Abstract
Plasma microRNA (miR)-423-5p is a potential biomarker for the detection of colon cancer. However, the expression and biological role of miR-423-5p in colon tumorigenesis remains unclear. In the current study, reverse transcription-quantitative polymerase chain reaction was used to determine miR-423-5p expression in malignant colon tissues and plasma from patients with colon cancer. Cell viability, colony formation and apoptosis assays, as well as western blotting, were performed to investigate the biological role and regulatory mechanisms of miR-423-5p in colon cancer. The results demonstrated that miR-423-5p expression was downregulated in tumor tissues and plasma from patients with colon cancer, as well as in colon cancer cell lines. Furthermore, overexpression of miR-423-5p promoted colon cancer cell apoptosis and resulted in the inhibition of cell proliferation and colony formation. Mechanistically, miR-423-5p induced the expression of caspases 3, 8 and 9, as well as p53 in colon cancer. The effect of z-VAD treatment indicated that the miR-423-5p-mediated colon cancer cell apoptosis is caspase-dependent. These results suggest that miR-423-5p is a tumor suppressor in colon cancer and a potential diagnostic target to enable the early detection of colon cancer.
Keywords: colon cancer, microRNA-423-5p, apoptosis, caspase
Introduction
The mortality rate of patients with colon cancer is one of the highest of all types cancer, due to late tumor presentation and rapid progression (1). Despite improvements in the diagnosis and treatment of colon cancer, there are ~1,000,000 cases of colon cancer reported annually, with >600,000 mortalities per year (1). Patients are usually diagnosed when they reach an advanced stage of colon cancer (2), when surgery is ineffective; however, early stage surgery may be an effective method of attenuating its progression (3). Therefore, novel prognostic biomarkers and targeted therapeutics are required to enable the early detection and treatment of colon cancer.
MicroRNAs (miRNAs) are a class of endogenous noncoding RNAs 21–23 nucleotides long, which regulate the expression of target genes in mammals either by degrading target mRNA or inhibiting the translation of target genes (4). A total of 2,042 mature human miRNAs have been identified, which may regulate >30% of target mRNAs (5). The results of previous studies have demonstrated that miRNAs serve important roles in tumorigenesis by regulating the expression of various oncogenes and tumor suppressor genes (6–8). miR-423-5p is an effective serum biomarker for colon cancer. Fang et al (9) demonstrated that the concentration of plasma miR-423-5p was decreased in patients with colon cancer and benign lesions, including polyps and adenoma, compared with healthy controls. Therefore, it has been suggested that plasma levels of miR-423-5p may serve as a biomarker for colon cancer detection, particularly for early stage colon cancer (9). Indeed, it was demonstrated that in stage I–II colon cancer, serum miR-423-5p was significantly elevated compared with controls, whereas in stage III–IV colon cancer, the differences in miR-423-5p expression between patients with colon cancer and healthy controls were not significant (10).
However, the expression and function of miR-423-5p in malignant colon tissues and colon cancer tumorigenesis remains unclear. The aim of the present study was to evaluate the expression of miR-423-5p in malignant colon tissues and colon cancer cell lines. The potential regulatory role of miR-423-5p on colon cancer cell proliferation and apoptosis was also determined. These results may provide a novel target for the diagnosis and treatment of colon cancer.
Materials and methods
Clinical samples
The present study was approved by the Ethics Committee of Beijing Hospital (Beijing, China). A total of 25 pairs of diagnostic primary malignant colon samples and adjacent normal colon tissues (used as controls) were obtained from the Department of General Surgery at the Beijing Hospital between May and October 2016. The 25 colon cancer patients, 11 male and 14 female, were between 48 and 78 years (Table I). Fasting peripheral blood (5 ml) was drawn from each patient and placed in anticoagulative tubes at room temperature for 30 min, followed by centrifugation at 1,500 × g for 5 min at 4°C. The plasma supernatant was collected and stored at −80°C until use. Written informed consent was obtained from the patients.
Table I.
