Abstract
The purpose of the present study was to investigate the role of latency-associated peptide (LAP)+CD4+T cells in hepatocellular carcinoma (HCC) immunity. Flow cytometric analysis was performed to detect the proportion of LAP+CD4+ T cells among the peripheral blood mononuclear cells (PBMCs) of 30 HBV-infected HCC patients at the pre-operative and post-operative stages, as well as 30 hepatitis B virus (HBV)-infected volunteers as a control group. Furthermore, tumor tissues and peri-tumor tissues from 28 patients with HCC, as well as hepatic tissues from 28 HBV-infected patients with benign lesions were subjected to immunohistochemical analysis with double staining for LAP and CD4, and the average number of the LAP+CD4+T cells in each visual field was quantified. The results indicated that the proportion of LAP+CD4+ T cells in the PBMCs of patients with HCC was significantly higher than that in the control group (1.84±0.85 vs. 0.73±0.39%, P=0.019), while it was significantly reduced after the operation (1.07±0.35, P=0.021), but still slightly, if not significantly, higher compared with that in the control group (P=0.342). Furthermore, the number of LAP+CD4+ T cells per high-magnification microscopic field (magnification, ×400) in the HCC tissues was 11.25±3.00, which was significantly higher than that in the peri-cancer tissues (5.75±1.00) and that in the HBV-infected hepatic tissues around benign lesions (2.61±0.83). In peri-cancer tissues, LAP+CD4+ T cells were also significantly more abundant than in control tissues. Furthermore, in the HCC tissues, LAP+CD4+ T cells were present as clusters in the tumor stroma and closely associated with CD4+ T lymphocytes. By contrast, in the peri-cancer liver tissues and HBV-infected hepatic tissues around benign lesions, LAP+CD4+ T cells were sparsely distributed. LAP+CD4+ T cells have marked inhibitory effects, and in the peripheral blood and tumor tissues of patients with HCC, they have an important role in the suppression of anti-tumor immunity and in the immune evasion of tumor cells.
Keywords: LAP+CD4+ T cells, flow cytometry, immunohistochemistry, peripheral blood mononuclear cells, tumor microenvironment, hepatocellular carcinoma
Introduction
T regulatory (Treg) cells are important suppressive immune regulatory cells that exert their immunosuppressive effects by producing cytokines with inhibitory functions and have an important role in the maintenance of immune homeostasis (1). Treg cells facilitate immune evasion via two mechanisms: Immune anergy and immunosuppression; they reduce the recognition of tumor antigens by the organism, induce immune tolerance of tumor antigens associated with immune anergy and negatively regulate the anti-tumor immune response associated with immunosuppression, resulting in immune escape of malignancies (2). Treg cells account for 5–10% of CD4+ T cells and may be divided into natural Treg (nTreg) cells and inducible Treg (iTreg) cells (3). nTreg mainly refers to CD4+CD25+ Treg cells and iTreg refers to transforming growth factor (TGF)-β-secreting T helper lymphocyte 3 (Th3) and IL-10-secreting type 1 regulatory T (Tr1) cells (4–6). Furthermore, immunosuppressive CD8+ Treg and natural killer T cells, which exhibit bidirectional immunity, represent two categories of Tregs (7,8). Previous studies on Treg cells mainly focused on CD4+CD25+ Treg, Th3 and Tr1 cells. In recent years, a novel subset of Treg cells with an immunosuppressive function was identified based on the presence of cell surface marker latency-associated peptide (LAP), which were therefore designated as LAP+CD4+ T cells (9). Certain animal studies indicate that LAP+CD4+ T cells participate in the initiation and development of several autoimmune and inflammatory diseases (10,11). In clinical practice, a close association between LAP+CD4+ T cells and tumors has been indicated (12). However, to date, the distribution of LAP+CD4+ T cells in hepatocellular carcinoma (HCC) has remained to be determined.
In the present study, flow cytometric (FCM) analysis was performed to determine the proportion of LAP+CD4+ T cells among peripheral blood mononuclear cells (PBMCs) and changes of LAP+CD4+ T cell levels in the peripheral blood after curative resection of HCC. Furthermore, immunohistochemical staining with double enzyme labeling was applied to determine the abundance of LAP+CD4+ T cells in HBV-infected HCC tissue, peri-cancer tissue and HBV-infected hepatic tissues around benign lesions. The differences in the distribution characteristics of LAP+CD4+ T cells in HCC tissue, peri-cancer tissue and HBV-infected hepatic tissues around benign lesions were analyzed. The function of LAP+CD4+ T cells in the local immune microenvironment of HCC and the expression characteristics of LAP+CD4+ T cells among PBMCs were also analyzed.
Materials and methods
Clinical information
The pre-operative group included 30 patients with HCC who were preliminarily diagnosed at the department of Hepatobiliary and Laparoscopic Surgery, Shenzhen Hospital (Peking University, Shenzhen, China) between October 2011 and December 2012, including 17 males and 13 females at the age of 42–60 years (median age, 51.4 years). Patients exhibited a chronic hepatitis B virus (HBV) infection and did not receive any treatment (including transcatheter hepatic arterial chemoembolization) prior to surgery. They were diagnosed with HCC after post-operative pathological examination. The post-operative group comprised 28 patients with HCC from the pre-operative group (two patients were unable to undergo the surgery due to metastasis in other organs), including 16 males and 12 females (age range, 42–60 years; median age, 52.1 years). The peripheral blood control group included 30 HBV-carrying volunteers (16 males, 14 female; age range, 39–57 years; median age, 48.3 years) who presented at the aforementioned hospital with chronic cholecystitis, inguinal hernia and chronic appendicitis between October 2011 and December 2012. The histopathology control group contained 28 HBV-carrying patients who received hepatic resection due to benign lesions, including 16 males and 12 females (age range, 35–52 years; median age, 45.7 years). None of the patients included in the present study had diabetes, hyperthyroidism or any autoimmune diseases. The differences in gender and age between the HCC pre or post-operative group and the peripheral blood control group, and the differences between the HCC or peri-cancer group and the hepatic tissue control group were not statistically significant.
