings, including liver transplantation, partial hepatic resection, shock and hepatic failure (4–6) At present, there is no effective prevention strategy for liver IR injury and there is there for an urgent need for the development of new treatments.
Baicalin is a flavonoid glycoside extracted from the root of the traditional Chinese medicinal herb Scutellaria baicalensis (7). It has been documented that baicalin possesses a number of pharmacological actions, including anti-inflammatory, anti-bacterial, anti-fibrotic, anti-oxidant and anticancer effects (7–11). Previous studies have reported that baicalin serves a protective effect in IR injury in various organs, including the kidneys, brain and heart (12–14). It has been reported that baicalin may be able to induce autophagy, as evidenced by elevated expression of microtubule-associated proteins 1A/1B light chain 3B LC3 and beclin-1 in human bladder T24 cells (15) and human hepatocellular carcinoma SMMC-7721 cells following baicalin treatment (16).
Autophagy is an important intracellular process for degrading macromolecules, as well as recycling cytosolic proteins and organelles, to maintain cellular homeostasis (17). When cellular components are engulfed into double or multiple-membrane cytoplasmic vesicles, autophagosomes are formed, which subsequently fuse with lysosomes, forming autolysosomes and degrading the captured proteins or organelles with lysosomal enzymes (15,18). Autophagy is believed to exert a beneficial effect in IR injury; autophagy ameliorates liver damage when hepatocytes suffer from anoxia or nutrient starvation during IR insults (19). It has also been reported that rapamycin, a mechanistic target of rapamycin (mTOR) inhibitor and potent autophagy inducer, attenuates tubular injury and renal dysfunction by promoting autophagy in acute kidney injury (20), demonstrating the protective role of autophagy in IR injury.
The aim of the present study was to investigate the effect of baicalin on liver IR injury and its relationship with autophagy. The results of the present study indicate that baicalin may be an effective clinical treatment for liver IR injury.
Materials and methods
Cell culture and establishment of hypoxia/reoxygenation model
Normal human liver LO2 cells were purchased from the Chinese Academy of Science (Shanghai, China) and maintained in Dulbecco's modified Eagle's medium/F-12 nutrient mixture (DMEM-F12; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin in 21% O2 at 37°C. Hypoxia/reoxygenation (H/R) was performed as previously described (21). Briefly, cells were seeded in 6-well plates at a density of 105 cells/well. At 80% confluence, the 6-well plates were incubated under hypoxic conditions (94% N2, 5% CO2 and 1% O2 at 37°C) for 24 h in a hypoxia chamber. Cells were then removed from the hypoxia chamber and incubated for 6 h in an atmosphere containing 5% CO2 at 37°C. A total of 6 groups (n=3 each) were established: Control group, H/R group, 50 µmol/l baicalin group, 100 µmol/l baicalin group, 200 µmol/l baicalin group and 3-methyladenine (3-MA; 5 mmol/l) group. In the control group, LO2 cells were cultured under normal culture conditions only. In the baicalin and 3-MA groups, baicalin (cat. no. 572667) or 3-MA (cat. no. M9281) (both Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added 1 h prior to H/R.
Cell viability analysis
Cells were seeded in a 96-well plate at a density of 2,000 cells/well. Following 24 h hypoxia and 6 h reoxygenation, cell survival was assessed using a Cell Counting kit-8 (CCK-8) kit (Beyotime Institute of Biotechnology, Haimen, China). Briefly, 10 µl of CCK-8 reagent was added to each well and incubated for 2 h at 37°C. Cell density was determined by measuring the absorbance at 450 nm using a microplate reader (MDC Vacuum Products, LLC, Hayward, CA, USA).
Lactate dehydrogenase (LDH) activity assay
The supernatant from the 96-well plates were collected by centrifugation at 500 × g for 5 min at 25°C. LDH activity in the supernatant (20 µl) was measured using a LDH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's protocol.
