Abstract
The present study determined the expression of microRNA (miR)-663 in hypertrophic scar (HS) tissues and investigate the regulatory mechanisms of miR-663 in HS. A total of 51 patients diagnosed with HS between December 2013 and February 2016 were included in the present study. HS tissues (experimental group) and HS-adjacent tissues (control group) were collected. Primary fibroblasts were obtained from HS tissue and transfected with small-interfering RNA against transforming growth factor (TGF)-β1 or miR-663 mimics. Reverse-transcription quantitative PCR was used to determine the levels of TGF-β1 mRNA and miR-663. Western blot analysis was performed to determine TGF-β1 protein expression. An MTT assay was employed to detect the proliferation of fibroblasts, and a dual luciferase reporter assay was performed to identify the binding of miR-663 with TGF-β1 mRNA. TGF-β1 was found to have a regulatory role in HS at the transcriptional level. The expression of TGF-β1 was upregulated in HS tissues, and knockdown of TGF-β1 in cultured fibroblasts led to inhibition of proliferation. The expression of miR-663 was downregulated in HS. miR-663 was revealed to regulate the expression of TGF-β1 by binding with the 3′-untranslated region of TGF-β1 mRNA. Elevated expression of miR-663 inhibited the proliferation of fibroblasts by regulating TGF-β1 expression. The present study demonstrated that upregulation of TGF-β1 in HS tissues is associated with the downregulation of miR-663 expression. miR-663 may regulate the proliferation of fibroblasts in HS and the expression of associated proteins.
Keywords: hypertrophic scar, microRNA-663, transforming growth factor-β1
Introduction
Hypertrophic scar (HS) is fibrotic abnormality that is commonly observed after wound healing of human skin (1). Treatments of wounds mainly focus on promoting wound healing and inhibiting excessive scar formation (2–4). However, certain contradictions remain between the two; for instance, growth factors that promote wound healing may also increase the risk of scar formation (5–7). Treatments of scars are still empirical (8,9), and are not always effective (10). Furthermore, certain scar treatments have severe side effects that may affect healing (11). Therefore, there is currently no effective way to promote wound healing and simultaneously inhibit the formation of scar.
Transforming growth factor (TGF)-β1 is a member of the TGF superfamily that is generated by multiple types of cell. TGF-β1 regulates the growth, differentiation and immune functions of cells. It regulates the production of interleukin (IL)-6 by human fibroblasts, and facilitates the growth of fibroblasts and osteoblasts (12). In HS, TGF-β1 exerts its effects via its downstream signal transduction factors, such as Smad3 and 2. Through this TGF-β1/Smad pathway, scar formation is promoted (13,14). Another study has demonstrated that in Smad3 knockout mice, the wound healing rate was significantly accelerated (15), suggesting that inhibition of TGF-β1-dependent pathways may achieve the goal of promoting wound healing and attenuating scar formation.
MicroRNAs (miRNAs or miRs) are a class of small non-coding RNAs of ~22 nucleotides in length, which have functions in RNA silencing and post-transcriptional regulation of gene expression (16). Upregulated microRNA-20a expression has been reported to inhibit the expression of TGF-β1 and finally suppresses the proliferation of fibroblasts (17). Overexpression of miRNAs upstream of TGF-β1 may be an effective method for the treatment of scars. For instance, miR-663 has been demonstrated to affect the proliferation, migration and invasion of tumor cells by regulating TGF-β1 (18,19). However, to the best of our knowledge, the effect of miR-663 on fibroblasts in HS has remained to be demonstrated. The present study therefore investigated the expression of TGF-β1 mRNA and protein as well as miRNA-663 in HS and HS-adjacent tissues as well as isolated fibroblasts in order to elucidate the association between TGF-β1 and miRNA-663 in HS.
Patients and methods
Patients
A total of 51 patients diagnosed with HS at the Department of Plastic and Cosmetic Surgery of the Second Affiliated Hospital of Soochow University (Suzhou, China) between December 2013 and February 2016 were included in the present study. HS tissues (experimental group) and HS-adjacent tissues (control group) were collected. Among the 51 patients, 18 were male and 33 were female, with an age range of 18–58 years and a median age of 41.6 years. None of the patients had a history of use of hormones, intake of Chinese medicine or radiochemotherapy. HS of all patients were formed by scalds or burns, and met the criteria of the Patient and Observer Scar Assessment Scale classification (20,21). All procedures were approved by the Ethics Committee of Soochow University (Suzhou, China). Written informed consent was obtained from all patients or their families.