Clinicopathological characteristics of patients with colon cancer.
| miR-423-5P expression | ||||
|---|---|---|---|---|
| Characteristics | Number of patients | Low | High | P-values |
| Sex (%) | >0.05 | |||
| Male | 11 (44) | 5 (45.5) | 6 (54.5) | |
| Female | 14 (56) | 6 (42.9) | 8 (57.1) | |
| Age (%) | >0.05 | |||
| ≤60 | 6 (24) | 3 (50.0) | 3 (50.0) | |
| >60 | 19 (76) | 8 (42.1) | 11 (57.9) | |
| TNM stage (%) | <0.05 | |||
| I | 6 (24) | 2 (33.3) | 4 (66.7) | |
| II–III | 11 (44) | 6 (54.5)a | 5 (45.5)a | |
| IV | 8 (32) | 7 (87.5)b | 1 (12.5)b | |
TNM, tumor node metastasis.
P<0.05 vs. stage I tumor
P<0.05 vs. stage II–III tumor.
Cell culture
The human colon cancer cell lines HT29, SW480, Caco-2, HCT116 and SW620 were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and antibiotics (penicillin and streptomycin; Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a 100% humid incubator with 5% CO2. Normal human colon epithelial cells (HCoEpiC) were purchased from Shanghai Hongshun Biotechnology (Shanghai, China).
Cell transfection
The pU6 vector-based miR-423-5p overexpression plasmid and miR-negative control (NC) expression plasmid were customized and purchased from GenePharma (Shanghai, China). A total of 2 µg miR-423-5p overexpression plasmid or miR-NC were transfected into SW620 and HCT116 cells using 5 µl FuGENE HP® (Promega Corporation, Madison, WI, USA), following the manufacturer's protocol. A total of 48 h after cell transfection, the cells were collected for further analysis. z-VAD-FMK was purchased from Selleck Chemicals (Shanghai, China) and used to treat cells at a concentration of 50 µM for 24 h following transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Human colon samples and transfected cells were lysed using TRIzol® reagent (Thermo Fisher Scientific, Inc.) and total RNA was extracted following the manufacturer's protocol. Reverse transcription was performed on the isolated total RNA using a PrimeScript® RT Master Mix kit (Perfect Real Time; cat. no. RR036A; Takara Bio, Inc., Kusatsu, Japan) and qPCR was performed using a SYBR Green I Real Time PCR kit (cat. no. RR420A; Takara Bio, Inc.), according to the manufacturer's protocol. Reverse transcription was performed at 65°C for 5 min, 30°C for 10 min, 42°C for 10 min and 2°C for 3 min. qPCR conditions were as follows: Denaturation at 94°C for 2 min, amplification for 30 cycles at 94°C for 30 sec, annealing at 59°C for 30 sec and extension at 72°C for 1 min, followed by a terminal elongation step at 72°C for 10 min. The primers for miR-423-5p were purchased from Guangzhou RiboBio Co., Ltd. (cat no. S170721170309; Guangzhou, China). Sequences were not supplied due to the rules of the company. U6 was used as an internal control. qPCR analysis was performed using a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data were quantified using the 2−ΔΔCq method (11,12).
Cell proliferation assay
Following transfection, cells were cultured in a 96-well plate at a density of 800 cells/well. A Cell Counting Kit-8 (CCK-8) assay was performed to measure cell proliferation. At 0, 24, 48 and 72 h post transfection, CCK-8 (Beyotime Institute of Biotechnology, Haimen, China) was added to the wells and cells were incubated at 37°C for 4 h. Optical density was measured at 450 nm using a microplate reader. Experiments were performed four times.
Colony formation assay
SW620 and HCT116 cells (1,000 cells/well) were seeded in a 6-well plate following transfection. Cells were cultured with DMEM containing 10% FBS. Following 12 days, cells were fixed with 4% paraformaldehyde at room temperature for 15 min and stained with crystal violet (Beyotime Institute of Biotechnology) at room temperature for 10 min. The number of colonies in 5 random fields were counted and analyzed with inverted microscope (BX51; Olympus, Tokyo, Japan) at a magnification of ×10. Experiments were performed three times.
Apoptosis analysis
Following transfection for 48 h, cells were harvested and stained using an Annexin V-fluorescein isothiocyanate/Propidium iodide Apoptosis Detection kit (Nanjing Keygentec Biotech, Nanjing, China) following the manufacturer's protocol. Apoptotic cells were assessed using a flow cytometer (BD FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA) and analyzed by FlowJo (version 10.4.1; FlowJo LLC, Ashland, OR, USA). Experiments were performed three times.