Annexin V and phosphatidylinositol (PI) binding staining
Cells were washed twice with PBS and resuspended in 500 µl staining buffer (Beyotime Institute of Biotechnology) at a concentration of 105 cells/ml with 5 µl Annexin V-fluorescein isothiocyanate (FITC; Beyotime Institute of Biotechnology) and 5 µl PI. Cells were gently mixed and incubated for 15 min at 37°C in the dark. A total of 400 µl cell suspension was transferred into flow tubes and analyzed using a BD FACS Aria II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The flow cytometry data were analyzed using FlowJo 6.0 software (FlowJo LLC, Ashland, OR, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extract from LO2 cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. A total of 3–5 µg of RNA was transcribed into cDNA using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). Reverse transcription was performed at 42°C for 60 min, followed by 70°C for 5 min. Primers used for RT-qPCR are listed in Table I. qPCR was conducted using the SYBR Green premix kit from Takara Bio, Inc. (Otsu, Japan). Thermocycling conditions were as follows: Initial denaturation at 95°C for 30 sec followed by 50 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 60 sec. Expression levels were normalized to β-actin in the same samples using the 2−ΔΔCq method (7).
Table I.
Gene specific primers used for reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence |
|---|---|
| Binding of immunoglobulin protein | Sense: AAAGAAGACGGGCAAAGATGT |
| Antisense: TGCTTGATGCTGAGAAGACAG | |
| CCAAT/enhancer-binding protein homologous protein | Sense: ACCACTCTTGACCCTGCTTCT |
| Antisense: CTCTGGGAGGTGCTTGTGAC | |
| β-actin | Sense: GTTGTCGACGACGAGCG |
| Antisense: GCACAGAGCCTCGCCTT |
Western blotting
LO2 cells were washed with PBS three times and ice-cold radioimmunoprecipitation assay buffer (Vazyme, Piscataway, NJ, USA) was added for cell lysis. Lysates were centrifuged at 12,000 × g at 4°C for 25 min. The supernatant was collected and total proteins were quantified by bicinchoninic acid assay. Samples (20 µg) were separated by 10% SDS-PAGE, transferred onto a polyvinylidene fluoride membranes and blocked with 5% skimmed milk for 1 h at room temperature. Membranes were subsequently incubated with primary antibodies against the following: B-cell lymphoma 2 (Bcl-2; cat. no. 2872), Bcl-2-associated X protein (Bax; cat. no. 5023), caspase 3 (cat. no. 9662), cleaved caspase 3 (cat. no. 9661), binding of immunoglobulin protein (BIP; cat. no. 3177), CCAAT/enhancer-binding protein homologous protein (CHOP; cat. no. 2895), beclin-1 (cat. no. 3495), LC3 (cat. no. 4108), activating transcription factor 4 (ATF4; cat. no. 11815), X-box binding protein 1 (XBP-1; cat. no. 83418) and GAPDH (cat. no. 5174; all 1:1,000; all Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. Membranes were washed with 1X TBS with 1% Tween-20 and incubated with horseradish-conjugated goat anti-rabbit secondary antibodies (cat. no. ab6721; 1:10,000; Abcam) for 1 h at room temperature. Immunoreactive bands were visualized using a chemiluminescence solution (Thermo Fisher Scientific, Inc.) and normalized to GAPDH using Image J software version 1.8.0 (National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Results are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Bonferroni's correction on SPSS 20 software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Baicalin pretreatment attenuates H/R-induced damage to LO2 cells
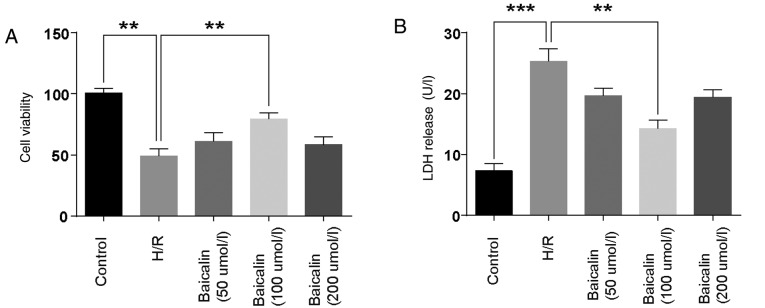
Cell viability and LDH levels were detected to investigate the effect of baicalin on LO2 cell damage. The results demonstrated that, compared with the control group, cell viability was decreased in the H/R group. Treatment with 100 µmol/l baicalin significantly ameliorated the effects of H/R (Fig. 1A). LDH, a marker of cell injury, was significantly elevated in the H/R group, while pretreatment with 100 µmol/l baicalin significantly reversed this effect (Fig. 1B). These results suggest that baicalin pretreatment attenuated H/R-induced damage in LO2 cells.