Sample collection
The blood specimens were collected using disposable vacuum blood collection tubes. Each sample contained 6 ml venous blood from the subject that was drawn in the morning after the subject had fasted. All specimens were processed within 4 h following collection and analyzed within 16 h. The blood samples were collected three days prior to the operation for the pre-operative group and 10 days after the operation for the post-operative group.
Fresh hepatic tissue was collected from patients after hepatectomy, fixed in formalin, embedded in conventional paraffin and continuously cut into 4-µm sections. Hepatic tumor samples and peri-cancer tissue samples (collected ≤2 cm away from the carcinoma nodule) were collected separately from patients with HCC, avoiding the necrotic region, and assigned to the HCC group and the hepatocellular peri-cancer group. For the control group, HBV-infected hepatic tissues around benign lesions were collected following hepatectomy due to benign lesions.
Analysis of LAP+CD4+ T cells in PBMCs by FCM analysis
The following major reagents and instruments were used: CD4-fluorescein isothiocyanate (FITC) monoclonal antibody (mAb; cat. no. 553650) and negative control group immunoglobulin (IgG)2α-FITC antibody (cat. no. 550056) for fluorescence staining were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Flow Cytometry Staining Buffer (1×; cat. no. FC001), monoclonal LAP-phycoerythrin (PE) antibody (cat. no. 246-LP-025) and the negative negative control group IgG1-PE (cat. no. FAB110P) were purchased from R&D Systems (Minneapolis, MN, USA). Lymphocyte separation medium (cat. no. B6385-10G) was provided by Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Prior to experimentation, the flow cytometer was calibrated to recognize positively stained cells using FITC calibration particles (5 peaks; BD Biosciences, Franklin Lakes, NJ, USA; cat. no. 559123). A four-color flow cytometer (Beckmann-Coulter, Brea, CA, USA) was used for FCM analysis.
The blood sample (3 ml) was diluted with an equal volume of PBS. The lymphocytes were isolated by mixing 3 ml sample with lymphocyte separation medium, followed by centrifugation at a speed of 500 × g for 20 min at 25°C. The cells were re-suspended with 200 µl buffer and 100 µl of this lymphocyte suspension was added to Tube A (experimental tube) and Tube B (isotype tube), followed by addition of monoclonal CD4-FITC antibodies (1:5,000) to each tube. The samples were incubated for 20 min at 25°C in the dark, fixed with 500 µl of 4% paraformaldehyde for 10 min at 25°C and washed twice with PBS. LAP-PE mAb (10 µl; 1:5,000) was added to Tube A and isotype control IgG1-PE (10 µl; 1:5,000) was added to Tube B. The samples were incubated for 45 min at 25°C in the dark. After one wash with PBS, the lymphocytes were re-suspended in 500 µl PBS and analyzed with the four-color flow cytometer. At least 50,000 lymphocytes were collected for each acquisition. Flow jo software (flowjo 7.6.1; BD Biosciences) was used to analyze the results.
Analysis of LAP+CD4+ T cells in HCC tissue by immunohistochemical staining with double enzyme labeling
As the major reagents, goat anti-human LAP polyclonal antibody (cat. no. AF-246-NA) was purchased from R & D Systems and was diluted at 1:100 for use furthermore, ready-to use rabbit anti-human CD4 mAb (cat. no. YB-0766R), enzyme-labeled rabbit anti-goat IgG (cat. no. EHJSW-135238), streptavidin-peroxidase (cat. no. SP KIT-D1), 3-amino-9-ethylcarbazole (AEC) color reagent kit (cat. no. HPBIO-JC583), and diaminobenzidine (DAB) color reagent kit (cat. no. YYJK-1009) were purchased from Fuzhou Maixin (Fuzhou, Fujian, China).
The tissue sections were de-waxed, incubated with pepsin (1:10,000; cat. no. P7012-250MG; Sigma-Aldrich; Merck KGaA) in a 37°C incubator for 25 min, washed with PBS and then soaked in 3% H2O2 for 20 min at room temperature. Samples were further washed with PBS twice for 5 min, to discard the H2O2 solution and then 10% fetal calf serum (cat. no. KL-P0015; Sigma-Aldrich; Merck KGaA) was added and the samples were incubated in a 37°C incubator for 30 min. Ready-to use rabbit anti-human CD4 monoclonal antibody (1:100) was added and the samples were incubated at 4°C overnight. After being washed with PBS, the samples were incubated with enzyme-labeled rabbit anti-goat IgG (1:100) in a 37°C incubator for 30 min. After washing with PBS, streptavidin-peroxidase was added and the samples were incubated in a 37°C incubator for 30 min. The samples were then washed with PBS, followed by incubation coloration with DAB for 4 min at 25°C and soaking in 3% H2O2 for 20 min at room temperature. After another wash with PBS, the samples were incubated with 10% fetal calf serum in a 37°C incubator for 30 min. Subsequently, the samples were incubated with goat anti-human LAP polyclonal antibody at a dilution of 1:100 at 4°C overnight. The samples were washed with PBS and incubated with enzyme-labeled rabbit anti-goat IgG (1:100) in a 37°C incubator for 30 min. After another wash with PBS, the samples were incubated with streptavidin-peroxidase in a 37°C incubator for 30 min. After being washed with PBS, the samples were incubated with AEC for 4 min to visualize the antibodies. Subsequently, the samples were dehydrated, cleared, sealed and examined under a microscope.
