Abstract.
Human immunodeficiency virus (HIV) infection is a major risk factor for the development of active tuberculosis (TB), one of the deadliest infectious diseases globally. The high mortality associated with the disease can be reduced by early diagnosis and prompt antituberculous treatment initiation. Facilities in TB-endemic regions are increasing the use of nucleic acid amplification (e.g., GeneXpert), which provides rapid results but may have suboptimal sensitivity in HIV-associated TB. Our objective was to evaluate the current practices for TB diagnosis at Edendale Hospital, a large regional hospital in KwaZulu-Natal, South Africa—a TB-endemic region with high HIV prevalence. In this cross-sectional study, all adult inpatients newly started on TB treatment at Edendale were identified over a 6-week period. Demographics, clinical information, diagnostic test results, and outcomes were documented. Pulmonary TB (PTB), extrapulmonary TB (EXTB), and PTB + EXTB were defined as disease evidence in the lungs, other organs, or both, respectively. Ninety-four cases were identified, of which 83% were HIV-associated. Only 30% of all TB patients were microbiologically confirmed, consisting of 7/16 (44%) HIV-uninfected and 21/78 (27%) HIV-infected TB patients. Smear microscopy and mycobacterial culture were seldom ordered. Ultrasound was performed in about one-third of suspected EXTB cases and was valuable in identifying abdominal TB. In this clinical setting with a high incidence of HIV-associated TB, TB diagnosis was more commonly based on clinical assessment and imaging results than on mycobacterial gold standard test confirmation.
INTRODUCTION
Active tuberculosis (TB) remains one of the most deadly infectious diseases worldwide, currently causing more deaths than human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS).1 In 2016, there were an estimated 10.4 million incident cases of TB globally, with 1.3 million associated deaths in HIV-uninfected and 374,000 in HIV-infected individuals. South Africa continues to have one of the highest TB burdens, and 59% of TB cases are HIV-associated, in contrast to an estimated proportion of 10% HIV coinfection globally.1 Because TB in the setting of HIV coinfection progresses rapidly and is the leading cause of death among people living with HIV, rapid diagnosis and early antituberculous treatment initiation are critical.2–4
The gold or reference standards for diagnosing TB are sputum or other body fluid culture or nucleic acid amplification tests (e.g., GeneXpert [GXP] from Cepheid) for Mycobacterium tuberculosis. Culture requires weeks of incubation, and thus GXP, which provides rapid results and can detect drug resistance, has been endorsed by the World Health Organization (WHO) for use in TB-endemic regions and as the first-line test in suspected multidrug resistant and/or HIV-associated TB.5 Smear microscopy for the detection of acid fast bacilli (AFB) is commonly used as a rapid diagnostic test for TB in resource-limited settings, though not considered a gold standard test because AFB are not specific for M. tuberculosis. However, smear microscopy and GXP can have low sensitivity in immunocompromised patients because of poor granuloma formation with less cavitation.6–8 In general, diagnostic confirmation of pulmonary TB (PTB) presents fewer challenges than that of extrapulmonary TB (EXTB) because sputum samples can be relatively easily obtained, as opposed to samples from many other body sites. Thus, diagnostic confirmation of HIV-associated TB, which presents with extrapulmonary manifestations in up to 50% of cases,9,10 often remains challenging regardless of the diagnostic method used.11
Many facilities in TB-endemic regions are increasingly using GXP as their main reference test to confirm TB, but its clinical value in high HIV prevalence settings might be limited. Studies have demonstrated that for PTB, GXP is 99% specific and 89% sensitive, ranging from 58% to 100%, with lower sensitivity in smear-negative than smear-positive PTB.10 In HIV-associated TB, GXP was found to be less sensitive, 79% overall, with a wide range from 0% to 100% in 10 individual studies.12 Sensitivities for EXTB can be quite low but vary depending on body fluid tested. Compared with culture, reported GXP sensitivities range from around 34% for pleural fluid to about 85% for cerebrospinal fluid (CSF) and 96% for lymph node (LN) aspirates, irrespective of HIV status.13
Because of the low sensitivity of confirmatory testing in HIV-infected patients, imaging studies are becoming increasingly important in the workup for HIV-associated TB. Recent studies suggest that abdominal ultrasound (US) may be useful in identifying extrapulmonary disease, but results have not been consistent. A study of Cambodian HIV-infected patients found that enlarged abdominal LNs on US had a low sensitivity (33%) but a high positive predictive value (71%) and specificity (97%) for abdominal TB.14 On the other hand, a prospective study in South Africa found that abdominal LN enlargement on US was highly suggestive of abdominal TB in HIV-infected patients and that the majority of TB cases identified based on US but without microbiological confirmation achieved symptomatic improvement with antituberculous treatment.15 Although the study was limited by the lack of gold standard diagnostic confirmation, the authors concluded that US can be a reliable tool for diagnosing HIV-associated TB.15 However, US is not a widely accepted method for EXTB diagnosis, and there are few studies examining its use and role in clinical practice.
