Abstract.
Powassan virus (POWV) is a tick-borne zoonosis maintained in natural enzootic cycles between ixodid ticks and wild mammals. Reported human cases have increased in recent years; these infections can be fatal or lead to long-term neurologic sequelae. However, both the geographic distribution and the role of common, potential mammalian hosts in POWV transmission are poorly understood, creating challenges to public health surveillance. We looked for evidence of POWV infection among candidate wildlife host species and ticks collected from mammals and birds in southern Ontario. Tissues (including blood) and ticks from trapped wild mammals were collected in the summers of 2015 and 2016. Ticks removed from dogs in 2015–2016 and wildlife diagnostic cases from 2011 to 2013 were also included. Tissue and tick (Ixodes spp.) homogenates were tested for POWV by reverse transcriptase–polymerase chain reaction (RT-PCR). In addition, sera from wild mammals were tested for antibodies to POWV, West Nile virus (WNV), and heartland virus (HRTV) by plaque reduction neutralization test. All 724 tissue samples were negative for POWV by RT-PCR. One of 53 pools of Ixodes cookei (among 98 total tick pools) was RT-PCR positive for deer tick virus (POWV) lineage. Antibodies to POWV and WNV were detected in 0.4% of 265 and 6.1% of 264 samples, respectively, and all of 219 serum samples tested negative for anti-HRTV antibodies. These results reveal low POWV detection rates in southern Ontario, while highlighting the challenges and need for continued efforts into understanding POWV epidemiology and targeted surveillance strategies.
INTRODUCTION
Powassan virus (POWV) is a tick-borne virus (family Flaviviridae; genus Flavivirus) maintained in enzootic cycles that involve small- and medium-sized wild mammals and ixodid ticks.1 The virus was first isolated in 1958 from the brain of a child that died of encephalitis in the village of Powassan, Ontario, Canada.2 It has since been documented across numerous regions of North America, including eastern Canada, the northeastern and north-central United States, and, less commonly, the western United States and Canada.3 Recently, reports of human POWV cases have increased both within and outside known endemic areas.1 These infections carry significant public health risks, as human infections often lead to long-term neurologic sequelae or death and tick-borne POWV transmission to mice occurs in as little as 15 minutes of feeding.3–5 However, the virus has rarely been shown to cause clinical disease in experimentally infected wild and domestic mammals, including eastern gray squirrels (Sciurus carolinensis), horses (Equus caballus), and rhesus monkeys (Macaca mulatta).6–8
Powassan virus activity and geographic range have primarily been defined by human case reports,3 thus underusing wildlife reservoir hosts and tick vectors as a potential source of valuable information. For example, several wildlife species have been documented with infectious virus or anti-POWV antibodies; these include small mammals such as striped skunks (Mephitis mephitis), groundhogs (Marmota monax), and red squirrels (Tamiasciurus hudsonicus).9–16 However, the recent diagnosis of POWV in humans in Ontario (R. Lindsay, unpublished data) and the increase in reported cases in the northeastern United States.17 underscore the need to further determine the potential importance of various wildlife reservoir hosts in POWV maintenance and transmission. Two genetically distinct but serologically indistinguishable POWV lineages have been described, prototype POWV and deer tick virus (DTV).1 Viruses in both lineages can cause fatal neurologic disease in humans and a distinct sylvatic transmission cycle has been described for each.1,5,18 Through furthering the understanding of regional virus–wildlife–tick relationships, candidate surveillance species may be identified to better evaluate public health risk. Southern Ontario is home to a high density of humans and an expanding Ixodes scapularis tick population,19 which further warrant enhanced vigilance for POWV and other tick- and mosquito-borne viruses in this region.
The present study was conducted based on the history of POWV in Ontario and recent increased human case reports in the northeastern United States, concurrent with a paucity of currently available information on its distribution and circulation among wildlife hosts. The study objectives were to 1) compare the prevalence of POWV infections among candidate wildlife reservoir host species through RNA detection and serology; 2) examine ticks removed from wildlife and companion animals in southern Ontario for POWV RNA; and 3) determine the seroprevalence and geographic distribution of other arthropod-borne viruses, West Nile virus (WNV) and heartland virus (HRTV), among local wildlife in southern Ontario.
MATERIALS AND METHODS
Animal trapping and sample collection.