Cells
To obtain primary fibroblasts, HS and HS-adjacent tissues were washed with 0.1 M PBS (pH 7.4) for three times. The tissues were then digested with 0.25% dispase (Roche Diagnostics, Basel, Switzerland) at 4°C overnight to remove the skin. After homogenization, the tissues were digested with 0.1% type I collagenase (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with agitation at 40 × g for 180 min. Digestion was then terminated by mixing the samples with an equal volume of low-glucose Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.). After centrifugation at 600 × g for 10 min, the supernatant was discarded and the fibroblasts were resuspended in high-glucose DMEM (Thermo Fisher Scientific, Inc.). The fibroblasts were seeded onto a culture plate with a diameter of 100 mm at a density of 4×104/cm2 and cultured at 37°C in an atmosphere containing 5% CO2 with 100% humidity. The medium was first replenished after 24 h, and replaced every other day thereafter. The cells were passaged when reaching confluence.
One day prior to transfection, 3×105 cells in the logarithmic growth phase (second passage) were seeded onto 24-well plates containing DMEM/F12 medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum, but without antibiotics. For the silencing of TGF-β1, the small-interfering RNA (siRNA) of TGF-β1 forward, 5′-ACCCACAACGAAAUCUAUGdTdT-3′ and reverse, 5′-CAUAGAUUUCGUUGUGGGUdTdT-3′ was used to transfect fibroblasts. Scrambled siRNA (sc-37007; both Sangon Biotech, Co., Ltd., Shanghai, China) was used as negative control. For transfection of fibroblasts with miR-663 mimics (agomiR-663), the cells were divided into control group (untreated), negative control (NC; transfected with empty plasmids) group and miR-663 mimics group. When reaching 70% confluence, 1.25 µl miR-663 mimics plasmid (Sangon Biotech, Co., Ltd.) and 1 µl Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) were added into two individual vials each containing 50 µl OptiMEM medium (Thermo Fisher Scientific, Inc.). Five minutes later, the liquids in the two vials were mixed and left to stand for another 20 min. The mixture was then added onto the cells for an incubation of 6 h. After replacing the medium with fresh DMEM/F12 supplemented with 10% fetal bovine serum, the fibroblasts were cultured under normal conditions for 48 h prior to use.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Tissues (100 mg) were ground into powder using liquid nitrogen, followed by addition of 1 ml TRIzol (10606ES60; Yeasen, Shanghai, China) for lysis. Total RNA was the extracted using the phenol chloroform method. The purity of RNA was determined by measuring the 260/280 nm absorbance using ultraviolet spectrophotometry (Nanodrop ND1000; Thermo Fisher Scientific, Inc.). The complementary (c)DNA was obtained by RT using the TIANScript II cDNA first-strand synthesis kit (cat. no. KR107; Tiangen, Beijing, China) from 1 µg RNA and stored at −20°C. The primers for TGF-β1 were: Forward, 5′-GGACACCAACTATTGCTTCAG-3′ and reverse, 5′-TCCAGACTCCAAATGTAG-3′. The primers for internal reference, β-actin, were as follows: Forward, 5′-TTCCAGCCTTCCTTCCTGG-3′ and reverse, 5′-TTGCGCTCAGGAGGAGGAAT-3′. The PCR reaction system (25 µl) was composed of 10 µl SuperReal PreMix (SYBR-Green; cat. no. FP204; Tiangen), 0.5 µl forward primer, 0.5 µl reverse primer, 2 µl cDNA and 7 µl double-distilled H2O. The thermocycling conditions were as follows: Initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 94°C for 45 sec, annealing at 55°C for 55 sec and elongation at 72°C for 1 min, and one final elongation step at 72°C for 10 min (iQ5; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq method (22) was used to calculate the relative expression of TGF-β1 mRNA against β-actin.
To assess the expression of miR-663, the miRcute miRNA qPCR detection kit (FP401; Tiangen) was selected, using U6 as an internal reference. The primers for miR-663 were as follows: Forward, 5′-GCGAGCACAGAATTAATACGAC-3′ and reverse, 5′-AGGCGGGGCGCCGCG-3′. The primers for U6 were: Forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The PCR thermocycling conditions were as follows: Initial denaturation at 95°C for 5 min; 40 cycles of denaturation at 95°C for 15 sec and annealing at 60°C for 30 sec; and final elongation at 72°C for 20 sec (iQ5). The 2−ΔΔCq method was used to calculate the relative expression of miR-663 against U6.