Figure 1.
Baicalin pretreatment attenuates H/R induced damage in LO2 cells. (A) LO2 cell viability was assessed using a Cell Counting Kit-8 assay. (B) LDH expression in the supernatant was assessed following baicalin treatment. **P<0.01 ***P<0.001. H/R, hypoxia/reperfusion; LDH, lactate dehydrogenase.
Baicalin pretreatment inhibits apoptosis following H/R
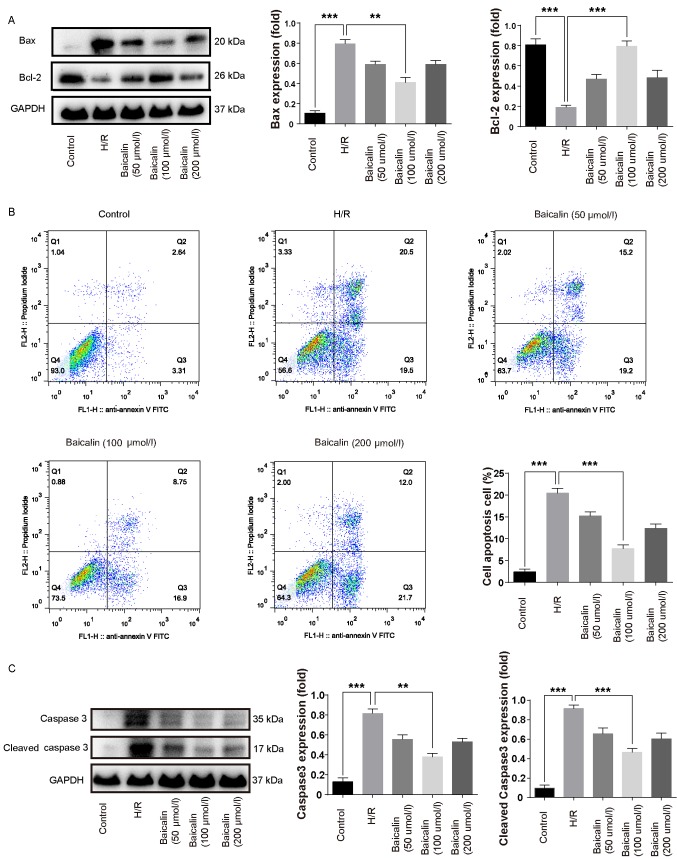
Bcl-2 is an anti-apoptotic protein, whereas Bax is a pro-apoptotic protein (12). Bcl-2 protein expression was lower in the H/R group compared with the control, whereas Bax expression was increased (Fig. 2A). Pretreatment with baicalin significantly increased the expression of Bcl-2 and inhibited the expression of Bax compared with the H/R group (Fig. 2A). The results of flow cytometry revealed that the percentage of apoptotic cells in the H/R group was increased compared with the control group; however, pretreatment with 100 µmol/l baicalin significantly decreased apoptosis compared with the H/R group (Fig. 2B). These results suggest that baicalin pretreatment inhibits apoptosis following H/R.
Figure 2.
Baicalin pretreatment inhibits apoptosis and caspase 3 expression following H/R. (A) Western blotting was used to measure the expression of Bcl-2 and Bax in LO2 cells following H/R. (B) Cell apoptosis in LO2 cells following H/R was evaluated using flow cytometry. (C) Western blotting was used to assess the expression of caspase 3 and cleaved caspase 3 in LO2 cells following H/R. **P<0.01 and ***P<0.001. H/R, hypoxia/reperfusion; Bcl-2, B-call lymphoma 2; Bax, Bcl-2-associated X protein; FITC, fluorescein isothiocyanate.