Ticks and tissues (including blood) were collected from wildlife carcasses of small-to medium-sized mammals submitted or donated to the Canadian Wildlife Heath Cooperative (CWHC) from mid-May to mid-October in 2015 and 2016, when seasonal transmission of POWV is expected to occur in southern Ontario.20 Additional ticks were collected from previous CWHC diagnostic cases from 2011 to 2013, which included a variety of wild mammal and bird species. In most cases, carcasses were frozen to −20°C before sample collection. Blood collection from carcasses via cardiac puncture was performed when possible. Heart, kidney, spleen, and brain samples were collected and approximately 0.5 cm3 samples of each tissue were pooled. Tissues were selected based on previous studies of Flavivirus infections (e.g., WNV and POWV) in wild mammals.21,22
Livetrapping of groundhogs and eastern gray squirrels took place in Guelph, Ontario, at Guelph Lake and the University of Guelph campus and arboretum. Tomahawk traps (sizes 106 and 108; Tomahawk Live Trap Co. Tomahawk, WI) were set in the evening, baited with apple and cantaloupe for groundhogs and oat–peanut butter mix for squirrels, and checked the following morning. Animals were anesthetized with ketamine (raccoons [Procyon lotor] and striped skunks; Bioniche Animal Health, Belleville, Ontario, Canada) at a dose of 6.5–13 mg/kg or isoflurane gas (groundhogs and gray squirrels; Baxter Corporation, Mississauga, Ontario, Canada) at a 1–3% concentration within an induction chamber for blood collection (via jugular or saphenous vein) and tick removal. The latter involved a 5-minute visual and tick grooming (with a fine-toothed comb) inspection period. Animals were also weighed and individually identified with ear tags (raccoons and striped skunks; National Band and Tag Co. Newport, KY) or pit tags (groundhogs and gray squirrels; Biomark, Boise, ID) before recovery and release. Blood volume collected from each animal was < 1% of body weight. Wildlife trapping and sampling were performed under institutional animal care and use committee approval (AUP#3471; University of Guelph, Guelph, Ontario, Canada). Additional blood samples and ticks were included from raccoons and striped skunks trapped as part of the Ontario Ministry of Natural Resources and Forestry rabies baiting program in Peterborough in June and Hamilton (Bronte Creek Provincial Park and Stony Creek) in September of 2016. Data recorded from each wild animal included species, sex, age (i.e., immature < 1 year and adult ≥ 1 year; determined by weight), geographic coordinates, and number of ticks collected.
In addition to ticks removed from wildlife, ticks from companion animals were contributed by seven veterinary clinics located within a 7-km radius of Guelph, Ontario, from May to November in 2015 and 2016. For each of these ticks, veterinary clinics provided the following data: host species, date of tick collection, and general location (i.e., the town center where the dog lived), as well as any reported travel history outside of Ontario.
Sample processing and storage.
Tissues (50–100 mg each of pooled brain, heart, kidney, and spleen) were immediately frozen and stored in cryovials at −80°C in 2015 or were placed in cryovials with RNA later (Thermo Fisher Scientific, Waltham, MA) and stored at −20°C in 2016. Blood samples were collected into serum separator tubes (BD Microtainer; Becton-Dickinson, Franklin Lakes, NJ), stored at 4°C for up to 12 hours, and centrifuged for 10 minutes at 2,500 × g. Sera were decanted into cryovials and stored at −20°C.
When more than one tick of the same life stage and species was collected from an individual animal in 2015–2016, up to 10 adults or 25 nymphs were pooled into one vial.23 For ticks previously collected from wildlife diagnostic cases (2011–2013), tick pools included multiple life stages. Ticks collected from wildlife were refrigerated at 4°C for up to 72 hours before identification24 via stereomicroscope and then stored at −80°C. Ticks from companion animals were immediately placed into cryovials with 70% ethanol and stored at room temperature. Just before testing, each tick was halved at midline with a sterile scalpel blade and half-ticks were homogenized by mincing with a scalpel blade and incubating at 56°C overnight in TissueLyser II and proteinase K (Qiagen, Inc., Valencia, CA). Remaining half-ticks were stored at −80°C. Pooled tissue samples were homogenized in TRIzol reagent (Thermo Fisher Scientific) with a ball bearing via mixer mill (Retsch, Haan, Germany) for 1 minute at 30 cycles/second.
Reverse transcriptase–polymerase chain reaction (RT-PCR).
For ticks, RNA was extracted according to the manufacturer’s protocols using the RNeasy mini kit (Qiagen, Inc.). For tissues, RNA was extracted via the TRIzol method according to the manufacturer’s protocol (Thermo Fisher Scientific). All extracted RNA samples were tested by real-time RT-PCR targeting the NS5 region of the POWV genome, using a method adapted from existing protocols (TIB Molbiol, Adelphia, NJ).