Western blot analysis
Following transfection for 48 h, cells were harvested and lysed in ice-cold radioimmunopreciptation assay lysis buffer (Beyotime Institute of Biotechnology) containing 1% protease inhibitor cocktail (Pierce; Thermo Fisher Scientific, Inc.) for 30 min and were then centrifuged at 13,000 × g for 15 min at 4°C. A BCA kit (Beyotime Institute of Biotechnology) was used to determine protein concentration and SDS-PAGE loading buffer (Beyotime Institute of Biotechnology) was added to prepare the loading sample. A total of 15 µg/lane protein was subjected- to electrophoresis on 10–12% SDS-PAGE gels and transferred to PVDF membranes (Merck KGaA, Darmstadt, Germany). Membranes were blocked with PBS containing 5% non-fat milk for 1 h at room temperature and then incubated with primary antibodies against caspase 3 (cat no. 9662; 1:1,000), caspase 8 (cat. no. 4790; 1:800), caspase 9 (cat. no. 9508; 1:800), p53 (cat. no. 2524; 1:1,000) and GAPDH (cat no. 5174; 1:5,000; all Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. Following washing, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (cat. no. TA140003) and mouse secondary antibodies (cat. no. TA140002; both 1:10,000, OriGene Technologies, Inc., Rockville, MD, USA) for 60 min at room temperature. The bands were detected by enhanced chemiluminescent (ECL) kit (Merck KGaA) and the band density were quantified by Image J software (version 1.48; National Institutes of Health, Bethesda, MD, USA). The quantities of samples were normalized based on the expression of GAPDH.
Statistical analysis
All values are expressed as the mean ± standard deviation of ≥3 results. Student's t test and one-way analysis of variance with Dunnett's multiple comparisons test were used to assess differences between groups using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Logistic regression analysis was used to assess the correlation between plasma miR-423-5p expression and tumor grade. P<0.05 was determined to indicate a statistically significant difference.
Results
Downregulation of miR-423-5p in colon cancer tissues and cell lines
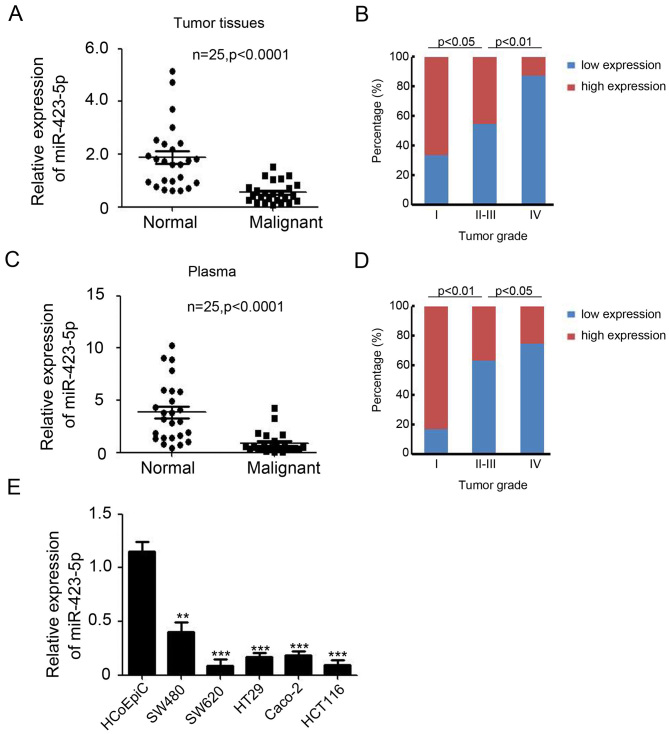
The expression of miR-423-5p in colon cancer tissues and cell lines was investigated using RT-qPCR. miR-423-5p expression was significantly lower in colon tumor tissues (0.55±0.08) compared with normal colon tissues (1.87±0.24; P<0.0001; Fig. 1A). Logistic regression analysis indicated that miR-423-5p expression was lower in patients with high grade tumors (P<0.05; Fig. 1B). The expression of miR-423-5p in the plasma of 25 patients with colon cancer compared with healthy controls was also determined. The results indicated that the expression of miR-423-5p in plasma was significantly lower in patients with colon cancer (0.88±0.20) compared with healthy controls (3.85±0.57; P<0.0001; Fig. 1C). The expression of miR-423-5p in plasma was also decreased as the tumor grade increased (P<0.05; Fig. 1D). miR-423-5p expression was decreased in malignant colon tissues and plasma from patients with colon cancer; therefore, the expression of miR-423-5p was subsequently assessed in colon cancer cell lines. The results indicated that it was significantly downregulated in all colon cancer cell lines compared with HCoEpiCs (all P<0.01; Fig. 1E). These results suggest that the expression of miR-423-5p is downregulated in colon cancer and decreases as tumor grade increases.