Edendale Hospital is a large regional and township hospital in KwaZulu-Natal (KZN), South Africa, a province with both a very high TB incidence and HIV prevalence. Despite improved TB rates over the past years, the province still has one of the highest TB incidences globally—685/100,000 in 2015 with 64% HIV coinfection.16 Few studies have evaluated the routine use and clinical value of TB diagnostics and imaging modalities in TB-endemic regions with high HIV prevalence. As such, our objective was to investigate current practices toward TB diagnosis in this region with high incidence of both HIV-associated and non–HIV-associated TB. We specifically focused on the rates of microbiological testing, the proportion of patients confirmed by gold standard tests, and the clinical value of imaging modalities, including US, chest X-ray (CXR), and computed tomography (CT) scans.
MATERIALS AND METHODS
The medical records of inpatients aged older than 13 years (considered to be adults in South Africa) and newly started on treatment of TB at Edendale Hospital over a 6-week observation period from June to July 2015 were reviewed. Patients on antituberculous treatment started before admission were excluded. Data collection included demographics; results of clinical tests such as HIV and CD4 counts, if applicable; all TB diagnostic tests ordered, such as smear, culture, or GXP on sputum, CSF, pleural fluid, LN fluid, ascitic fluid, and pericardial fluid; and imaging studies such as CXR, US, and CT scans. We further recorded patients’ in-hospital length of stay and mortality. The study was approved by the University of KZN Biomedical Research Ethics Review Committee and Albert Einstein College of Medicine Institutional Review Board.
Case definitions.
Pulmonary TB was defined as disease confined to the lungs according to clinical evaluation. This was assessed based on symptoms, pulmonary abnormalities on CXR, and lack of clinical suspicion for disease dissemination. Extrapulmonary TB was defined as disease detected in organs or locations other than the lungs, such as the meninges, pleural space, abdomen, or LNs. Cases defined as PTB + EXTB had evidence of disseminated disease including the lungs and other sites.
Clinical diagnostic algorithms.
Patients requiring further evaluation for TB are identified by medical staff, based on symptoms of cough, weight loss, fever, or sweats. A CXR is routinely taken on all medical admissions for TB workup. Sputum for GXP testing is collected from patients who are able to produce a specimen, and the CXR is reviewed on the post-intake ward round. Diagnostic categories outlined by the WHO for smear-negative PTB and EXTB are used to guide empiric prescription of antituberculous treatment.17 Patients with TB symptoms who do not have features of one or more of the WHO-defined categories may also have an abdominal US to screen for intra-abdominal lymphadenopathy, ascites, or splenic lesions.
Statistical methods.
Statistical analysis was carried out using STATA software, version 14.0 (StataCorp, College Station, TX). χ2 tests were used to compare categorical variables, with the Fisher exact test used when two or more groups contained less than five observations. Continuous variables were assessed for normality. For those with normal distributions, means were compared using analysis of variance. For not normally distributed variables, the Kruskal–Wallis test was used for multiple group comparison.
RESULTS
Demographic and clinical data.
We identified 94 patients newly started on antituberculous treatment during the study period, consisting of 36 (38%) with PTB, 33 (35%) with EXTB, and 25 (26%) with PTB + EXTB. All patients were Zulu Africans and there was no statistical difference in demographics between the disease manifestation groups (Table 1). All patients presented with symptoms consistent with TB. Overall, 78 (83%) TB patients were known to be HIV infected, with a median CD4 count of 99.5 cells/μL (interquartile range: 35–242). As anticipated, the portion of HIV coinfection was significantly higher in patients with EXTB than in those with PTB alone, and among those with HIV coinfection, those with EXTB manifestations had a nonsignificant trend toward lower CD4 counts (Table 1). Eighteen (19%) TB patients died in the hospital, of whom 15 (83%) were HIV infected, but there was no significant difference in mortality rates between HIV-infected and HIV-uninfected patients (HIV infected: 15/78, 19%; HIV uninfected: 3/16, 19%; P = 0.96). Among those who died, the median CD4 count was 81 cells/μL (interquartile range: 52–147), compared with 104 cells/μL (interquartile range: 32–320) for those who survived, although this difference was not significant (P = 0.41). There was also no significant difference in mortality based on disease location (PTB: 4/36, 11%; EXTB: 7/33, 21%; PTB + EXTB: 7/25, 28%; P = 0.24), although statistical power was limited by small numbers.