LAP+CD4+ T cells were identified as follows: Complete cellular morphology, clear structure, brown/yellow and purple/red granules specifically co-localized in the cytoplasm and on cell the membrane (brown granules). CD4+ T cells were identified as follows: Complete morphology, clear structure, brown/yellow granules specifically located in the cytoplasm and on the cell membrane. The slides were observed under a microscope by an observer blinded to the sample identity. First, the whole slide was observed at a low magnification. Subsequently, 5 high-power fields (magnification, ×400) were randomly selected for quantification of stained cells and calculation of the mean value.
Statistical analysis
Values are expressed as the mean ± standard deviation of the percentage. A paired Student's t-test was used for comparisons between HCC and corresponding peri-cancer samples, as well as between HCC pre-operative peripheral blood samples and their corresponding post-operative samples. Two-way analysis of variance was performed for multi-group comparisons involving the control and the HCC groups, and Tukey's post-hoc test was used for comparisons between the control and the HCC groups (peripheral blood control group vs. pre/post-operative group for FCM results and hepatic tissue control group vs. HCC/peri-cancer group for immunohistochemistry results). SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data processing. P<0.05 was considered to indicate a statistically significant difference.
Results
LAP+CD4+T cells are increased in the PBMCs of patients with HCC, and are reduced after surgical resection
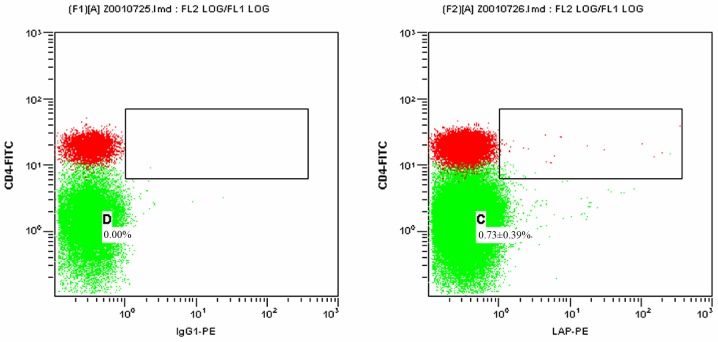
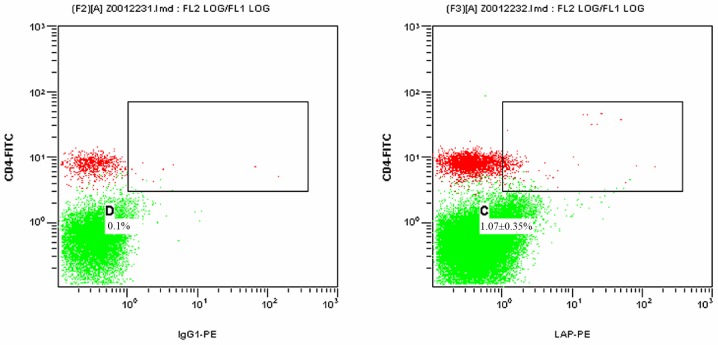
Figs. 1 and 2 present scatter plots of LAP+CD4+ T cells in PBMCs from the control group and the HCC pre-operative group, respectively, detected by FCM. As indicated in Table I, the proportion of LAP+CD4+ T cells among the PBMCs was 0.73±0.39% in the control group and 1.84±0.85% in the HCC pre-operative group. The proportion of LAP+CD4+ T cells in the PBMCs of the HCC pre-operative group was significantly higher than that in the control group (P=0.019).
Figure 1.
Proportion of LAP+CD4+ T cells among the PBMCs of the control group detected by flow cytometry. The left and right panels display the results for the isotype control and the experimental sample, respectively. The green dots represent CD4−LAP− T cells and the red dots represent CD4+LAP− T cells. The dots in the upper right box represent LAP+CD4+ T cells. C and D represent the proportion of LAP+CD4+ T cells among the PBMCs in the experimental and isotype control sample, respectively. Percentages represent the proportion of LAP+CD4+ T cells among the PBMCs in different groups presented. PBMCs, peripheral blood mononuclear cells; FITC, fluorescein isothiocyanate; PE, phycoerythrin; LAP, latency-associated peptide; IgG, immunoglobulin.
Figure 2.
Proportion of LAP+CD4+ T cells among the PBMCs from the hepatocellular carcinoma group at the pre-operative stage detected by flow cytometry. The left and right panels display the results for the isotype control and the experimental sample, respectively. The green dots represent CD4−LAP− T cells and the red dots represent CD4+LAP− T cells. The dots in the upper right box represent LAP+CD4+ T cells. C and D represent the proportion of LAP+CD4+ T cells among the PBMCs of the experimental and isotype control sample, respectively. Percentages represent the proportion of LAP+CD4+ T cells among the PBMCs in different groups presented. PBMCs, peripheral blood mononuclear cells; FITC, fluorescein isothiocyanate; PE, phycoerythrin; LAP, latency-associated peptide; IgG, immunoglobulin.
Table I.
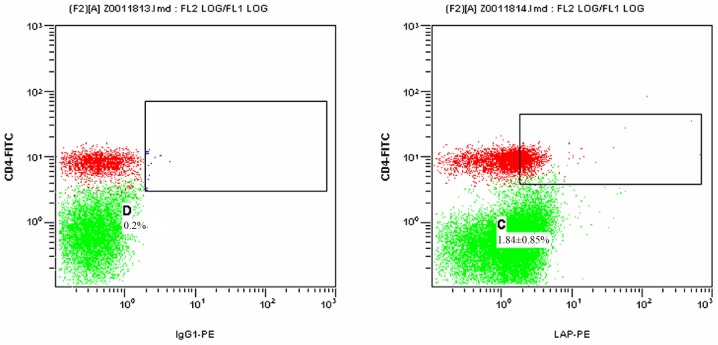
Proportion of LAP+CD4+ T cells among the peripheral blood mononuclear cells of the control group as well as the HCC pre-operative and post-operative groups.
| Group | N | CD4+ cells (%) | LAP+CD4+ T cells (%) |
|---|---|---|---|
| Control | 30 | 32.11±5.12 | 0.73±0.39 |
| HCC pre-operative | 30 | 21.91±6.62 | 1.84±0.85a |
| HCC post-operative | 28 | 29.58±6.24 | 1.07±0.35b |
Values are expressed as the mean ± standard deviation.