Figure 1.
Downregulation of miR-423-5p in colon cancer tissues. (A) RT-qPCR analysis of miR-423-5p expression in 25 pairs of normal colon and colon cancer tissue. (B) Logistic regression analysis between miR-423-5p expression in tumor tissues and tumor grade (tumor grade I, n=6; tumor grade II–III, n=11; tumor grade IV, n=8). (C) RT-qPCR analysis of miR-423-5p expression in the plasma of 25 patients and healthy controls. (D) Logistic regression analysis between plasma miR-423-5p expression and tumor grade (tumor grade I, n=6; tumor grade II–III, n=11; tumor grade IV, n=8). (E) RT-qPCR analysis of miR-423-5p expression in HCoEpiCs and colon cancer cell lines. n=3. Values are expressed as the mean ± standard deviation. **P<0.01 and ***P<0.001 vs. HCoEpiC. miR-423-5p, microRNA-423-5p; reverse transcription-quantitative polymerase chain reaction; HCoEpiCs, human colon epithelial cells.
Overexpression of miR-423-5p inhibits colon cancer growth and colony formation
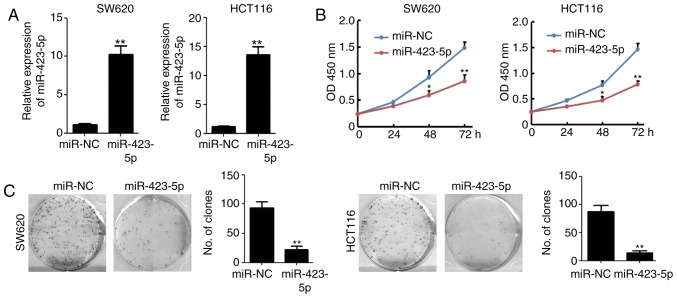
miR-423-5p was cloned into a plasmid expression system and transfected into cells to validate its effects on colon cancer. miR-423-5p transfection significantly upregulated miR-423-5p levels in SW620 and HCT116 cells (Fig. 2A). The results of the CCK8 assay indicated that the overexpression of miR-423-5p in SW620 and HCT116 cells significantly reduced cell viability by 42.5 and 46.6%, respectively, compared with that of the miR-NC-transfected groups at 72 h (n=4; P<0.01; Fig. 2B). Colony formation was also significantly decreased in HCT116 (87.3±11.3 vs. 13.7±3.7, miR-NC vs. miR-423-5p) and SW620 (93.3±10.6 vs. 22.3±5.9, miR-NC vs. miR-423-5p) cells transfected with miR-423-5p (both P<0.01; Fig. 2C). These results suggest that the overexpression of miR-423-5p inhibits colon cancer cell proliferation and colony formation.
Figure 2.
Overexpression of miR-423-5p inhibits colon cancer growth and colony formation. (A) Reverse transcription-quantitative polymerase chain reaction analysis of miR-423-5p expression in SW620 and HCT116 cells transfected with miR-NC and miR-423-5p. n=3. (B) A Cell Counting Kit-8 assay was used to detect the proliferation of SW620 and HCT116 cells following transfection with miR-NC and miR-423-5p. n=3. (C) A colony formation assay was performed on SW620 and HCT116 cells transfected with miR-NC and miR-423-5p. n=3. Values are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. miR-NC group. miR-423-5p, microRNA-423-5p; NC, negative control; OD, optical density.