Baicalin pretreatment decreases caspase 3 expression following H/R
Caspase 3 is a downstream effector in the caspase cascade that directly mediates apoptosis upon activation by multiple upstream signals (22). Therefore, caspase 3 is regarded as an important pro-apoptotic indicator. Caspase 3 and cleaved caspase 3 were increased in the H/R group compared with the control group (Fig. 2C). In contrast, pretreatment with 100 µmol/l significantly decreased the expression of caspase 3 and cleaved caspase 3 compared with the H/R group (Fig. 2C).
Baicalin pretreatment inhibits endoplasmic reticulum (ER) stress following H/R
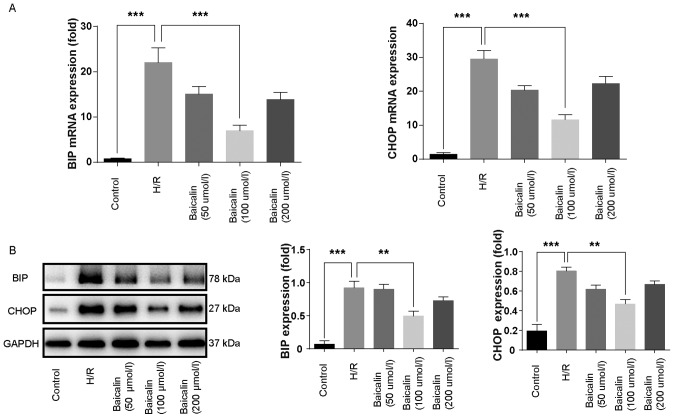
ER stress was assessed by measuring the expression of BIP and CHOP. Compared with the control group, levels of BIP and CHOP mRNA were higher in the H/R group, whereas pretreatment with 100 µmol baicalin significantly ameliorated the effects of H/R (Fig. 3A). Furthermore, the expression of BIP and CHOP at the protein level was increased in the H/R group compared with the control and significantly decreased in the 100 µmol/l baicalin group compared with the H/R group (Fig. 3B). These results suggest that baicalin pretreatment inhibits ER stress following H/R.
Figure 3.
Baicalin pretreatment inhibits endoplasmic reticulum stress following H/R. (A) Reverse transcription-quantitative polymerase chain reaction was used to measure BIP and CHOP mRNA expression in each group following H/R. (B) Western blotting was used to measure BIP and CHOP protein expression in each group following treatment. **P<0.01 and ***P<0.001. H/R, hypoxia/reperfusion; BIP, binding of immunoglobulin protein; CHOP, CCAAT/enhancer-binding protein homologous protein.
Baicalin pretreatment induced autophagy in vitro
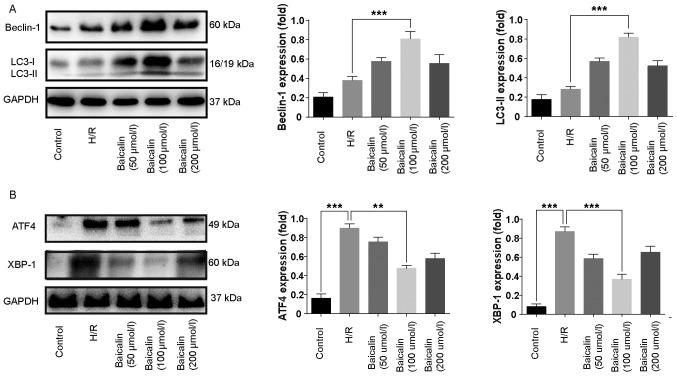
To investigate whether baicalin pretreatment is able to induce autophagy, the expression of LC3 and beclin-1 was assessed. The results of western blotting revealed that the expression of LC3-II and beclin-1 were increased in the H/R group compared with the control (Fig. 4A). Furthermore, 100 µmol/l baicalin pretreatment significantly increases the expression of LC3-II and beclin 1 following H/R (Fig. 4A).
Figure 4.