To determine whether positive samples contained prototype lineage POWV or DTV lineage, they were tested by two additional real-time RT-PCR assays specific for prototype lineage POWV env (TIB Molbiol) and DTV lineage NS5 genes.23 All real-time PCR tests were prepared with TaqMan Fast Virus Master Mix (Thermo Fisher Scientific) and the following thermocycling conditions were used according to the manufacturer’s guidelines: reverse transcriptase at 50°C for 5 minutes, initial denaturation at 95°C for 20 seconds, followed by 40 cycles of 95°C for 1 second and 60°C for 20 seconds.
Samples that tested positive for POWV RNA were amplified by conventional RT-PCR using primers targeting the POWV NS5 gene for sequence analysis. Reactions were prepared using Qiagen OneStep RT-PCR Kit (Qiagen, Inc.) and PCR was performed with the following thermocycling conditions: reverse transcriptase at 50°C for 30 minutes, initial denaturation at 95°C for 15 minutes, followed by 40 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 1 minute. A final extension step of 72°C for 7 minutes was also performed.25 Amplicons were purified before sequencing using an ABI 3730XL DNA analyzer (Applied Biosystems, Foster City, CA).
Phylogenetic analysis.
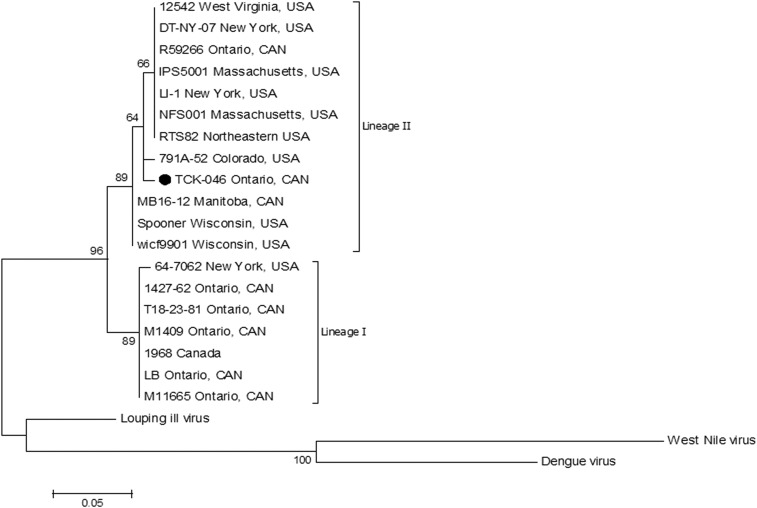
Sequence data from the POWV-PCR positive Ixodes cookei tick were assembled and analyzed using the DNASTAR Lasergene software suite (DNASTAR, Inc., Madison, WI) and the POWV lineage was determined by basic local alignment search tool (BLAST) analysis. A maximum likelihood tree (Figure 1) was constructed using the Jukes–Cantor substitution model.26,27 Molecular Evolution Genetics Analysis (MEGA v7.0.26; http:\\www.megasoftware.net) was used to construct the tree with 17 GenBank NS5 sequences and an unpublished DTV NS5 sequence from the National Microbiology Laboratory (NML) in Winnipeg, Manitoba, Canada. Strain characteristics for prototype POWV and DTV sequences used for phylogenetic analysis are available in Table 1. Louping ill virus (KF056331), WNV (DQ164202), and dengue virus (KX274130) were used as outgroups. Sequences were trimmed to 430 nucleotides and an alignment of NS5 sequences was generated using ClustalX (v2.0; Heidelburg, Germany).28 Genetic distance was determined using the BioNJ algorithm and their robustness was estimated by performing 100 bootstrap replicates.
Figure 1.
Maximum likelihood tree based on a 430-bp fragment of the NS5 gene of Powassan (lineage I) and deer tick virus (lineage II). The phylogenetic tree was produced with 100 bootstrap replications and branch length is proportional to genetic distance using BioNJ algorithm analyses. Louping ill virus (KF056331), West Nile virus (DQ164202), and Dengue virus (KX274130) were used as outgroups. The ruler shows the branch length for a pairwise distance equal to 0.05.
Table 1.