Western blot analysis
Tissues (100 mg) were ground using liquid nitrogen and mixed with 200 µl pre-cooled radioimmunoprecipitation assay lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for lysis on ice for 30 min. The mixture was then centrifuged at 12,000 × g and 4°C for 15 min. The supernatant was used to determine the protein concentration by using a bicinchoninic acid protein concentration determination kit (cat no. RTP7102; Real-Times Biotechnology Co., Ltd., Beijing, China). Protein samples (20 µg) were then mixed with sodium dodecyl sulfate loading buffer prior to denaturation in a boiling water bath for 5 min. Afterwards, the samples were subjected to 10% SDS-PAGE. The resolved proteins were transferred onto polyvinylidene difluoride membranes (Merck KGaA; Darmstadt, Germany) on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. The membranes were then incubated with rabbit anti-human TGF-β1 polyclonal primary antibody (1:500 dilution; cat. no. ab92486) and rabbit anti-human β-actin primary antibody (1:5,000 dilution; cat. no. ab129348; both Abcam, Cambridge, UK) at 4°C overnight. After extensive washing with PBS containing Tween-20 (PBST) 3 times for 15 min each, the membranes were incubated with polyclonal goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3,000 dilution; cat. no. ab6721; Abcam) for 1 h at room temperature prior to washing with PBST 3 times for 15 min each. The membrane was developed with an enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck KGaA) for imaging with GelDoc XR+ (Bio-Rad Laboratories, Inc.). Image lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to acquire and analyze imaging signals. The relative content of TGF-β1 protein was expressed as the TGF-β1/β-actin ratio.
MTT assay
After transfection, cells were seeded into 96-well plates at a density of 2×103 cells per well. Each condition was tested in triplicate wells. At 24, 48 and 72 h following transfection, 20 µl MTT (5 g/l; JRDC000003, JRDUN Biotechnology, Shanghai, China) was added to each well, followed by incubation for 4 h at 37°C. Following the aspiration of medium, DMSO (150 µl per well) was added to dissolve purple crystals. Then, the absorbance of each well was measured at 490 nm with a microplate reader (Bio-Rad Laboratories, Inc.) and cell proliferation curves were plotted.
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool for the study of the functions of miRNAs. To understand the regulatory mechanism of TGF-β1 in HS, miRanda (http://www.microma.org/rnicroma/home.do), TargetScan (http://www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html), RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and PICTA (http://pictar.mdc-berlin.de/) were used to predict miRNA molecules that regulate TGF-β1, and miR-663 identified as a potential regulator of TGF-β1 (Fig. 1). According to these bioinformatics results, wild-type (WT) and mutant seed regions of miR-663 of the 3′-untranslated region (3′UTR) of TGF-β1 mRNA were chemically synthesized as described previously (23). SpeI and HindIII restriction sites were added, and the sequences were cloned into pMIR-REPORT luciferase reporter plasmids (Ambion; Thermo Fisher Scientific, Inc.). Plasmids (0.8 µg) with WT or mutant 3′-UTR DNA sequences were co-transfected with agomiR-663 (100 nM; Sangon Biotech, Co., Ltd.) into 293T cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China). The cells in negative control (NC) group were transfected with the aforementioned empty plasmid. After cultivation for 24 h, the cells were lysed using a dual luciferase reporter assay kit according to the manufacturer's manual, and the fluorescence intensity was measured using a GloMax 20/20 luminometer (both Promega Corp., Madison, WI, USA). Using Renilla fluorescence activity as an internal reference, the fluorescence values of each group of cells were measured.
Figure 1.

Bioinformatics prediction of direct interactions between miR-663 and TGF-β1. Bioinformatics prediction is a powerful tool for the study of the functions of miRNAs. To determine the regulatory mechanism of TGF-β1 in hypertrophic scars, miRanda (http://www.microma.org/rnicroma/home.do), TargetScan (http://www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html), RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and PICTAR (http://pictar.mdc-berlin.de/) were used to predict miRNA molecules that regulate TGF-β1, indicating that miR-663 was potentially able to regulate TGF-β1. TGF, transforming growth factor; miR, microRNA; hsa, Homo sapiens.
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ± standard deviation. Data were tested for normality. In the case of homogeneity of variance, the Least Significant Differences and Student-Newman-Keuls tests were used, while in the case of heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was used. P<0.05 was considered to indicate a statistically significant difference.