Baicalin pretreatment inhibits H/R-induced autophagy and is a potent inductor of autophagy in LO2 cells. Western blotting was used to assess the expression of (A) beclin and LC3, as well as (B) ATF4 and XBP-1 in LO2 cells from each group. **P<0.01 and ***P<0.001. H/R, hypoxia/reperfusion; LC3, microtubule-associated proteins 1A/1B light chain 3B; ATF4, activating transcription factor 4; XBP-1, X-box binding protein 1.
Baicalin pretreatment inhibits ER stress-induced autophagy in vitro
It has been reported that ER stress induces autophagy, whereas ATF4 and XBP1 are regarded as important inducers of autophagy following ER stress (23,24). Western blotting revealed that ATF4 and XBP1 was upregulated in the H/R group compared with the control (Fig. 4B). In contrast, baicalin pretreatment significantly reduced the expression of ATF4 and XBP1 compared with the H/R group (Fig. 4B).
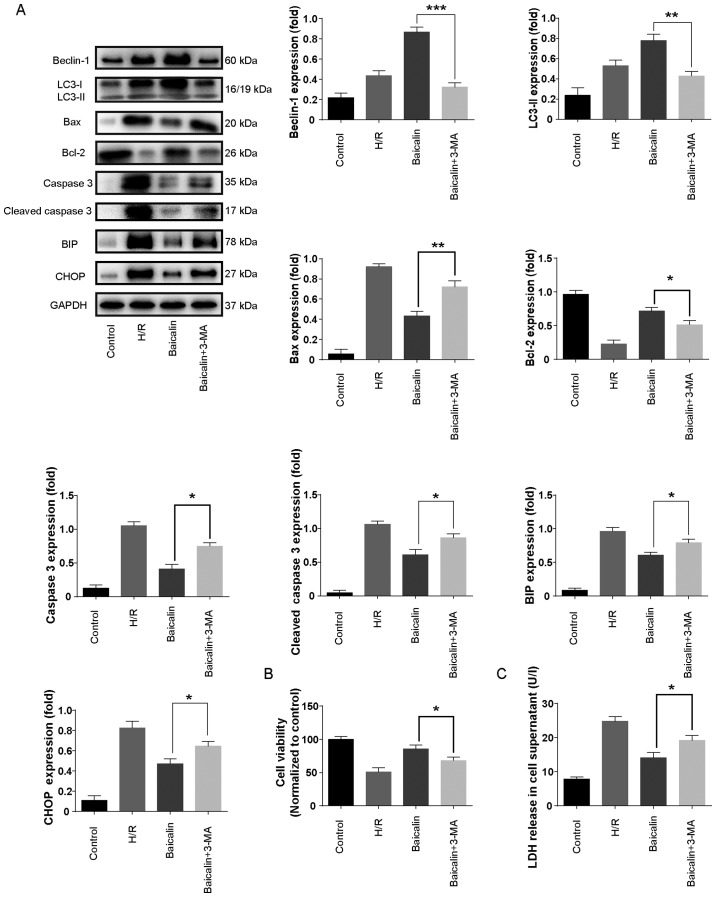
Autophagy inhibition abrogates the protective effect of baicalin in vitro
To further confirm the role of autophagy in H/R injury, the autophagy inhibitor 3-MA was used. The results revealed that LC3-II and beclin-1 levels were increased in the H/R group compared with the control and further increased with baicalin pretreatment (Fig. 5A). However, co-administration with 3-MA significantly decreased the expression of LC3-II and beclin-1 compared with baicalin treatment alone (Fig. 5A).
Figure 5.
Autophagy inhibition abrogated the protective effect of baicalin in vitro. (A) Western blotting was used to measure the expression of beclin, LC3, Bcl-2, Bax, caspase 3 and cleaved caspase 3 in LO2 cells. (B) LO2 cell viability was measured using a Cell Counting Kit-8 assay. (C) Effects of 3-MA on LDH release in LO2 cells subjected to hypoxia/reoxygenation. **P<0.05, **P<0.01 and ***P<0.001. LC3, microtubule-associated proteins 1A/1B light chain 3B; Bcl-2, B-call lymphoma 2; Bax, Bcl-2-associated X protein; 3-MA, 3-methyladenine; LDH, lactate dehydrogenase.