P=0.019 vs. control
P=0.342 vs. control; P=0.021 vs. post-operative. LAP, latency-associated peptide; HCC, hepatocellular carcinoma; ANOVA, analysis of variance.
Fig. 3 presents a 2-dimensional scatter plot displaying LAP+CD4+ T cells among PBMCs from the HCC post-operative group detected by FCM. As displayed in Table I, the proportion of LAP+CD4+ T cells among the PBMCs was 1.07±0.35% in the HCC post-operative group, which was significantly declined compared with that in the HCC pre-operative group (1.84±0.85; P=0.021), while it remained slightly, but not significantly higher than that in the control group (0.73±0.39%; P=0.342).
Figure 3.
Proportion of LAP+CD4+ T cells among the PBMCs from the hepatocellular carcinoma group at the post-operative stage detected by flow cytometry. The left and right panels display the results for the isotype control and the experimental sample, respectively. The green dots represent CD4−LAP− T cells and the red dots represent CD4+LAP− T cells. The dots in the upper right box represent LAP+CD4+ T cells. C and D represent the proportion of LAP+CD4+ T cells among the PBMCs in the experimental and isotype control sample, respectively. PBMCs, Percentages represent the proportion of LAP+CD4+ T cells among the PBMCs in different groups presented. peripheral blood mononuclear cells; FITC, fluorescein isothiocyanate; PE, phycoerythrin; LAP, latency-associated peptide; IgG, immunoglobulin.
Histopathological analysis of LAP+CD4+ T cells in HCC tissues
As exemplified in the representative image in Fig. 4, LAP was localized in the cytoplasm and on the cell membrane of CD4+ T cells in HCC tissues. In normal hepatic tissues and peri-cancer tissue, LAP+CD4+ T cells were scattered, but were mostly clustered in tumor stroma and located close to other lymphocytes (Figs. 5–7). Of note, a large number of LAP+CD4+ T cells were observed in HCC nests (cancer nests have a clear boundary and are primarily composed of heavily stained basal cells. Cancer cells are also well differentiated and nuclear fission is rare) and they were in close contact with CD4+ T cells (Fig. 8).
Figure 4.
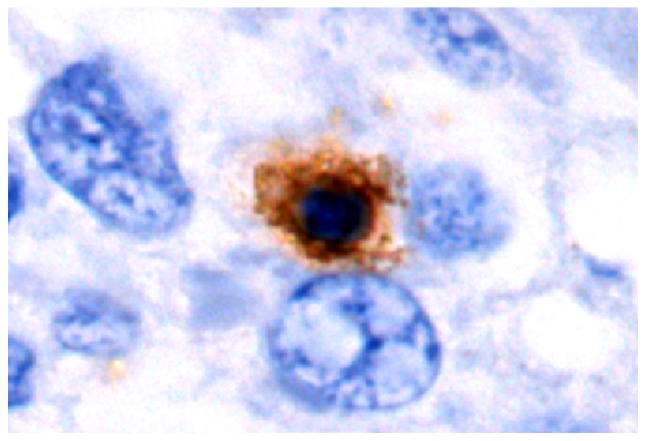
Representative immunohistochemistry image of latency-associated peptide+CD4+ T cells in HBV-infected hepatic tissues from the control group (high-power field; magnification, ×1,000). Positive cells were indicated by brown staining and blue staining indicates hepatic or tumor cells that are counterstained with hematoxylin in IHC images.
Figure 5.
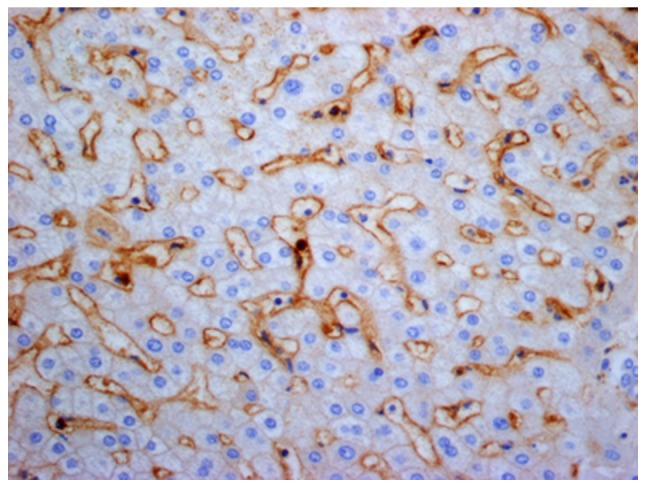
Distribution of latency-associated peptide+CD4+ T cells in HBV-infected hepatic tissues from the hepatic tissue control group (high-power field; magnification, ×400).
Figure 7.
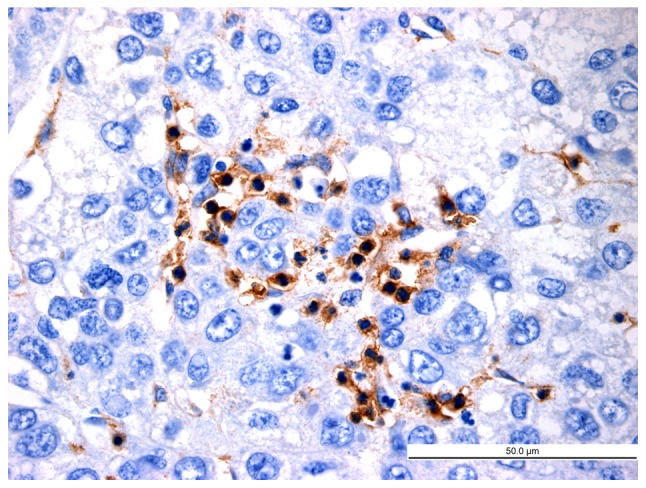
Distribution of latency-associated peptide+CD4+ T cells in hepatocellular carcinoma tissues (high-power field; magnification, ×400; scale bar, 50 µm).