Results
TGF-β1 has a regulatory role in HS
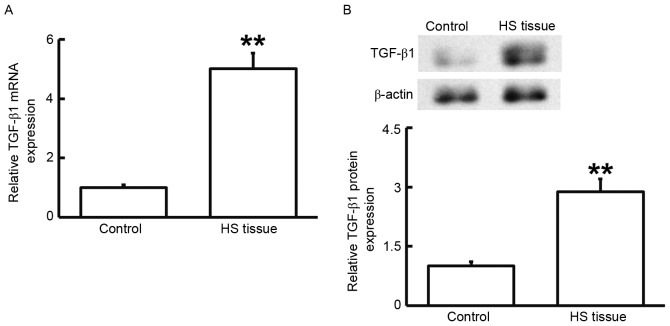
To measure the mRNA and protein expression of TGF-β1, RT-qPCR and western blot analysis were performed. As presented in Fig. 2, the levels of TGF-β1 mRNA and protein in HS tissues were significantly higher than those in HS-adjacent tissues (P<0.01). These results suggested that TGF-β1 has a regulatory role in HS.
Figure 2.
(A) mRNA and (B) protein expression of of TGF-β1 in HS and HS-adjacent tissue. Reverse-transcription quantitative polymerase chain reaction was used to determine the expression of mRNA and western blotting was employed to measure protein expression. **P<0.01 compared with control. HS, hypertrophic scar; TGF, transforming growth factor.
Reduced TGF-β1 expression leads to inhibited proliferation of fibroblasts
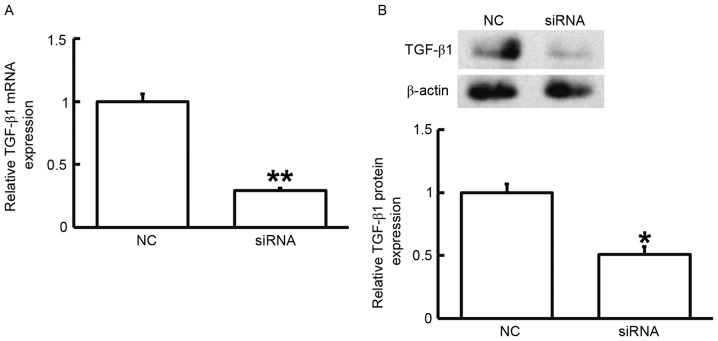
To assess the effect of TGF-β1 on the proliferation of fibroblasts, the cells were transfected with siRNA targeting TGF-β1 and subjected to an MTT assay. RT-qPCR and western blot analysis demonstrated that the expression of TGF-β1 mRNA and protein was significantly reduced after transfection with the siRNA targeting TGF-β1 (P<0.05; Fig. 3). In addition, an MTT assay demonstrated that the proliferation of fibroblasts transfected with siRNA targeting TGF-β1 was significantly reduced at 72 h compared with that in the control group (P<0.05; Fig. 4). The results indicated that reduced TGF-β1 expression leads to inhibited proliferation of fibroblasts.
Figure 3.
(A) mRNA and (B) protein expression of TGF-β1 in fibroblasts prior to and after silencing of TGF-β1. Reverse-transcription quantitative polymerase chain reaction was used to determine the expression of mRNA and western blotting was employed to measure protein expression. *P<0.05; **P<0.01 compared with NC. TGF, transforming growth factor; NC, negative control transfected with scrambled control siRNA; siRNA, small interfering RNA; TGF, transforming growth factor.
Figure 4.
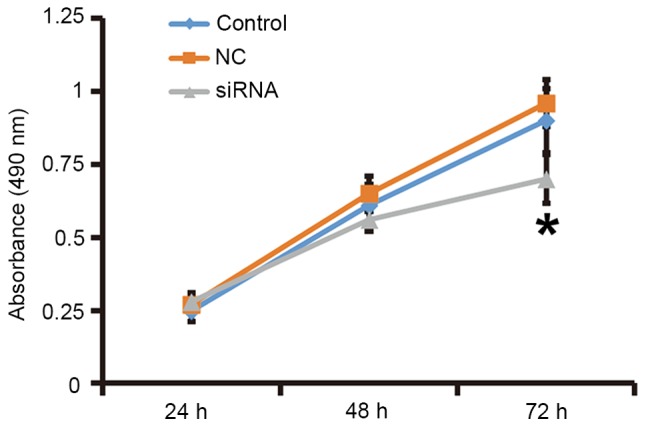
Proliferation of fibroblasts at 24, 48 and 72 h after silencing of transforming growth factor-β1. An MTT assay was used to determine the proliferation of fibroblasts. Absorbance of each well was measured at 490 nm with a microplate reader and cell proliferation curves were plotted. *P<0.05 compared with control and NC. NC, negative control siRNA; siRNA, small interfering RNA.