Table 1.
Demographic and clinical data
| PTB, N = 36 | PTB + EXTB, N = 25 | EXTB, N = 33 | P value | |
|---|---|---|---|---|
| Male (%) | 18 (50%) | 14 (56%) | 16 (49%) | 0.84* |
| Age (years), mean ± SD | 40.7 ± 15.3 | 38.5 ± 12.7 | 35.2 ± 11.8 | 0.25† |
| HIV+ (%) | 24 (67%) | 23 (93%) | 31 (99%) | 0.004* |
| CD4, median (IQR) | 141 (37–359) | 64.5 (20–137) | 104 (44–307) | 0.11‡ |
| Mortality (%) | 4 (11%) | 7 (28%) | 7 (21%) | 0.24* |
| Microbiologically confirmed TB (%) | 17 (47%) | 5 (20%) | 6 (18%) | 0.014* |
EXTB = extrapulmonary tuberculosis; IQR = interquartile range; PTB = pulmonary tuberculosis; SD = standard deviation; TB = tuberculosis.
χ2.
Analysis of variance.
Kruskal–Wallis.
Microbiological evaluation—GXP testing.
About half of all TB patients (49/94, 52%) received GXP testing on any body fluid, consisting mostly of sputum samples in patients with pulmonary manifestations (Table 2). There was no significant difference in GXP testing performed based on HIV status. However, among PTB cases, HIV-uninfected patients were significantly more likely to have a positive sputum GXP when tested (6/6, 100%) compared with HIV-infected patients (9/20, 45%; P = 0.024). For patients with EXTB manifestations, GXP was rarely performed. However, when used, GXP was positive in the majority of pleural fluid and in the one ascitic fluid specimen tested, but was rarely positive in CSF samples (Table 3).
Table 2.
Use and results of microbiological testing of sputum samples
| PTB, N = 36 | PTB + EXTB, N = 25 | EXTB, N = 33 | P value | |
|---|---|---|---|---|
| GXP (%) | 26 (72%) | 11 (44%) | 8 (24%) | 0.000* |
| GXP+ (%) | 15/26 (58%) | 3/11 (27%) | 2/8 (25%) | 0.15† |
| Smear (%) | 6 (17%) | 2 (4%) | 2 (6%) | 0.34† |
| Smear+ (%) | 4/6 (67%) | 0/2 (0%) | 0/2 (0%) | 0.33† |
| Culture (%) | 6 (17%) | 3 (12%) | 4 (12%) | 0.87† |
| Culture+ (%) | 1/6 (17%) | 1/3 (33%) | 0/4 (0%) | 0.69† |
| Total microbiological tests performed | 29/36‡ (81%) | 16/33 (48%) | 14/25 (56%) | 0.015* |
| Total microbiological tests + | 17/29 (59%) | 6/16 (38%) | 5/14 (36%) | 0.24* |
EXTB = extrapulmonary tuberculosis; GXP = GeneXpert; PTB = pulmonary tuberculosis.
χ2.
Fisher exact test.
Five PTB patients received both sputum GXP and sputum smear, and four PTB patients received both sputum GXP and sputum culture.
Table 3.