Overexpression of miR-423-5p promotes apoptosis and caspase protein expression in colon cancer cells
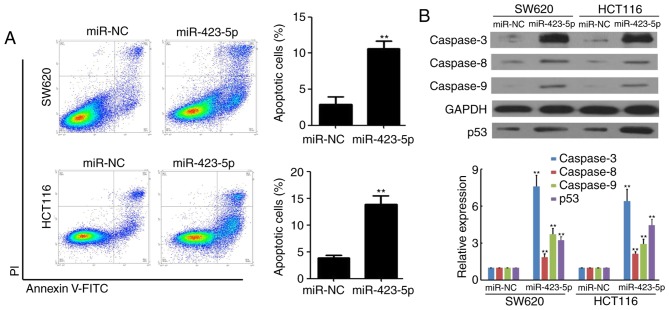
The mechanism underlying the miR-423-5p-mediated inhibition of colon cancer cell growth was determined using flow cytometry in SW620 and HCT116 cells following transfection with miR-423-5p and miR-NC. The results indicated that the rate of apoptosis was increased in SW620 (2.9±0.9% vs. 10.6±1.1%, miR-NC vs. miR-423-5p) and HCT116 (3.8±0.5% vs. 13.8±1.6%, miR-NC vs. miR-423-5p) cells following miR-423-5p transfection (both P<0.01; Fig. 3A). Furthermore, the expression of caspases 3, 8 and 9 in miR-NC and SW620 and HCT116 cells transfected with miR-423-5p was measured using western blotting. The expression of caspases 3, 8, 9 and p53 were increased in SW620 and HCT116 cells transfected with miR-423-5p compared with the respective cells transfected with miR-NC (all P<0.01; Fig. 3B). These results suggest that the overexpression of miR-423-5p promotes apoptosis and the expression of caspases in colon cancer cells.
Figure 3.
Overexpression of miR-423-5p promotes apoptosis and upregulates caspase expression in colon cancer cells. (A) Flow cytometric analysis of cell apoptosis by Annexin V and PI staining in SW620 and HCT116 cells transfected with miR-NC and miR-423-5p. n=3. (B) Western blot analysis of caspases 3, 8 and 9 and p53 expression in HCT116 and SW620 cells transfected with miR-NC and miR-423-5p. GAPDH was used as a loading control. n=3. Values are expressed as the mean ± standard deviation. **P<0.01 vs. miR-NC group. miR-423-5p, microRNA-423-5p; PI, propidium iodide; FITC, fluorescein isothiocyanate; NC, negative control.
miR-423-5p induces caspase-dependent apoptosis in colon cancer cells
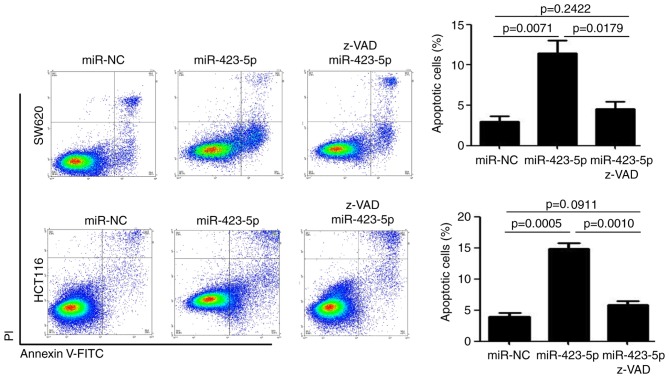
z-VAD is an inhibitor of caspase activity and was used to treat miR-423-5p-transfected colon cancer cells. z-VAD treatment attenuated the miR-423-5p-mediated upregulation of apoptosis in SW620 cells (11.5±1.5% vs. 4.5±0.9%, miR-423-5p vs. miR-423-5p+z-VAD; P<0.05) and HCT116 cells (14.9±0.9% vs. 5.8±0.5%, miR-423-5p vs. miR-423-5p+z-VAD; P<0.01; Fig. 4). There were no significant differences in the rates of apoptosis between the miR-NC-transfected and miR-423-5p+z-VAD groups in SW620 cells (2.9±0.7% vs. 4.5±0.9%, miR-NC vs. miR-423-5p+z-VAD) and HCT116 cells (3.9±0.6% vs. 5.8±0.5%, miR-NC vs. miR-423-5p+z-VAD). These results indicate that miR-423-5p induces caspase-dependent apoptosis in colon cancer cells.
Figure 4.
miR-423-5p induces caspase-dependent apoptosis in colon cancer cells. Flow cytometric analysis of cell apoptosis by Annexin V and PI staining in HCT116 and SW620 cells transfected with miR-NC, miR-423-5p and miR-423-5p+z-VAD. n=3. Values are expressed as the mean ± standard deviation. miR-423-5p, microRNA-423-5p; PI, propidium iodide; FITC, fluorescein isothiocyanate; NC, negative control.