To further investigate the effect of autophagy inhibition in H/R, the expression of caspase-3, cleaved caspase 3, Bcl-2, Bax, BIP and CHOP, as well as cell viability and LDH levels were measured. Co-treatment with 3-MA significantly increased the expression of caspase-3, cleaved caspase 3, Bcl-2, Bax, BIP and CHOP (Fig. 5A). Furthermore, cell viability was significantly decreased in the baicalin + 3-MA treatment group compared with baicalin treatment alone (Fig. 5B). LDH levels were significantly increased with 3-MA treatment compared with baicalin treatment alone (Fig. 5C). These results suggest that autophagy inhibition abrogates the protective effect of baicalin on apoptosis and ER stress in LO2 cells.
Discussion
As a flavonoid glycoside extracted from the root of the traditional Chinese medicinal herb Scutellaria baicalensis, baicalin has been reported to possess a protective effect against IR injury in multiple organs (12–14). Zheng et al (13) demonstrated that baicalin treatment led to significantly reduced neurological deficit, smaller infarct volume and enhanced expression of induced myeloid leukemia cell differentiation protein-1 and BCL-2 in a rat model of cerebral artery occlusion/reperfusion, suggesting that baicalin's protective effect against cerebral IR injury is achieved via the inhibition of ischemia-induced neuronal apoptosis. Kong et al (14) revealed that baicalin improved left ventricular function, reduced creatine kinase and LDH release in the coronary effluent, and increased SOD and MDA activity in rats with IR injury. Lin et al (12) demonstrated that baicalin ameliorates kidney IR injury by inhibiting the production of proinflammatory cytokines, including tumor necrosis factor-α and interleukin-1β. In agreement with previous studies, the results of the present study demonstrated that baicalin pretreatment is able to attenuate hepatic injury and inhibit LO2 apoptosis, possibly via the induction of autophagy.
The upregulation of Bcl-2, downregulation of Bax and decreased percentage of apoptotic cells in the baicalin group indicated that apoptosis was decreased following baicalin pretreatment. Decreased apoptosis is associated with deactivation of the caspase cascade (22). As a downstream effector in the caspase cascade, caspase 3 directly mediates apoptosis when activated by various upstream signals (22) and as such has been reported to be a crucial modulator of apoptosis. In the present study, caspase 3 and cleaved caspase 3 were upregulated in the H/R group, but baicalin pretreatment was able to significantly decrease their expression, indicating that baicalin inhibits LO2 cells apoptosis.
ER stress is a cellular stress condition that disrupts ER function and results in massive accumulation of unfolded or misfolded proteins in the ER lumen (25). To maintain ER homeostasis, a series of adaptive responses called the unfolded protein response (UPR) is triggered upon ER stress (12). This process is mediated by 3 major stress sensors, namely inositol-requiring protein 1 (IRE1), ATF6 and protein kinase RNA-like ER kinase (22). UPR is associated with decreased protein load and upregulated ER chaperones, including BIP (22). With persisting ER stress, UPR fails and cells progress into the apoptosis phase, in which CHOP accumulation, IRE1 phosphorylation and c-JUN N-terminal kinase are activated (22). Herein, BIP and CHOP are regarded as vital markers of ER stress. In the present study, the expression of BIP and CHOP were increased in the H/R group and decreased by baicalin pretreatment, implying that ER stress occurs following H/R and may be successfully inhibited by baicalin pretreatment.