Expression of miR-663 is downregulated in HS
To determine the expression of miR-663, RT-qPCR was performed. As presented in Fig. 5, miR-663 levels in HS tissues were significantly lower than those in HS-adjacent tissues (P<0.05). This result suggested that the expression of miR-663 is downregulated in HS.
Figure 5.
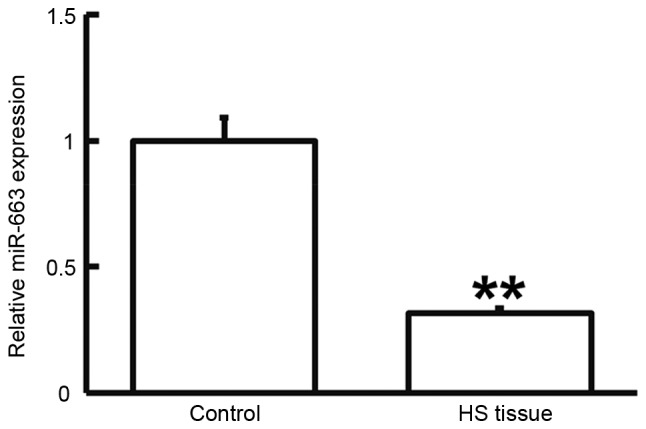
Expression of miR-663 in HS and HS-adjacent tissue. Reverse-transcription quantitative polymerase chain reaction was used to determine the expression of miR-663. **P<0.01 compared with control. HS, hypertrophic scar; miR, microRNA.
miR-663 regulates the expression of TGF-β1 by binding with the 3′-UTR of TGF-β1
To test whether miR-663 targets TGF-β1, a dual luciferase reporter assay was performed. As displayed in Fig. 6, transfection with agomiR-663 and pMIR-REPORT in the WT group resulted in a significantly reduced fluorescence intensity compared with that in the negative control group (P<0.05), while fluorescence intensity in the mutant group was not significantly different from that in the negative control group (P>0.05). This result indicated that miR-663 regulates the expression of TGF-β1 by binding with the 3′-UTR of TGF-β1.
Figure 6.
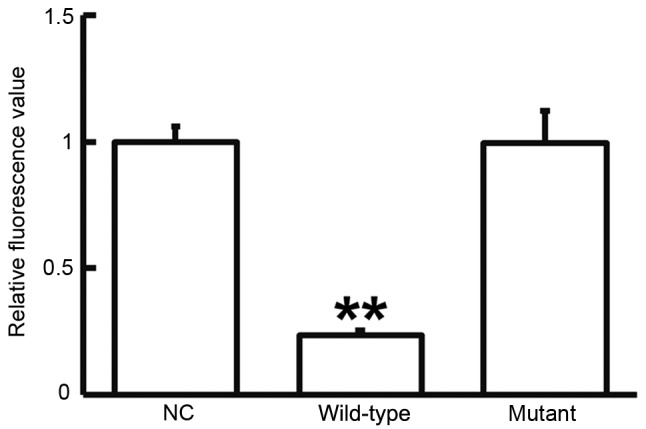
Fluorescence values of HEK293T cells transfected with luciferase reporter plasmid containing wild-type or mutant 3′-untranslated region DNA sequences of TGF-β1 and miR-663 mimics. A dual luciferase reporter assay was used to evaluate the interaction between miR-663 and TGF-β1. **P<0.01 compared with NC. TGF, transforming growth factor; NC, negative control transfected with empty plasmid; miR, microRNA.