Discussion
The results of the current study demonstrated that miR-423-5p expression was significantly downregulated in colon malignant tissues, as well as in colon cancer cell lines. It was demonstrated that miR-423-5p inhibits colon cancer cell proliferation and colony formation, thereby promoting cell apoptosis. Furthermore, the effects of z-VAD demonstrated that miR-423-5p-mediated colon cancer cell apoptosis is caspase-dependent. These results suggest that miR-423-5p is downregulated in colon cancer and functions as a tumor suppressor.
miR-423-5p is upregulated in the myocardium following heart failure and is associated with the prohormone brain natriuretic peptide and ejection fraction (13). However, it is not an effective biomarker of systemic ventricular and left ventricular remodeling (14,15). Furthermore, it has been demonstrated that miR-423-5p significantly downregulates the expression of β-linked N-acetylglucosamine transferase and its associated downstream targets, and also induces apoptosis in cardiomyocytes (16). Furthermore, miR-423-5p expression is increased in the serum of patients with hepatocarinoma following treatment with sorafenib and miR-423-5p promotes autophagy (17). It has been determined that miR-423-5p knockdown enhances the sensitivity of glioma stem cells to apigenin via the mitochondrial pathway (18). In addition, plasma miR-423-5p may be a biomarker for colon cancer (9,10). The current study demonstrated that miR-423-5p is downregulated in colon malignant tissues and colon cancer cell lines. Overexpression of miR-423-5p impaired colon cancer cell proliferation and colony formation in vitro. These results improve understanding of the role miR-423-5p serves in colon cancer.
Environmental factors and accumulation of mutations serve a role in the development of colon cancer, resulting in increased cell proliferation, uncontrolled angiogenesis, inhibition of apoptosis and immune system evasion (19–22). Evasion of apoptosis is one of the hallmarks of human cancer (23). Damaged or unnecessary cells are normally eliminated by apoptosis via the extrinsic and intrinsic apoptotic pathways (23). Thus, promoting the apoptosis of tumor cells may be an effective method of treating colon cancer. Agents that induce cellular DNA damage and cause cell apoptosis, including irinotecan and cisplatin, are commonly used to treat patients with colon cancer (24,25). In the current study, the role of miR-423-5p in promoting cell apoptosis and caspase expression in colon cancer was determined. The results indicated that miR-423-5p-mediated cell apoptosis is caspase-dependent, thus improving the understanding of the mechanisms by which miR-423-5p regulates apoptosis.
In conclusion, the results of the current study demonstrated that miR-423-5p is downregulated in colon malignant tissues and colon cancer cell lines. Overexpression of miR-423-5p induces caspase-dependent apoptosis, resulting in inhibition of cell proliferation and colony formation. Taken together, the results of the current study suggest that miR-423-5p may serve be an effective target for the detection and treatment of colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data and materials supporting the results of the current study are available within the article.
Authors' contributions
WZJ and TY were involved in the acquisition of the data. QA was involved in the analysis and interpretation of the data. XLC were involved in the collection of human tissues. HDP was involved in the conception and design of the present study.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Beijing Hospital (Beijing, China). Written informed consent was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jonas S, Thelen A, Benckert C, Spinelli A, Sammain S, Neumann U, Rudolph B, Neuhaus P. Extended resections of liver metastases from colorectal cancer. World J Surg. 2007;31:511–521. doi: 10.1007/s00268-006-0140-3. [DOI] [PubMed] [Google Scholar]