Use and results of microbiological testing of extrapulmonary samples
| Pleural TB,* N = 9 | TB meningitis,† N = 13 | Abdominal TB,‡ N = 20 | TB lymphadenitis,§ N = 3 | TB pericarditis,‖ N = 2 | |
|---|---|---|---|---|---|
| GXP | 3 (33%) | 3 (23%) | 1 (5%%) | 0 (0%) | 0 (0%) |
| GXP+ | 2/3 (67%) | 0/3 (0%) | 1/1 (100%) | 0/0 (0%) | 0/0 (0%) |
| Smear microscopy | 2 (22%) | 1 (8%) | 0 (0%) | 2 (67%) | 0 (0%) |
| Smear+ | 0/2 (0%) | 0/1 (0%) | 0/0 (0%) | 2/2 (100%) | 0/0 (0%) |
| Mycobacterial culture | 1 (11%) | 1 (8%) | 0 (0%) | 1 (33%) | 1 (50%) |
| Culture+ | 1/1 (100%) | 1/1 (100%) | 0/0 (0%) | 1/1 (100%) | 1/1 (100%) |
| Total extrapulmonary microbiological tests performed | 5/9¶ (56%) | 5/13 (38%) | 1/20 (5%) | 2/3# (67%) | 1/2 (50%) |
| Total extrapulmonary microbiological tests+ | 3/5 (60%) | 1/5 (20%) | 1/1 (100%) | 2/2 (100%) | 1/1 (100%) |
GXP = GeneXpert; TB = tuberculosis. Obtained for 47/58 TB patients with extrapulmonary disease manifestations (11/58 patients with extrapulmonary manifestations had miliary or spinal TB and no samples obtained).
Pleural fluid tested.
CSF = cerebrospinal fluid tested.
Ascites tested.
Lymph node aspirate tested.
Pericardial fluid tested.
One pleural sample had both smear and culture performed.
One lymph node sample had both smear and culture performed.
Smear microscopy and mycobacterial cultures.
Sputum smear microscopy was performed in only 10/94 (11%) TB patients, of which six were sputum smears for PTB evaluation (Table 2). Smear microscopy was infrequently used for extrapulmonary samples which, except for LN aspirates, were typically negative (Table 3). Culture of any body fluid was performed in only 17/94 (18%) TB patients, consisting mostly of sputum cultures (Tables 2 and 3). Whereas sputum cultures were rarely positive, all of the few extrapulmonary samples sent for culture were positive (Table 3).
Microbiological evaluation by site of disease.
Pulmonary TB patients were significantly more likely to have gold standard testing performed—29/36 (81%) received sputum GXP, smear microscopy, or culture, versus 16/33 (49%) EXTB and 14/25 (56%) PTB + EXTB patients receiving these tests on any body fluid (P = 0.016). About a quarter of PTB patients did not receive sputum GXP testing (10/36, 28%). Of these, one patient had sputum microscopy performed and two patients had cultures performed. Of the seven remaining PTB patients who did not receive any gold standard diagnostic testing, two died on hospital days 2 and 7. The majority of EXTB patients did not receive GXP testing on any body fluid (21/33, 64%). Of these, 17/21 (81%) had no microbiological testing performed. Most of those (12/17, 71%) had no extrapulmonary samples obtained and several (7/17, 41%) died while hospitalized. Similar to EXTB patients, more than half of the PTB + EXTB patients did not receive GXP testing of sputum or other body fluid samples (14/25, 56%). Of those, one had culture and two had smears performed. Of the 11/25 (44%) PTB + EXTB patients who had no microbiological testing performed, 8/11 (73%) had no extrapulmonary sample obtained and 3/11 (27%) died at hospital days 5, 12, and 15.
Summary of microbiological evaluation.
Thirty-five of 94 (37%) patients diagnosed with TB had no gold standard testing performed and 12 of these 35 (34%) died in the hospital. Irrespective of testing, 18/94 (19%) of patients diagnosed with TB died in the hospital. There was a significant difference in mortality between patients who received TB gold standard testing (6/59, 10%), compared with patients who did not receive TB testing (12/35, 34%; P = 0.004). Only 28/94 (30%) patients received a microbiologically confirmed diagnosis. Pulmonary TB patients were significantly more likely to be confirmed than EXTB or PTB + EXTB patients (P = 0.14; Table 1). There was no significant difference in gold standard testing based on HIV status (HIV infected: 48/78, 62%; HIV uninfected: 11/16, 69%; P = 0.59), but there was a nonsignificant trend for lower diagnostic confirmation in HIV-associated TB (HIV infected: 21/78, 27%; HIV uninfected: 7/16, 44%; P = 0.18).
Imaging.
Imaging studies were the most common method used to support TB diagnosis and initiate antituberculous treatment. Radiographic findings suggestive of TB on CXR were used as the only diagnostic tool, in addition to clinical assessment, before starting treatment in 39% of PTB and 56% of PTB + EXTB patients, and CT of the chest was rarely performed (Table 4). In patients with PTB alone, findings ranged from reticulonodular infiltrates to cavitary lesions, whereas patients with PTB + EXTB showed mostly patterns of miliary TB on CXR. For EXTB, CXR was only useful for detection of pleural TB. Of the nine pleural TB patients, 4 (44%) were initiated on treatment based on imaging, whereas the rest had follow-up testing on pleural fluid samples.