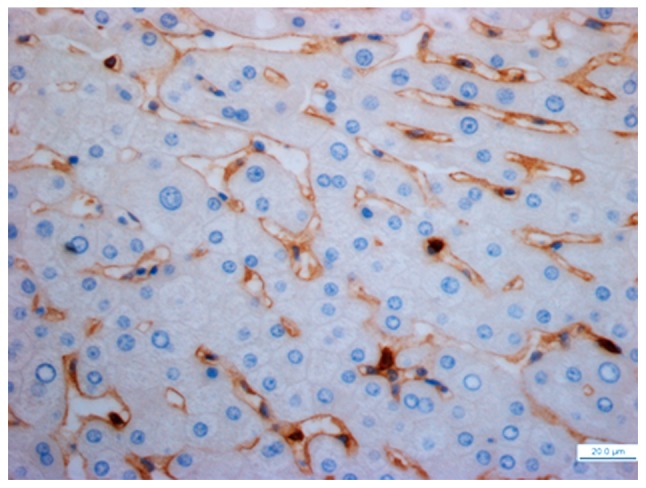
Figure 8.
Distribution of latency-associated peptide+CD4+ T cells in hepatocellular carcinoma nests (high-power field; magnification, ×400; scale bar, 50 µm).
As presented in Table II, the average number of LAP+CD4+ T cells per high-power field in the HCC group, peri-cancer group and normal control group was 11.25±3.00, 5.75±1.00 and 2.61±0.83, respectively. The difference in the abundance of LAP+CD4+ T cells was significant between the HCC group and the control group (P=0.013), between the peri-cancer group and the control group (P=0.025), and between the peri-cancer group and the HCC group (P=0.018).
Table II.
Distribution of LAP+CD4+ T cells in HCC, peri-cancer and control HBV-infected hepatic tissues.
| Group | N | Average number of LAP+CD4+T cells |
|---|---|---|
| Control | 28 | 2.61±0.83 |
| HCC | 28 | 11.25±3.00a |
| Peri-cancer | 28 | 5.75±1.00b |
Values are expressed as the mean ± standard deviation. The number of cells in the table is the average number of a single high-power field per group.
P=0.013 vs. control
P=0.025 vs. control; P=0.018 vs. peri-cancer. LAP, latency-associated peptide; HCC, hepatocellular carcinoma; ANOVA, analysis of variance.
Discussion
In 1995, Sakaguchi et al (13) first reported that 10% of CD4+ T cells in the peripheral blood of normal adult non-immune mice with T lymphocyte defects can express the α chain (CD25) of interleukin (IL)-2. They named these CD4+CD25+ T cells Treg cells and demonstrated that these cells inhibit the activation of other T cells. To date, various types of Treg cell have been identified among CD4+ T cells, but the most widely studied are CD4+CD25+forkhead box protein 3 (FOXP3)+ Treg cells (14). FOXP3 is the specific transcription factor of Treg cells and is specifically expressed on their surface. It is the most specific surface marker of Treg cells and regulates their development, activation and functions (15).
LAP was first discovered by Miyazono et al (16) in 1993. It is a pro-peptide that binds non-covalently to the amino terminus of TGF-β. TGF-β is a multifunctional polypeptide growth factor that is usually secreted out of the cells in its inactive or latent precursor form and exerts its biological activity after activation and binding to TGF-β receptor (TβR). Pre-activated complexes of TGF-β include TGF-β homodimer, as well as those with LAP and latent TGF-β binding protein (LTBP). LAP remains connected to TGF-β via a non-covalent bond after being cleaved from TGF-β precursor by a specific protease and forms an inactive complex with LTBP to prevent uncontrolled activation of TβR (17). In addition to keeping TGF-β in a latent state, LAP also has an important role in releasing and targeting latent TGF-β to the extracellular matrix, whereas LTBP guides the assembly and secretion of latent TGF-β complexes. Activation of TGF-β is achieved by partial or total enzymatic cleavage of LAP (18).
In 2001, Nakamura et al (19) reported that TGF-β precursor is expressed in mouse CD4+ T cells, drawing attention to the functions of LAP in CD4+ T cells. Oida et al (20) indicated that CD4+ T cells express LAP on their surface regardless of whether CD25 is expressed. A previous study also suggested that CD25 expression in CD4+CD25+ Treg cells is closely linked to the regulatory activity of these cells (14). However, Nakamura et al (21) demonstrated that LAP+ T cells with TGF-β1 on their cell surface exert inhibitory effects, which is independent of the expression of CD25. Therefore, they reasoned that LAP as a surface marker of Treg cells has more advantages than CD25. Chen et al (9) performed a study on CD4+CD25+LAP+ Treg cells from mice, indicating that TGF-β and TβR were expressed on their surface. The immune regulatory function of these CD4+CD25+LAP+ Treg cells is more effective than that of CD4+CD25+LAP− T cells due to intercellular contact and TGF-β-dependent mechanisms.