Autophagy is a lysosome-dependent dynamic intracellular pathway that involving the delivery and degradation of unfolded or misfolded proteins (26). It has been documented that ER stress is a potent trigger of autophagy (27). In the process of UPR, ATF4 and XBP-1 induce autophagy (23,24). In the present study, increased ATF4 and XBP-1 levels were observed in the H/R group. Similarly, beclin 1 and LC3-II were upregulate in the H/R group. Pretreatment with baicalin inhibited the expression of ATF4 and XBP-1; however, beclin 1 and LC3-II levels remained high in the baicalin groups, particularly at the 100 µmol/l dosage, revealing that baicalin pretreatment inhibited ER-induced autophagy and that baicalin is a potent inducer of autophagy. This ability is similar to that of a previously reported immunosuppressive drug, rapamycin (28). Zhu et al (28) revealed that, in a mouse model of, ischemia reperfusion injury and thapsigargin-treated primary hepatocytes, ATF4 and XBP1 were markedly upregulated, whereas rapamycin treatment downregulated their expression; in addition, it was also revealed that rapamycin is able to enhance autophagy.
To demonstrate the role of autophagy in the baicalin-mediated protective effect on H/R, the autophagy inhibitor 3-MA was administered. 3-MA is a commonly used autophagy inhibitor and is a specific inhibitor of autophagic/lysosomal protein degradation and the formation of autophagosomes (29). The phosphoinositide 3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway has been implicated in autophagy (29). AKT is downstream of class I and class III PI3K (30). Class I PI3K is a major upstream modulator of mTOR that causes the suppression of autophagy, while the class III PI3K pathway has been recognized to activate autophagy through sequestration of cytoplasmic material (30). It has been reported that 3-MA is able to inhibit the class III PI3K pathway, thus inhibiting autophagy (31). The results of the present study revealed that pretreatment with 3-MA markedly inhibited autophagy, as evidenced by low expression of beclin-1 and LC3-II. Furthermore, high expression of BIP and CHOP were detected following the inhibition of autophagy, suggesting that autophagy inhibition aggravates ER stress. This is consistent with a previous study, in which autophagy was reported to eliminate accumulated misfolded proteins and degrade damaged organelles to ameliorate ER stress (32). Autophagy inhibition also increased LDH levels, increased the expression of caspase-3, cleaved caspase 3 and Bax and decreased cell viability and Bcl-2 expression, which indicates a protective effect of baicalin in H/R. In conclusion, the results of the present study demonstrate that baicalin may have a protective effect in H/R, possibly mediated by the induction of autophagy. Although the action and mechanism of baicalin on liver hypoxia/reoxygenation injury were investigated, there are certain limitations to the present study. The effect of baicalin on liver hypoxia/reoxygenation injury was only performed in vitro and requires further study in vivo. In addition the PI3K/AKT/mTOR signaling pathway was not investigated in the present study and represents an important pathway in autophagy. This should also be further investigated in any subsequent animal studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grants from the National Natural Science Foundation of China (grant nos. 81400675 and 81603406).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZM and FL conceived the study and participated in the design of the study. JZ and JQ performed the experiments. FL analyzed the data and drafted the manuscript. GW revised the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53:2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, Tian Y, Jiao C, Wang X, Li X. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One. 2014;9:e98834. doi: 10.1371/journal.pone.0098834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, Zheng S. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant Proc. 2007;39:1332–1337. doi: 10.1016/j.transproceed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/S0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 5.Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: Established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Shimazu M, Wakabayashi G, Tanabe M, Morikawa Y, Hoshino K, Harada H, Kadomura T, Obara H, Urakami H, et al. Significance of portal venous flow in graft regeneration after living related liver transplantation. Transplant Proc. 2001;33:1484–1485. doi: 10.1016/S0041-1345(00)02562-8. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Zhang C, Li L, Hu C, Hu M, Sidikejiang N, Wang X, Lin M, Rong R. Baicalin ameliorates renal fibrosis via inhibition of transforming growth factor β1 production and downstream signal transduction. Mol Med Rep. 2017;15:1702–1712. doi: 10.3892/mmr.2017.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Wang J, Sheng Y, Zou Y, Bo L, Wang F, Lou J, Fan X, Bao R, Wu Y, et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One. 2012;7:e35523. doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waisundara VY, Siu SY, Hsu A, Huang D, Tan BK. Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. 2011;88:1016–1025. doi: 10.1016/j.lfs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Shieh DE, Cheng HY, Yen MH, Chiang LC, Lin CC. Baicalin-induced apoptosis is mediated by Bcl-2-dependent, but not p53-dependent, pathway in human leukemia cell lines. Am J Chin Med. 2006;34:245–261. doi: 10.1142/S0192415X06003801. [DOI] [PubMed] [Google Scholar]