Table 4.
Performance of radiographic imaging modalities
| PTB, N = 36 | PTB + EXTB, N = 25 | EXTB, N = 33 | P value | |
|---|---|---|---|---|
| CXR performed (%) | 36 (100%) | 25 (100%) | 33 (100%) | – |
| CXR-based diagnosis* (%) | 14/36 (39%) | 14/25 (56%)† | 4/33 (12%) | 0.002‡ |
| US performed (%) | 3 (8%) | 9 (36%) | 11 (33%) | 0.016‡ |
| US-based diagnosis* (%) | 0/3 (0%) | 8/9 (89%)† | 9/11 (82%) | 0.024§ |
| CT performed (%) | 3 (8%) | 1 (4%) | 5 (15%) | 0.33§ |
| CT-based diagnosis* (%) | 2/3 (67%) | 0/1 (0%) | 0/5 (0%) | 0.17§ |
| Total imaging-based diagnosis (%) | 16 (44%) | 18 (72%) | 13 (36%) | 0.034‡ |
CT = computed tomography; CXR = chest X-ray; EXTB = extrapulmonary tuberculosis; PTB = pulmonary tuberculosis; US = ultrasound.
Proportion of patients whose treatment was initiated based on imaging modalities alone without microbiological testing.
Four patients were diagnosed based on both CXR and US results.
χ2.
Fisher exact test.
Abdominal US was frequently performed in patients with extrapulmonary manifestations (Table 4). Of those receiving abdominal US, 82% of EXTB and 89% of PTB + EXTB patients had results suggestive of TB disease and treatment initiated based on these results (Table 4). Among the 17 patients diagnosed by abdominal US, the most common findings suggestive of TB were abdominal lymphadenopathy (12/17, 70%) and splenic microabscesses (10/17, 59%).
Of the 47 patients initiated on treatment following suggestive imaging, 26 (55%) received follow-up microbiological testing to confirm the diagnosis. As can be anticipated, PTB and PTB + EXTB patients were more likely to receive follow-up microbiological testing compared with EXTB alone (PTB: 12/16, 75%; EXTB: 3/13, 23%; PTB + EXTB: 11/18, 61%; P = 0.016). All of these tests in PTB and the majority of PTB + EXTB (10/11, 91%) patients were from sputum samples. Only 8/26 (31%) tested positive, including 4/12 (33%) PTB patients, 1/3 (33%) EXTB patients, and 3/11 (27%) PTB + EXTB patients.
Empirical TB diagnosis.
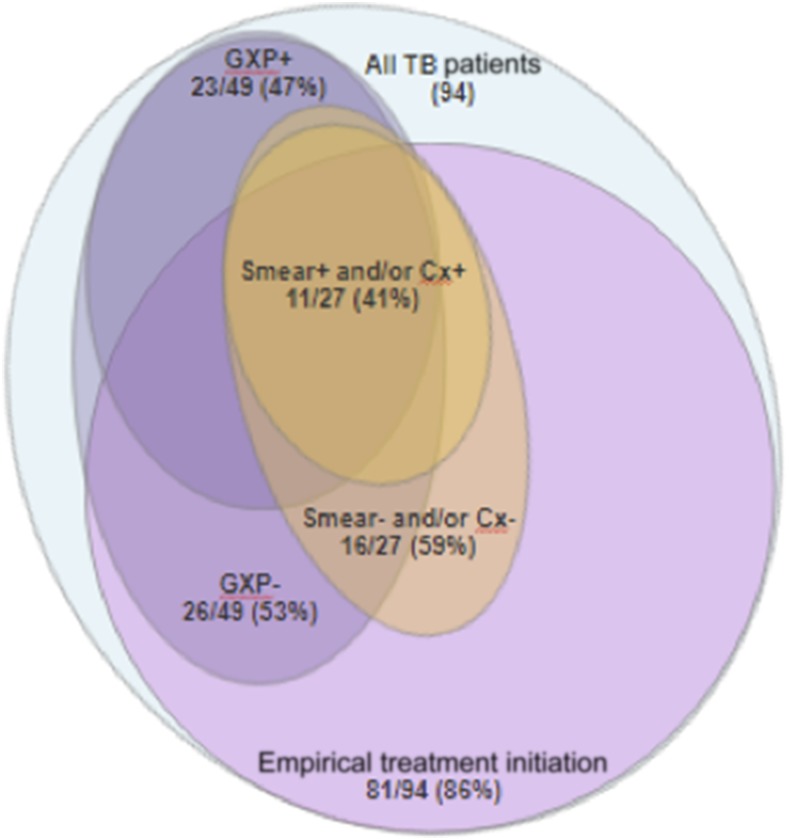
Empirical TB diagnosis was common with antituberculous treatment initiated in 81/94 (86%) patients based on clinical presentation alone (23/81, 28%), or a combination of clinical assessment with imaging (47/81, 58%), pleural fluid (3/81, 4%), or CSF (9/81, 11%) analysis suggestive of TB in the absence of microbiological evidence. Suggestive fluid analyses included high adenosine deaminase in pleural fluid or high protein and low glucose in CSF. Of the 81 patients empirically initiated on antituberculous treatment, 15 (16%) were later microbiologically confirmed (Figure 1).