Elevated expression of miR-663 inhibits the proliferation of fibroblasts by regulating TGF-β1 expression
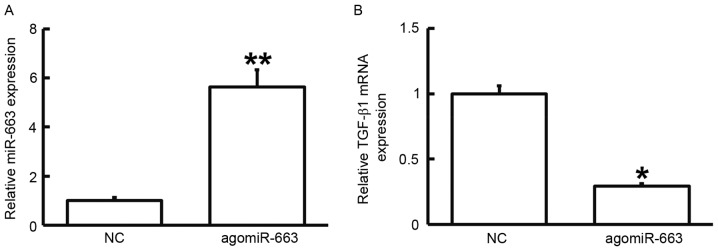
To examine how miR-663 affects the proliferation of fibroblasts, the cells were transfected with agomiR-663 and subjected to an MTT assay. RT-qPCR analysis revealed that the levels of miR-663 in fibroblasts were significantly enhanced after transfection with agomiR-663 (P<0.01; Fig. 7A). In addition, the expression of TGF-β1 mRNA was significantly reduced after transfection with agomiR-663 (P<0.05; Fig. 7B). Of note, the MTT assay demonstrated that the proliferation of fibroblasts transfected with agomiR-663 was significantly reduced at 48 and 72 h compared with that in the control group (P<0.05; Fig. 8). These results suggested that elevated expression of miR-663 inhibits the proliferation of fibroblasts by regulating TGF-β1 expression.
Figure 7.
Expression of (A) miR-663 and (B) TGF-β1 mRNA in fibroblasts prior to and after transfection with agomiR-663. Reverse-transcription quantitative polymerase chain reaction was used to determine the expression of miRNA and mRNA. *P<0.05; **P<0.01 compared with NC. TGF, transforming growth factor; NC, empty plasmid-transfected; miR, microRNA; agomiR-663, miR-663 mimics.
Figure 8.
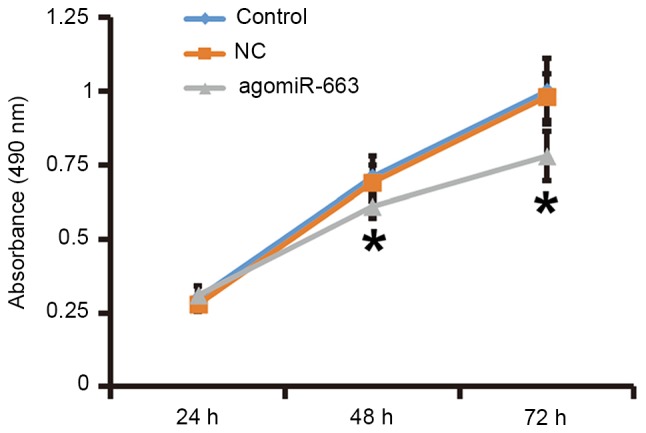
Proliferation of fibroblasts at 24, 48 and 72 h after transfection with agomiR-663. An MTT assay was used to determine the proliferation of fibroblasts. Absorbance of each well was measured at 490 nm with a microplate reader and cell proliferation curves were plotted. *P<0.05 compared with control and NC. NC, empty plasmid-transfected; agomiR-663, microRNA-663 mimics.
Discussion
The pathological essence of HS is the excessive proliferation of cells (mainly fibroblasts) and the excessive deposition of extracellular matrix (mainly collagen). However, the pathogenesis of HS has remained to be fully elucidated. The mechanism of HS formation is the metabolic imbalance between the synthesis and decomposition of extracellular matrix components such as collagen. Excessive contracture caused by scar hyperplasia often leads to a variety of abnormalities, leading to pain or functional disorders of the human body (24). Several studies have reported the inhibition of cell proliferation by TGF-β1, with the underlying mechanism mainly comprising the regulation of Smad3 by TGF-β1 (25,26). Certain studies have revealed that reduced expression of TGF-β1 is a significant characteristic of non-scar healing of fetuses, leading to a faster healing rate, more active cell proliferation, lower type I collagen content and no scar formation compared with the skin healing in adults (27–29). Therefore, non-scar healing of fetuses is an ideal tissue repair status, and reduced expression of TGF-β1 has an important role in achieving this ideal healing (30). In the present study, TGF-β1 expression was found to be elevated in HS tissues, and the proliferation of primary fibroblasts transfected with siRNA targeting TGF-β1 was inhibited. These results suggested that abnormally elevated expression of TGF-β1 may have an important role in HS.