The immunosuppressive effects of LAP+CD4+ T cells have been demonstrated in mouse models of cerebrospinal meningitis, allergic inflammation, type II diabetes, colitis, arthritis, systemic lupus erythematosus and atherosclerosis. Oida et al (20) identified CD4+ T cells that express LAP+ on the cell surface by using goat LAP antibody. Compared with LAP−CD4+ T cells, LAP+CD4+ T cells produce more TGF-β and IL-10, cytokines which are important for the immunomodulatory effects of Treg cells in several systems. In a mouse model of autoimmune encephalomyelitis, Ochi et al (22) identified that CD4+CD25+LAP+ T cells express TGF-β and more FOXP3 and cytotoxic T-lymphocyte-associated protein 4 than CD4+CD25+LAP− T cells and exert stronger immune inhibitory effects in vitro and in vivo. In a mouse model of arthritis, LAP+CD4+ T cells were induced to secrete IL-10 in the joint to inhibit arthritis-specific reactive T-cell proliferation, the generation of specific antibodies and the development of arthritis (23). Different from the LAP+CD4+ T cells in the mouse model of arthritis, LAP+CD4+ T cells induced in models of systemic lupus erythematosus and diabetes inhibited the development of T helper cells in a TGF-β-dependent manner to alleviate the disease (24,25). The above studies not only reveal the generality of immunosuppressive action, but also suggest the differentiation of immunosuppression. For instance, TGF-β mainly works in models of systemic lupus erythematosus, diabetes and encephalomyelitis, high levels of IL-10 are secreted in arthritis models to inhibit effector T cells, and TGF-β and IL-10 work jointly in the colitis model.
Previous studies (9,19,22,23) have primarily focused on the functional mechanism of LAP+CD4+ T cells, the results of which in animal models are promising, but only few clinical studies are available. Mahalingam et al (12) have assessed LAP+CD4+ T cells in the frame of clinical tumor research, revealing that the levels of LAP+CD4+ T cells in the PBMCs and solid tumor mass of colorectal carcinoma patients are markedly higher than those in non-tumor tissue. The present study indicated that in patients with HCC, the proportion of LAP+CD4+ T cells among the PBMCs is higher than that in the normal control group. The mechanism may be associated with IL-8, TGF-β and/or Foxp3. Akiba et al (26) also reported that IL-8 was abnormally increased in the peripheral blood of patients with HCC and in seven HCC cell lines and proved that HCC cells secrete large amounts of IL-8. In the study by Gandhi et al (27) IL-8 was the most effective stimulator of LAP+CD4+ T-cell activation among other cytokines. While increases of IL-8 activated LAP+CD4+ T cells, the levels of active LAP+CD4+ T cells exhibited a marked decline when IL-8 mAb was provided. These results indicate that IL-8 in the peripheral blood of patients with HCC acts as a modulator to activate LAP+CD4+ T cells and is an important factor involved in the negative regulation of tumor immunology via LAP+CD4+ T cells. Almost all types of tumor cell secrete TGF-β. The HCC and HepG2 human hepatoma cell line also secretes TGF-β actively (17). Chen et al (9) reported that activated LAP+CD4+ T cells secrete TGF-β and that the negative regulatory effects of LAP+CD4+ T cells are contact-dependent and TGF-β-dependent. Therefore, it was further indicated that TGF-β is important in the negative immune regulation of LAP+CD4+ T cells. FOXP3+ Treg cells are a T-cell subset with a marked negative regulatory cellular immunologic function. Abnormal expression of FOXP3+ Treg cells has been identified in various inflammatory pathologies and tumor types (28). FOXP3+ Treg cells are able to inhibit the anti-tumor immunity and increase the immune tolerance of tumor cells, which is important for the immune evasion of tumors. Duan et al (10) indicated that in a mouse model of asthma-associated pneumonia, elevated levels of LAP+CD4+ T cells were accompanied with an increased number of FOXP3+ Treg cells. They reasoned that FOXP3 expressed on LAP+CD4+ T cells may have an important regulatory function.
The present results have demonstrated that the proportion of LAP+CD4+ T cells among the PBMCs of patients with HCC at the post-operative stage was significantly declined compared with that at the pre-operative stage, while it remained slightly but not significantly higher compared with that in the control group. However, the advanced stage of these two patients with metastasis may have affected the results of flow cytometry. This result indicates that radical resection affects the levels of LAP+CD4+ T cells in the peripheral blood of patients with HCC and it balanced out the immunologic derangement, reducing LAP+CD4+ T cells to the normal level. The possible mechanisms include the following: i) In the absence of tumor antigens, the anti-tumor immune response, which mostly comprises cellular immunity, decreases and the inhibitory effect of LAP+CD4+ T cells on the anti-tumor immune response also decreases. ii] Tumor cells secrete large amounts of IL-8, TGF-β and other cytokines. Radical resection may reduce the levels of these cytokines and change the microenvironment where LAP+CD4+ T cells exert their function, leading to the inhibition of LAP+CD4+ T-cell activation. Thus, this provides a valuable direction of future research. Radical resection affects the levels of LAP+CD4+ T cells in the peripheral blood of patients with HCC and reduces LAP+CD4+ T cells to the normal level. As LAP+CD4+ T cells in the blood of patients with HCC also (partially) originate from the tumor environment itself, where upon they are released into the circulation, it can be hypothesized that these trends may be more marked in hepatic tissues. Due to the difficulty of hepatic tissue sampling from patients with HCC following radical resection, it is difficult to validate this hypothesis. Future studies should therefore determine the difference in LAP+CD4+ T cell expression in hepatic tissue following radical resection in animal models.