Figure 1.
Performance and positive yield of microbiological testing with empirical treatment initiation. Proportion of tuberculosis (TB) cases who were empirically initiated on treatment after suggestive imaging results, clinical assessment, and/or extrapulmonary fluid analysis, without microbiological evidence of TB (81/94, 86%). The remaining 13 patients were initiated on TB treatment following positive GeneXpert (GXP). Overall, 49/94 (52%) patients had GXP performed, of which 23 (47%) were positive. Twenty-seven of 94 (29%) patients received either smear microscopy or culture (Cx), of which 11 (41%) were positive. Fifteen patients who were empirically initiated on treatment were later confirmed microbiologically, of which 10 (67%) had positive GXPs, 9 (60%) had positive smear microscopy and/or cultures, and 4 (27%) were positive with GXP, smear, and/or culture. Only 3/27 (11%) patients who received smear and/or culture were not empirically initiated on treatment. These three patients also had positive GXPs and two (67%) had positive smear and/or culture.
Patients with extrapulmonary manifestations were more likely to be diagnosed empirically compared with PTB patients (PTB: 25/36, 69%; EXTB: 31/33, 94%; PTB + EXTB: 25/25, 100%; P = 0.001). HIV-infected patients were more likely to be empirically diagnosed (70/78, 90%) than HIV-uninfected patients (11/16; 69%; P = 0.027).
DISCUSSION
Our study shows that in a South African clinical setting with a high incidence of both HIV-associated and non–HIV-associated TB, TB diagnosis was less commonly confirmed by gold standard tests, in only around a third of patients, than based on clinical assessment supported by imaging results. Smear microscopy and culture were rarely used and were of limited clinical value regardless of samples obtained. GeneXpert, although more frequently used than other microbiological tests, was performed in only half of the patients treated for TB, and in those who did receive GXP, less than half tested positive.
Our findings are consistent with prior studies indicating that GXP may be a less useful TB diagnostic test in high than in low HIV prevalence settings, given its lower sensitivity in HIV-infected patients (range of 0–100% sensitivity across 10 studies) compared with HIV-uninfected patients (range of 56–100% sensitivity across nine studies).11–13 Despite WHO recommendations for GXP use as a first-line test in HIV-associated TB, there was no significant difference between the frequency of GXP ordered based on HIV status. Chest X-ray was often used as the only diagnostic tool before initiating antituberculous treatment. Consistent with prior findings,18 it is conceivable that doctors in our and possibly other high HIV prevalence settings have found a low adjunctive value of GXP beyond routine performance of CXRs for all TB suspects. Another study in an inpatient setting with high rates of HIV-associated TB similarly found that GXP had little diagnostic value in addition to clinical assessment and imaging. In that study, this was mostly because of operational challenges of GXP performance, such as sputum collection and slow turnaround time in a busy hospital setting.19
As can be anticipated, GXP was more commonly performed in PTB compared with EXTB or PTB + EXTB patients. However, even for PTB, the overall GXP sensitivity remained less than 60%, with HIV-infected patients significantly less likely to have a positive result (45%) than HIV-uninfected patients (100%). We note that the sensitivity for GXP in HIV-infected PTB patients in our study is lower than that reported in other studies. A 2014 Cochrane review identified 27 unique studies investigating the diagnostic accuracy of GXP in PTB patients. Seven studies provided data for both HIV-uninfected and HIV-infected PTB patients, with pooled sensitivities of 86% in HIV-uninfected and 79% in HIV-infected individuals.12 Among these seven studies, four reported mean or median CD4 counts of the HIV-infected subjects, which ranged from 182 to 276 cells/μL.20–23 The median CD4 count in HIV-infected PTB patients in our study was 141 cells/μL, lower than those reported in the other studies. This suggests that the lower GXP positivity rate could have been because of the severity of immunosuppression which typically leads to less cavity formation and organized granulomas and higher rates of disease dissemination.24 This observation is consistent with the decreased sensitivity of GXP associated with lower CD4 counts in another sub-Saharan African study.23 In addition, sputum quality and collection methods have been shown to influence GXP diagnostic yield.25 Whether these variables could have led to a lower rate of positivity in our population would need to be investigated in future studies. Furthermore, a study of smear-negative TB suspects at an outpatient clinic in Uganda found that CXR-based diagnosis may overestimate TB cases.26 Given the high rates of empirical and imaging-based diagnosis found in our study, it cannot be excluded that TB was overdiagnosed.