- 11.Motoo Y, Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994;86:91–95. doi: 10.1016/0304-3835(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 12.Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R, Zhu T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14:19. doi: 10.1186/1472-6882-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng WX, Cao XL, Wang F, Wang J, Ying TZ, Xiao W, Zhang Y, Xing H, Dong W, Xu SQ, et al. Baicalin inhibiting cerebral ischemia/hypoxia-induced neuronal apoptosis via MRTF-A-mediated transactivity. Eur J Pharmacol. 2015;767:201–210. doi: 10.1016/j.ejphar.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Kong F, Luan Y, Zhang ZH, Cheng GH, Qi TG, Sun C. Baicalin protects the myocardium from reperfusion-induced damage in isolated rat hearts via the antioxidant and paracrine effect. Exp Ther Med. 2014;7:254–259. doi: 10.3892/etm.2013.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC, Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int J Oncol. 2013;42:993–1000. doi: 10.3892/ijo.2013.1791. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep. 2012;27:1128–1134. doi: 10.3892/or.2011.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shintani T, Klionsky DJ. Autophagy in health and disease: A double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geronimo-Olvera C, Montiel T, Rincon-Heredia R, Castro-Obregón S, Massieu L. Autophagy fails to prevent glucose deprivation/glucose reintroduction-induced neuronal death due to calpain-mediated lysosomal dysfunction in cortical neurons. Cell Death Dis. 2017;8:e2911. doi: 10.1038/cddis.2017.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney Int. 2012;82:1250–1253. doi: 10.1038/ki.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao W, Li H, Han X, Chen C, Zhang Y, Tai WL, Xia Z, Hei Z. MG53 anchored by dysferlin to cell membrane reduces hepatocyte apoptosis which induced by ischaemia/reperfusion injury in vivo and in vitro. J Cell Mol Med. 2017;21:2503–2513. doi: 10.1111/jcmm.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong R, Zhu T. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci. 2014;15:12507–12522. doi: 10.3390/ijms150712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue LX, Liu HY, Cui Y, Dong Y, Wang JQ, Ji QY, He JT, Yao M, Wang YY, Shao YK, et al. Neuroprotective effects of Activin A on endoplasmic reticulum stress-mediated apoptotic and autophagic PC12 cell death. Neural Regen Res. 2017;12:779–786. doi: 10.4103/1673-5374.206649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishino A, Hayashi K, Hidai C, Masuda T, Nomura Y, Oshima T. XBP1-FoxO1 interaction regulates ER stress-induced autophagy in auditory cells. Sci Rep. 2017;7:4442. doi: 10.1038/s41598-017-02960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyer-Hansen M, Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 26.B'Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang Z, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 2013;288:859–872. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Hua X, Li D, Zhang J, Xia Q. Rapamycin attenuates mouse liver ischemia and reperfusion injury by inhibiting endoplasmic reticulum stress. Transplant Proc. 2015;47:1646–1652. doi: 10.1016/j.transproceed.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Hermann R, Vélez DE, Rusiecki TM, Fernández Pazos Mde L, Cordero Mestre VE, Prendes Marina MG, Rossini Perazzo JC, Savino EA, Varela A. Effects of 3-methyladenine on isolated left atria subjected to simulated ischaemia-reperfusion. Clin Exp Pharmacol Physiol. 2015;42:41–51. doi: 10.1111/1440-1681.12323. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Son KM, Kim KY, Yu SN, Park SG, Kim YW, Nam HW, Suh JT, Ji JH, Ahn SC. Deoxypodophyllotoxin induces cytoprotective autophagy against apoptosis via inhibition of PI3K/AKT/mTOR pathway in osteosarcoma U2OS cells. Pharmacol Rep. 2017;69:878–884. doi: 10.1016/j.pharep.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Sun Y, Liu K, Sun X. Autophagy: A double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 2014;9:1210–1216. doi: 10.4103/1673-5374.135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.