The immunity of patients with primary HCC is weak, and the local immune microenvironment of the tumor is abnormal, resulting in failure of immune defense. It is an important factor for the immune evasion, recurrence and metastasis of HCC (29). A previous study revealed that immunosuppressive Treg cells were abnormally increased in the peripheral blood and tumor tissues of patients with HCC (30). Therefore, the extent of Treg cell infiltration in tumor tissues is representative of the suppression of anti-tumor immunity and is a key mechanism resulting in tumor immune tolerance and immune escape. In the present study, immunohistochemical staining with double enzyme labeling indicated that i) LAP is located in the cytoplasm and on the membrane of CD4+ T cells; ii) LAP+CD4+ T cells are clustered in HCC tissues; and iii) the extent of LAP+CD4+ T-cell infiltration in HCC, peri-cancer and normal control groups is significantly different. These results suggest the presence of LAP+CD4+ T cell infiltration in HCC tissues, which increased with the proximity to the tumor tissue. The results of the present study indicate that the LAP+CD4+ T cell infiltration (to reduce the body's anti-tumor immune response) was highest in HCC tissue but lower in peri-cancer tissue. However, this result is unexpected as it would be more likely that LAP+CD4+T cell infiltration is highest at the border between tumor and normal tissue (peri-cancer tissue), where the body's anti-tumor immune response is most inhibited. (31) This may be due to tumor cells secreting large quantities of IL-8 (which is increased in the peripheral blood and microenvironments of various types of cancer), TGF-β and other cytokines; furthermore, IL-8 is closely associated with immune cell chemotaxis and cell proliferation (26,27) as aforementioned. Therefore, it is hypothesized that LAP+CD4+ T cells migrate to HCC tissues via the IL-8 chemotactic effect and that LAP+CD4+ T cells alter the quantity and function of infiltrating lymphocytes within HCC tissues, thereby inhibiting tumor-specific and non-specific immune responses and promoting tumor progression, invasion or metastasis. The full elucidation of this mechanism should thus be assessed in future studies. Immunohistochemical analysis indicated that LAP+CD4+ cells were present not only in the stroma but at the surface of sinusoids or capillaries. This may be due to the breakage of certain LAP+CD4+ T cells during the preservation and fixation process of the tissue sections, resulting in positive staining in sinusoids and capillaries. Therefore, it may be speculated that LAP+CD4+ T cells are gathered in local tissue of HCC to negatively regulate effector T cells, which then inhibit specific and non-specific anti-tumor immune responses, possibly resulting in changes in the local microenvironment of the tumor in favor of tumor cell proliferation and migration.
Treg cells mediate the immune evasion of tumors via multiple molecular mechanisms. At present, the immune regulation effects of Treg cells and the underlying mechanisms have remained to be fully elucidated. An in vitro study indicated that CD4+CD25+ Treg cells may mediate immunosuppression by cell-to-cell contact-dependent and cytokine-independent mechanisms, whereas the subsequent in vivo study indicates that a cytokine-dependent mechanism may also exist. For instance, IL-10 has an important role in immune inhibition and TGF-β may participate in this process (32). Studies on animal models with autoimmune diseases and inflammation indicated that LAP+CD4+ T cells exert their immunosuppressive activity by inhibiting effector T cells (23,24). At present, little is known regarding LAP+CD4+ T cells in the tumor microenvironment. The present study indicated that in HCC tissues, LAP+CD4+ T cells are mostly clustered and localize close to lymphocytes; furthermore, CD4+ T cells accumulate in HCC nests. Therefore, the present study suggests that LAP+CD4+ T cells may inhibit the proliferation of effector T cells by cell-to-cell contact and facilitate the evasion of HCC cells of the anti-tumor immune response of the body.
The results of the present study allow for the following conclusions: i) High levels of LAP+CD4+ T cells with immune inhibitory effects exist in the peripheral blood and tumor tissues of patients with HCC, which is important for the negative regulation of the local microenvironment of HCC and is one of the mechanisms of HCC immune evasion. ii) After curative resection, the level of LAP+CD4+ T cells in the peripheral blood of patients with HCC decreased and approached the normal level, which may aid in the attenuation of the immunosuppressive state and improve the microenvironment of the local tissue. iii) LAP+CD4+ T cells are closely associated with CD4+ T cells in HCC tissues, suggesting that LAP+CD4+ T cells may inhibit local anti-tumor immunity by cell-cell contact and have an important role in the immune escape of HCC. Further research is required to study the cytokines and molecular mechanisms involved in the function of LAP+CD4+ T cells in the tumor microenvironment. Zhong et al (33) observed a positive correlation between the proportion of LAP+CD4+ T cells and the tumor-nodes-metastasis stage, the presence of distant metastasis and the serum levels of carcinoembryonic antigen. In the present study, the distribution of LAP+CD4+ T cells in patients with HCC with different grades of differentiation and clinical stages was not analyzed due to the limited funding and the number of cases included; this will be investigated in a future study by our group. Elucidation of the functions of LAP+CD4+ T cells in the tumor microenvironment and the underlying regulatory mechanisms, as well as identification of methods to regulate LAP+CD4+ T-cell proliferation, may lead to the development of more effective anti-tumor immunotherapies.
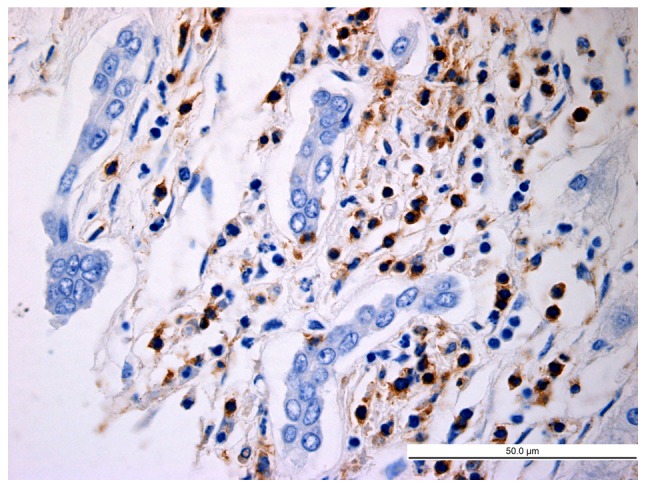
Figure 6.
Distribution of latency-associated peptide+CD4+ T cells in peri-cancer tissues (high-power field; magnification, ×400; scale bar, 20 µm).
Acknowledgements
The authors would like to thank Professor Yao-Ting Gui and Dr Cai-Ling Li from the Department of Central Laboratory at Shenzhen Hospital (Peking University, Shenzhen, China) for their respective scientific consultation and discussion, and help with laboratory analysis.