Strain characteristics for Powassan and deer tick virus sequences used for phylogenetic analysis
| Strain | Location origin | Year isolated | Source | GenBank accession |
|---|---|---|---|---|
| 12542 | West Virginia, United States | 1977 | Vulpes spp. | AF310949 |
| 1427-62 | Ontario, Canada | 1962 | Unknown | AF310942 |
| 1968 | Canada | 1968 | Unknown | EU303217 |
| 64-7062 | New York, United States | 1964 | Ixodes sp. | AF310945 |
| 791A-52 | Colorado, United States | 1952 | Ixodes sp. | AF310950 |
| DT-NY-07 | New York, United States | 2007 | Human brain | EU338403 |
| IPS5001 | Massachusetts, United States | 1995 | Unknown | AF310947 |
| LB | Ontario, Canada | 1958 | Human brain | L06436 |
| LI-1 | New York, United States | 2013 | Ixodes scapularis | KJ746872 |
| M11665 | Ontario, Canada | 1960s | Ixodes sp. | AF310937 |
| M1409 | Ontario, Canada | 1960s | Unspecified tick species | AF310940 |
| MB16-12 | Manitoba, Canada | 2016 | I. scapularis | Unpublished |
| NFS001 | Massachusetts, United States | 1996 | I. scapularis | HM440559 |
| R59266 | Ontario, Canada | 1997 | Human brain | AF310948 |
| RTS82 | Northeastern United States | 2016 | I. scapularis | MG647780 |
| Spooner | Wisconsin, United States | Unknown | Ixodes dammini | AF135459 |
| T18-23-81 | Ontario, Canada | 1981 | Ixodes cookei | AF310943 |
| wicf9901 | Wisconsin, United States | 1999 | I. dammini | HM440558 |
Serologic assays.
Serum samples were screened at a 1:10 dilution for anti-Flavivirus antibody reactivity by hemagglutination inhibition (HI) test.29 Samples positive by HI at a ≥ 1:10 dilution were further assessed for anti-POWV antibodies by a plaque reduction neutralization test (PRNT) and considered positive at a reciprocal end point 90% neutralization (PRNT90) titer of ≥ 1:20.23 Similarly, samples were screened for WNV by competitive enzyme-linked immunosorbent assay (cELISA) and any positive samples (> 30% inhibition) were confirmed for WNV-neutralizing antibodies by PRNT30 and were considered positive at a PRNT90 titer of ≥ 1:20. Any POWV or WNV PRNT-positive samples underwent serial 2-fold dilutions and a ≥ 4-fold increase of titer for one virus over the other determined it as the causative virus.31 All samples with sufficient remaining volume (N = 219) were also screened for antibodies to HRTV via PRNT and considered positive at ≥ 70% neutralization at a 1:10 dilution.32
Hemagglutination inhibition test and cELISA were completed in biosafety level-2 facilities and PRNT was completed in biosafety level-3 facilities at the NML (POWV and WNV) and the Animal Disease Laboratory at Colorado State University, Fort Collins, CO (HRTV).
Statistical analysis.
Prevalence and exact confidence intervals (CI) for sample collection and test results were estimated using STATA14® Intercooled (StataCorp, College Station, TX). For sample sets with positive test results, 95% CI were used; for those with no positive test results, 97.5% CI were used.
RESULTS
Ticks.
A total of 234 ticks of three ixodid species, I. cookei (N = 178; 127 adult females, 48 nymphs, and three larvae), I. scapularis (N = 41; 30 adult females, eight adult males, and three nymphs), and Ixodes marxi (N = 15; 11 adults and four nymphs), were collected from 80 wildlife and companion animals from May to November of 2015 and 2016. In addition, I. cookei (N = 49) were collected from wildlife diagnostic cases from 2011 to 2013. These ticks comprised 98 pools. Host species and number of ticks collected varied among tick species. Ixodes cookei were removed from 21 striped skunks, 12 raccoons, five groundhogs, three red foxes (Vulpes vulpes), two dogs (Canis lupus familiaris), and one each from a beaver (Castor canadensis), Canada goose (Branta canadensis), cat (Felis catus), fisher (Pekania pennant), and porcupine (Erethizon dorsatum). Ixodes scapularis were removed from 24 dogs and one black bear (Ursus americanus), coyote (Canis latrans), fisher, porcupine, raccoon, red fox, and red squirrel. Ixodes marxi were removed from two raccoons and one each from a fisher and striped skunk. Some hosts were infested with multiple tick species, including a red fox with I. scapularis and I. cookei, a fisher with I. cookei and I. marxi, and a raccoon with I. scapularis and I. marxi. Additional tick species collected from these animals will be reported elsewhere (K. Smith, unpublished data).