Thirty-seven percent of patients diagnosed with TB received no gold standard testing. A third of these died in the hospital, and it is conceivable that these patients may have been too weak to produce sputum or undergo invasive testing. It is further conceivable that for the other two-thirds who did not receive testing, the physicians who are highly experienced in evaluating and treating TB patients in this high HIV prevalent TB-endemic setting may have had sufficient clinical and radiologic evidence to feel confident about their TB diagnosis.
Our study was limited by the small sample size and the lack of gold standard TB confirmation in the empirically treated cases. In addition, we note that our study was based in a hospitalized setting with more resources available than in ambulatory and community health-care clinics. Therefore, our results are likely not generalizable to all resource-poor settings in this region, which often do not have capabilities to perform imaging or GXP, and might have less trained and experienced health-care providers compared with the physicians in our hospital setting.
Images suggestive of EXTB on abdominal US were recorded in 80–90% of patients with extrapulmonary manifestations and led to initiation of antituberculous treatment in these cases. These findings were especially useful for documenting abdominal TB manifestations and indicate that US is a useful adjunctive method for TB diagnosis in a high HIV prevalence setting. Because of the known challenges of TB confirmation in HIV coinfected patients, our data warrant validation in larger prospective cohort studies with documentation of clinical and radiographic response in patients empirically treated for TB. Such cohort studies would be especially important in evaluating the usefulness of US and potential other biomarkers for the diagnosis of EXTB. Our data highlight that despite recent advances in TB diagnostics, a large proportion of TB cases, especially those that are extrapulmonary and/or HIV associated, remain microbiologically unconfirmed. These data support the critical need for accurate non–sputum-based TB biomarkers that could inform the development of simple adjunctive diagnostic tests.11 An example is the lateral flow test based on the detection of lipoarabinomannan in urine. Because this test lacks sensitivity in less immunocompromised patients, its use has been endorsed by WHO for HIV-infected TB suspects with CD4 counts less than 100 cells/μL.27–29 Thus, further research is needed to identify and validate easily detectable non–sputum-based biomarkers for both HIV-infected and uninfected populations.11
In conclusion, our study gives insight into the current diagnostic approaches for TB in a region of high HIV–TB coinfection rates. Our observations demonstrate that although GXP has improved TB diagnosis, continued advancement in TB diagnostic tools is needed, especially for HIV-associated TB. As emphasized by WHO, Centers for Disease Control and Prevention (CDC), and other major TB stakeholders, these should include easily detectable non–sputum-based TB biomarkers. Our findings further suggest that US can be a valuable adjunctive tool for EXTB that warrants further validation.
REFERENCES
- 1.WHO , 2017. Global Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Toossi Z, 2003. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis 188: 1146–1155. [DOI] [PubMed] [Google Scholar]
- 3.Perkins MD, Cunningham J, 2007. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis 196 (Suppl 1): S15–S27. [DOI] [PubMed] [Google Scholar]
- 4.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C, 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 163: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 5.WHO ; WHO Guidelines Approved by the Guidelines Review Committee , 2013. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. Geneva, Switzerland: World Health Organization.