The regulatory mechanisms of TGF-β1 have remained to be fully elucidated. Studies have demonstrated that Nemo-like kinase and Smad proteins regulate the expression of TGF-β1 (31,32). In addition, miRNAs have been identified to target the mRNA of TGF-β1 to regulate the translation of TGF-β1, and this process is important in normal development and physiology, as well as disease conditions. Certain studies have shown that miR-663 regulates TGF-β1 by direct targeting. Li et al (18) reported that miR-663 affects the proliferation, migration and invasion of glioma cells via TGF-β1. Hong et al (33) discovered that miR-663 is upregulated in epithelial cells in a high uric acid environment, and affects the phosphatase and tensin homolog gene by targeting TGF-β1. Another study reported that miR-663 regulates TGF-β1 expression in a pattern of negative feedback and reduces the radiation-induced bystander effect of cells (34). The results of the present study demonstrated that expression of miR-663 was downregulated in HS, while TGF-β1 was upregulated, revealing an inverse association. After stimulation with miR-663 mimics, the proliferation of fibroblasts from HS tissues was reduced, and TGF-β1 mRNA and protein levels were decreased, suggesting that miR-663 regulates TGF-β1. The dual luciferase reporter assay confirmed this regulatory association. Taken into consideration the various factors associated with HS other than TGF-β1 (6,7), a vast variety of miRNA molecules other than miR-663 regulate these factors.
In conclusion, miRNA-663 was found to negatively regulate the expression of TGF-β1 in HS, which may provide a novel approach for the prevention and treatment of HS. However, the actual effect and mechanism of action of miR-663 still require further study in cells, animals and clinical experiments.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology Development Project of Suzhou City (grant no. SYSD2015094). The authors would also like to thank Dr Joel Thomson from Department of Dermatology, Bestway Medical Company, Taiwan for English-proofreading of this manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QC and TZ designed the present study; QC, XX, DY, LW, WY and WS performed the experiments; QC, TZ and XX analyzed the data. The final version of the manuscript was read and approved by all authors and each collaborated to interpret results and develop the manuscript.
Ethics approval and consent to participate
All procedures were approved by the Ethics Committee of Soochow University (Suzhou, China). Written informed consent was obtained from all patients or their families.
Patient consent for publication
Written informed consent for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: A review. Plast Reconstr Surg. 1999;104:1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 3.Berman B, Viera MH, Amini S, Huo R, Jones IS. Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg. 2008;19:989–1006. doi: 10.1097/SCS.0b013e318175f3a7. [DOI] [PubMed] [Google Scholar]
- 4.Menke MN, Menke NB, Boardman CH, Diegelmann RF. Biologic therapeutics and molecular profiling to optimize wound healing. Gynecol Oncol. 2008;111(2 Suppl):S87–S91. doi: 10.1016/j.ygyno.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younai S, Venters G, Vu S, Nichter L, Nimni ME, Tuan TL. Role of growth factors in scar contraction: An in vitro analysis. Ann Plast Surg. 1996;36:495–501. doi: 10.1097/00000637-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–590. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grella A, Kole D, Holmes W, Dominko T. FGF2 overrides TGFβ1-driven integrin ITGA11 expression in human dermal fibroblasts. J Cell Biochem. 2016;117:1000–1008. doi: 10.1002/jcb.25386. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson MW, O'Kane S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reish RG, Eriksson E. Scar treatments: Preclinical and clinical studies. J Am Coll Surg. 2008;206:719–730. doi: 10.1016/j.jamcollsurg.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Mofikoya BO, Adeyemo WL, Abdus-salam AA. Keloid and hypertrophic scars: A review of recent developments in pathogenesis and management. Nig Q J Hosp Med. 2007;17:134–139. doi: 10.4314/nqjhm.v17i4.12693. [DOI] [PubMed] [Google Scholar]
- 11.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: A meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362–368. doi: 10.1001/archfaci.8.6.362. [DOI] [PubMed] [Google Scholar]
- 12.Janda K, Krzanowski M, Dumnicka P, Kuśnierz-Cabala B, Kraśniak A, Sułowicz W. Transforming growth factor beta 1 as a risk factor for cardiovascular diseases in end-stage renal disease patients treated with peritoneal dialysis. Clin Lab. 2014;60:1163–1168. doi: 10.7754/Clin.Lab.2013.130337. [DOI] [PubMed] [Google Scholar]