Funding
The present study was supported by the Science and Technology Development Fund Project of Shenzhen (grant nos. JC200903180670A, JCYJ20150403091443302 and JCYJ20160428164539088), the Sanming Project of Medicine in Shenzhen (grant no. SZSM201612021) and the Science and Technology Developing Project of Guangdong Province (grant no. 2017B090904010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XO conceived and designed the work that led to the submission, carried out literature search, data acquisition, manuscript editing and played an important role in interpreting the results. XPL carried out the concepts, design, definition of intellectual content, literature search, data acquisition, data analysis and manuscript preparation. JG collected the blood specimens, performed the flow cytometry and participated in the data acquisition, data analysis and statistical analysis. JSC collected the tissue samples, performed the immunohistochemical analysis and participated in the data acquisition, data analysis and statistical analysis. JCY, PKT and JKL carried out the literature search, data acquisition and manuscript editing, and participated in data analysis and statistical analysis. LPN participated in the flow cytometry, assisted in the data acquisition, data analysis and data interpretation. YZ and GYY participated in the immunohistochemical analysis, assisted in the data acquisition, data analysis and data interpretation.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Shenzhen Hospital (Peking University, Shenzhen, China). All patients and volunteers who participated in the study were informed of the possible health risks and potential effects of blood sampling and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sayour EJ, Mclendon P, Mclendon R, de Leon G, Reynolds R, Kresak J, Sampson JH, Mitchell DA. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother. 2015;64:419–427. doi: 10.1007/s00262-014-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan CH, Sun XM, Zhu CL, Liu SP, Wu L, Chen H, Feng MH, Wu K, Wang FB. Amphiregulin activates regulatory T lymphocytes and suppresses CD8+T cell-mediated anti-tumor response in hepatocellular carcinoma cells. Oncotarget. 2015;6:32138–32153. doi: 10.18632/oncotarget.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 4.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 5.Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000;12:676–683. doi: 10.1016/S0952-7915(00)00162-X. [DOI] [PubMed] [Google Scholar]
- 6.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filaci G, Fenoglio D, Indiveri F. CD8(+) T regulatory/suppressor cells and their relationships with autoreactivity and autoimmunity. Autoimmunity. 2011;44:51–57. doi: 10.3109/08916931003782171. [DOI] [PubMed] [Google Scholar]
- 8.Wong CH, Kubes P. Imaging natural killer T cells in action. Immunol Cell Biol. 2013;91:304–310. doi: 10.1038/icb.2013.6. [DOI] [PubMed] [Google Scholar]
- 9.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan W, So T, Mehta AK, Choi H, Croft M. Inducible CD4+LAP+Foxp3-regulatory T cells suppress allergic inflammation. J Immunol. 2011;187:6499–6507. doi: 10.4049/jimmunol.1101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahalingam J, Lin YC, Chiang JM, Su PJ, Fang JH, Chu YY, Huang CT, Chiu CT, Lin CY. LAP+CD4+ T cells are suppressors accumulated in the tumor sites and associated with the progression of colorectal cancer. Clin Cancer Res. 2012;18:5224–5233. doi: 10.1158/1078-0432.CCR-12-0211. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 15.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Miyazono K, Ichijo H, Heldin CH. Transforming growth factor-beta: Latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- 17.Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol. 2004;14:657–659. doi: 10.1016/j.tcb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.McMahon GA, Dignam JD, Gentry LE. Structural characterization of the latent complex between transforming growth factor beta 1 and beta 1-latency-associated peptide. Biochem J. 1996;313:343–351. doi: 10.1042/bj3130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 22.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, et al. Oral CD3-specific antibody suppresses autoimmun encephalomyelitis by inducing CD4+ CD25-LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 23.Wu HY, Maron R, Tukpah AM, Weiner HL. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J Immunol. 2010;185:3401–3407. doi: 10.4049/jimmunol.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25-LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 26.Akiba J, Yano H, Ogasawara S, Higaki K, Kojiro M. Expression and function of interleukin-8 in human hepatocellular carcinoma. Int J Oncol. 2001;18:257–264. doi: 10.3892/ijo.18.2.257. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. Cutting edge: Human latency-associated peptide+ T cells: A novel regulatory T cell subset. J Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY, Gao YT, Du Z. Tumor-infiltrating FoxP3+Tregs and CD8+T cells affect the prognosis of hepatocellular carcinoma patients. Digestion. 2012;86:329–337. doi: 10.1159/000342801. [DOI] [PubMed] [Google Scholar]
- 29.Brunner SM, Itzel T, Rubner C, Kesselring R, Griesshammer E, Evert M, Teufel A, Schlitt HJ, Fichtner-Feigl S. Tumor-infiltrating B cells producing antitumor active immunoglobulins in resected HCC prolong patient survival. Oncotarget. 2017;8:71002–71011. doi: 10.18632/oncotarget.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Wang B, Wu J, Zhang C, Zhou Y, Yang X, Zhou J, Guo W, Fan J. Association of preoperative EpCAM circulating tumor cells and peripheral treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer. 2016;16:506. doi: 10.1186/s12885-016-2526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Jiang G, Yao F, Liang G, Wang F, Xu H, Wu Y, Yu X, Liu H. Osthole promotes anti-tumor immune responses in tumor-bearing mice with hepatocellular carcinoma. Immunopharmacol Immunotoxicol. 2015;37:301–307. doi: 10.3109/08923973.2015.1035391. [DOI] [PubMed] [Google Scholar]
- 32.Miyara M, Sakaguchi S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhong W, Jiang ZY, Zhang L, Huang JH, Wang SJ, Liao C, Cai B, Chen LS, Zhang S, Guo Y, et al. Role of lap+cd4+t cells in the tumor microenvironment of colorectal cancer. World J Gastroenterol. 2017;23:455–463. doi: 10.3748/wjg.v23.i3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.