- 3.Smith JJ, Deane NG, Dhawan P, Beauchamp RD. Regulation of metastasis in colorectal adenocarcinoma: A collision between development and tumor biology. Surgery. 2008;144:353–366. doi: 10.1016/j.surg.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore MJ, Scheel TK, Luna JM, Park CY, Fak JJ, Nishiuchi E, Rice CM, Darnell RB. miRNA-target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat Commun. 2015;6:8864. doi: 10.1038/ncomms9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slezak-Prochazka I, Durmus S, Kroesen BJ, van den Berg A. MicroRNAs, macrocontrol: Regulation of miRNA processing. RNA. 2010;16:1087–1095. doi: 10.1261/rna.1804410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rupaimoole R, Wu SY, Pradeep S, Ivan C, Pecot CV, Gharpure KM, Nagaraja AS, Armaiz-Pena GN, McGuire M, Zand B, et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Commun. 2014;5:5202. doi: 10.1038/ncomms6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SY, Rupaimoole R, Shen F, Pradeep S, Pecot CV, Ivan C, Nagaraja AS, Gharpure KM, Pham E, Hatakeyama H, et al. A miR-192-EGR1-HOXB9 regulatory network controls the angiogenic switch in cancer. Nat Commun. 2016;7:11169. doi: 10.1038/ncomms11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tajima K, Yae T, Javaid S, Tam O, Comaills V, Morris R, Wittner BS, Liu M, Engstrom A, Takahashi F, et al. SETD1A modulates cell cycle progression through a miRNA network that regulates p53 target genes. Nat Commun. 2015;6:8257. doi: 10.1038/ncomms9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Z, Tang J, Bai Y, Lin H, You H, Jin H, Lin L, You P, Li J, Dai Z, et al. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J Exp Clin Cancer Res. 2015;34:86. doi: 10.1186/s13046-015-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu X, Lu J. The significance of detection of serum miR-423-5p and miR-484 for diagnosis of colorectal cancer. Clin Lab. 2015;61:187–190. doi: 10.7754/Clin.Lab.2014.140625. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 13.Kumarswamy R, Anker SD, Thum T. MicroRNAs as circulating biomarkers for heart failure: Questions about MiR-423-5p. Circ Res. 2010;106:e8. doi: 10.1161/CIRCRESAHA.110.220616. author reply e9. [DOI] [PubMed] [Google Scholar]
- 14.Nabiałek E, Wańha W, Kula D, Jadczyk T, Krajewska M, Kowalówka A, Dworowy S, Hrycek E, Wludarczyk W, Parma Z, et al. Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in acute myocardial infarction and stable coronary heart disease. Minerva Cardioangiol. 2013;61:627–637. [PubMed] [Google Scholar]
- 15.Bauters C, Kumarswamy R, Holzmann A, Bretthauer J, Anker SD, Pinet F, Thum T. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int J Cardiol. 2013;168:1837–1840. doi: 10.1016/j.ijcard.2012.12.074. [DOI] [PubMed] [Google Scholar]
- 16.Luo P, He T, Jiang R, Li G. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Mol Med Rep. 2015;12:1163–1168. doi: 10.3892/mmr.2015.3491. [DOI] [PubMed] [Google Scholar]
- 17.Stiuso P, Potenza N, Lombardi A, Ferrandino I, Monaco A, Zappavigna S, Vanacore D, Mosca N, Castiello F, Porto S, et al. MicroRNA-423-5p promotes autophagy in cancer cells and is increased in serum from hepatocarcinoma patients treated with sorafenib. Mol Ther Nucleic Acids. 2015;4:e233. doi: 10.1038/mtna.2015.8. [DOI] [PubMed] [Google Scholar]
- 18.Wan Y, Fei X, Wang Z, Jiang D, Chen H, Wang M, Zhou S. miR-423-5p knockdown enhances the sensitivity of glioma stem cells to apigenin through the mitochondrial pathway. Tumour Biol. 2017;39:1010428317695526. doi: 10.1177/1010428317695526. [DOI] [PubMed] [Google Scholar]
- 19.Dai L, Cui X, Zhang X, Cheng L, Liu Y, Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al. SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat Commun. 2016;7:11996. doi: 10.1038/ncomms11996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Offit K, Kohut K, Clagett B, Wadsworth EA, Lafaro KJ, Cummings S, White M, Sagi M, Bernstein D, Davis JG. Cancer genetic testing and assisted reproduction. J Clin Oncol. 2006;24:4775–4782. doi: 10.1200/JCO.2006.06.6100. [DOI] [PubMed] [Google Scholar]
- 21.Calvert PM, Frucht H. The genetics of colorectal cancer. Ann Intern Med. 2002;137:603–612. doi: 10.7326/0003-4819-137-7-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 22.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28:4052–4057. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell MJ. Oxaliplatin or irinotecan as adjuvant therapy for colon cancer: The results are in. J Clin Oncol. 2009;27:3082–3084. doi: 10.1200/JCO.2009.22.2919. [DOI] [PubMed] [Google Scholar]
- 25.Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: Selecting optimal treatments for individual patients. J Clin Oncol. 2015;33:1787–1796. doi: 10.1200/JCO.2014.60.0213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials supporting the results of the current study are available within the article.