- 13.Massagué J. Wounding Smad. Nat Cell Biol. 1999;1:E117–E119. doi: 10.1038/12944. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Wang DR, Cao YL. TGF-beta: A fibrotic factor in wound scarring and a potential target for anti-scarring gene therapy. Curr Gene Ther. 2004;4:123–136. doi: 10.2174/1566523044578004. [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 16.Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X, Wang T, Zhou X, Zhu W, Li P, et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced fibrogenesis in human cardiac fibroblasts. Sci Rep. 2016;6:24747. doi: 10.1038/srep24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correia AC, Moonen JR, Brinker MG, Krenning G. FGF2 inhibits endothelial-mesenchymal transition through microRNA-20a-mediated repression of canonical TGF-β signaling. J Cell Sci. 2016;129:569–579. doi: 10.1242/jcs.176248. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Cheng Q, Chen Z, Peng R, Chen R, Ma Z, Wan X, Liu J, Meng M, Peng Z, Jiang B. MicroRNA-663 inhibits the proliferation, migration and invasion of glioblastoma cells via targeting TGF-β1. Oncol Rep. 2016;35:1125–1134. doi: 10.3892/or.2015.4432. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Liu J, Fan L, Wang F, Yu H, Wei W, Sun G. miR-663 overexpression induced by endoplasmic reticulum stress modulates hepatocellular carcinoma cell apoptosis via transforming growth factor beta 1. Onco Targets Ther. 2016;9:1623–1633. doi: 10.2147/OTT.S96902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the patient and observer Scar assessment scale. Plast Reconstr Surg. 2005;116:514–522. doi: 10.1097/01.prs.0000172982.43599.d6. [DOI] [PubMed] [Google Scholar]
- 21.Hoogewerf CJ, van Baar ME, Middelkoop E, van Loey NE. Patient reported facial scar assessment: Directions for the professional. Burns. 2014;40:347–353. doi: 10.1016/j.burns.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen T, Chen Z, Huang S, Gu J, Li J, et al. MiR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology. 2015;62:1132–1144. doi: 10.1002/hep.27929. [DOI] [PubMed] [Google Scholar]
- 24.Gorney M. Scar: The trigger to the claim. Plast Reconstr Surg. 2006;117:1036–1037. doi: 10.1097/01.prs.0000200666.67277.b2. [DOI] [PubMed] [Google Scholar]
- 25.Hong F, Wu N, Ge Y, Zhou Y, Shen T, Qiang Q, Zhang Q, Chen M, Wang Y, Wang L, Hong J. Nanosized titanium dioxide resulted in the activation of TGF-β/Smads/p38MAPK pathway in renal inflammation and fibration of mice. J Biomed Mater Res A. 2016;104:1452–1461. doi: 10.1002/jbm.a.35678. [DOI] [PubMed] [Google Scholar]
- 26.Zhou B, Zeng S, Li L, Fan Z, Tian W, Li M, Xu H, Wu X, Fang M, Xu Y. Angiogenic factor with G patch and FHA domains 1 (Aggf1) regulates liver fibrosis by modulating TGF-β signaling. Biochim Biophys Acta. 2016;1862:1203–1213. doi: 10.1016/j.bbadis.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front Biosci. 2003;8:s1240–s1248. doi: 10.2741/1183. [DOI] [PubMed] [Google Scholar]
- 28.Colwell AS, Longaker MT, Lorenz HP. Mammalian fetal organ regeneration. Adv Biochem Eng Biotechnol. 2005;93:83–100. doi: 10.1007/b99972. [DOI] [PubMed] [Google Scholar]
- 29.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 30.Beanes SR, Dang C, Soo C, Wang Y, Urata M, Ting K, Fonkalsrud EW, Benhaim P, Hedrick MH, Atkinson JB, Lorenz HP. Down-regulation of decorin, a transforming growth factor-beta modulator, is associated with scarless fetal wound healing. J Pediatr Surg. 2001;36:1666–1671. doi: 10.1053/jpsu.2001.27946. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Ye K, Wu H, Sun Y, Shi H, Huo K. Human SMAD4 is phosphorylated at Thr9 and Ser138 by interacting with NLK. Mol Cell Biochem. 2010;333:293–298. doi: 10.1007/s11010-009-0230-2. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z, Zhang J, Peng X, Dong Y, Jia L, Li H, Du J. The Notch γ-secretase inhibitor ameliorates kidney fibrosis via inhibition of TGF-β/Smad2/3 signaling pathway activation. Int J Biochem Cell Biol. 2014;55:65–71. doi: 10.1016/j.biocel.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Hong Q, Yu S, Geng X, Duan L, Zheng W, Fan M, Chen X, Wu D. High concentrations of uric acid inhibit endothelial cell migration via miR-663 which regulates phosphatase and tensin homolog by targeting transforming growth factor-β1. Microcirculation. 2015;22:306–314. doi: 10.1111/micc.12200. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, Xu S, Yao B, Hong M, Wu X, Pei H, Chang L, Ding N, Gao X, Ye C, et al. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol. 2014;11:1189–1198. doi: 10.4161/rna.34345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.