- 6.Lawn SD, Butera ST, Shinnick TM, 2002. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect 4: 635–646. [DOI] [PubMed] [Google Scholar]
- 7.Di Perri G, Cazzadori A, Vento S, Bonora S, Malena M, Bontempini L, Lanzafame M, Allegranzi B, Concia E, 1996. Comparative histopathological study of pulmonary tuberculosis in human immunodeficiency virus-infected and non-infected patients. Tuber Lung Dis 77: 244–249. [DOI] [PubMed] [Google Scholar]
- 8.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF, 1993. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis 148: 1292–1297. [DOI] [PubMed] [Google Scholar]
- 9.Poprawski D, Pitisuttitum P, Tansuphasawadikul S, 2000. Clinical presentations and outcomes of TB among HIV-positive patients. Southeast Asian J Trop Med Public Health 31 (Suppl 1): 140–142. [PubMed] [Google Scholar]
- 10.Naing C, Mak JW, Maung M, Wong SF, Kassim AIBM, 2013. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung 191: 27–34. [DOI] [PubMed] [Google Scholar]
- 11.Denkinger CM, et al. 2015. Defining the needs for next generation assays for tuberculosis. J Infect Dis 211 (Suppl 1): S29–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N, 2014. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 1: CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maynard-Smith L, Larke N, Peters JA, Lawn SD, 2014. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis 14: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sculier D, Vannarith C, Pe R, Thai S, Kanara N, Borann S, Cain KP, Lynen L, Varma JK, 2010. Performance of abdominal ultrasound for diagnosis of tuberculosis in HIV-infected persons living in Cambodia. J Acquir Immune Defic Syndr 55: 500–502. [DOI] [PubMed] [Google Scholar]
- 15.Heller T, Goblirsch S, Wallrauch C, Lessells R, Brunetti E, 2010. Abdominal tuberculosis: sonographic diagnosis and treatment response in HIV-positive adults in rural South Africa. Int J Infect Dis 14 (Suppl 1): e108–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massyn N, Peer N, English R, Padarath A, Barron P, Day C, eds, 2016. District Health Barometer 2015/16. Durban, South Africa: Health Systems Trust. [Google Scholar]
- 17.WHO , 2007. Improving the Diagnosis and Treatment of Smear-Negative Pulmonary and Extra-Pulmonary Tuberculosis among Adults and Adolescents: Recommendations for HIV-Prevalent and Resource-Constrained Settings. Geneva, Switzerland: World Health Organization.
- 18.Heidebrecht CL, Podewils LJ, Pym AS, Cohen T, Mthiyane T, Wilson D, 2016. Assessing the utility of Xpert(®) MTB/RIF as a screening tool for patients admitted to medical wards in South Africa. Sci Rep 6: 19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla KS, Kanyama C, Mbewe A, Matoga M, Hoffman I, Ngoma J, Hosseinipour MC, 2016. Policy to practice: impact of GeneXpert MTB/RIF implementation on the TB spectrum of care in Lilongwe, Malawi. Trans R Soc Trop Med Hyg 110: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rie A, Page-Shipp L, Hanrahan CF, Schnippel K, Dansey H, Bassett J, Clouse K, Scott L, Stevens W, Sanne I, 2013. Point-of-care Xpert® MTB/RIF for smear-negative tuberculosis suspects at a primary care clinic in South Africa. Int J Tuberc Lung Dis 17: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanrahan CF, Selibas K, Deery CB, Dansey H, Clouse K, Bassett J, Scott L, Stevens W, Sanne I, Van Rie A, 2013. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One 8: e65421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, Venter WF, Duse A, Stevens W, 2011. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med 8: e1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theron G, et al. 2011. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 184: 132–140. [DOI] [PubMed] [Google Scholar]
- 24.Achkar JM, Jenny-Avital ER, 2011. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis 204 (Suppl 1): S1179–S1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer AJ, et al. 2017. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLoS One 12: e0180572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wekesa C, Kirenga BJ, Joloba ML, Bwanga F, Katamba A, Kamya MR, 2014. Chest X-ray vs. Xpert® MTB/RIF assay for the diagnosis of sputum smear-negative tuberculosis in Uganda. Int J Tuberc Lung Dis 18: 216–219. [DOI] [PubMed] [Google Scholar]
- 27.WHO , 2015. The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV: Policy Update. Geneva, Switzerland: World Health Organization.
- 28.Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, Gupta-Wright A, Nicol MP, Meintjes G, 2017. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkhoff AD, Barr DA, Schutz C, Burton R, Nicol MP, Lawn SD, Meintjes G, 2017. Disseminated tuberculosis among hospitalised HIV patients in South Africa: a common condition that can be rapidly diagnosed using urine-based assays. Sci Rep 7: 10931. [DOI] [PMC free article] [PubMed] [Google Scholar]