Among the 98 tick pools tested for POWV by RT-PCR (including 53 I. cookei pools), one pool of I. cookei tested positive (1/98; 1.0%; 95% CI: 0.0–5.6% and 1/53; 1.9%; 95% CI: 0.0–10.1%, respectively). Ticks from this pool were removed from a Canada goose on September 16, 2012, and consisted of two engorged adult females and two nymphs.
Phylogenetic data.
Sequencing and BLAST analysis of the PCR-positive tick sample (GenBank accession MH092660) revealed DTV lineage with closest (94%) sequence homology to GenBank accessions AF135459 (from an Ixodes dammini tick in Spooner, Wisconsin, 1997–1998) and EU338403 (from the brain of a human in New York State, 2007) (Figure 1).25,33
Tissues.
Tissues were collected from 724 individuals of 15 species, including raccoon (N = 225), eastern gray squirrel (N = 217), striped skunk (N = 96), groundhog (N = 56), eastern chipmunk (Tamias striatus; N = 44), beaver (N = 30), red squirrel (N = 19), deer mice (Peromyscus maniculatus; N = 9), Virginia opossum (Didelphis virginiana; N = 6), eastern cottontail (Sylvilagus floridanus; N = 5), muskrat (Ondatra zibethicus; N = 5), fisher (N = 4), North American river otter (Lontra canadensis; N = 3), red fox (N = 3), and porcupine (N = 1). All pooled tissue samples tested negative by RT-PCR for prototype POWV and DTV lineage RNA.
Serology.
Blood was collected from 266 wild animals of seven species across southern Ontario; 58 of these were animals from which tissues were also collected (see the previous section). Most of the blood samples were collected from raccoons (N = 177), followed by striped skunks (N = 36). Initial screening by HI detected anti-Flavivirus antibodies in 36/266 (13.5%) samples. Powassan virus–neutralizing antibodies were subsequently confirmed in one of these suspect positive samples (0.4%) from a groundhog (PRNT90 of 20).
Anti-WNV antibodies were detected by cELISA in 21/264 (8.0%) samples, including 11 raccoons, seven skunks, and three groundhogs. West Nile virus–neutralizing antibodies were confirmed by PRNT in 16 (6.1%) wild mammals, including seven raccoons, six skunks, and three groundhogs (PRNT90 titers from 80 to 160). Inclusive, 4.0% of raccoons tested were seropositive, 17.1% of skunks, and 17.6% of groundhogs. All 219 serum samples tested negative for HRTV by PRNT (Table 2).
Table 2.
Wildlife species and number tested for antibodies to POWV, WNV, and HRTV byPRNT from May to October in 2015 and 2016 in southern Ontario, Canada
| Source species | HI test (1:10)* | POWV PRNT | WNV PRNT | HRTV PRNT | ||||
|---|---|---|---|---|---|---|---|---|
| No. positive/N (%) | 95% CI | No. positive/N (%) | 95% CI | No. positive/N (%) | 95% CI | No. positive/N (%) | 95% CI | |
| Raccoon (Procyon lotor) | 20/177 (11.3) | 7.0–16.9 | 0/176 (0.0)† | 0.0–2.1 | 7/176 (4.0)† | 1.6–8.0 | 0/155 (0.0) | 0.0–2.4 |
| Striped skunk (Mephitis mephitis) | 9/36 (25.0) | 12.1–42.2 | 0/36 (0.0) | 0.0–9.7 | 6/35 (17.1)‡ | 6.6–33.6 | 0/40 (0.0) | 0.0–8.8 |
| Groundhog (Marmota monax) | 7/17 (41.2) | 18.4–67.1 | 1/17 (5.9) | 0.1–28.7 | 3/17 (17.6) | 3.8–43.4 | 0/11 (0.0) | 0.0–28.5 |
| Eastern gray squirrel (Sciurus carolinensis) | 0/22 (0.0) | 0.0–15.4 | 0/22 (0.0) | 0.0–15.4 | 0/22 (0.0) | 0.0–15.4 | 0/1 (0.0) | 0.0–97.5 |
| Beaver (Castor canadensis) | 0/12 (0.0) | 0.0–26.5 | 0/12 (0.0) | 0.0–26.5 | 0/12 (0.0) | 0.0–26.5 | 0/11 (0.0) | 0.0–28.5 |
| Virginia opossum (Didelphis virginiana) | 0/1 (0.0) | 0.0–97.5 | 0/1 (0.0) | 0.0–97.5 | 0/1 (0.0) | 0.0–97.5 | 0/1 (0.0) | 0.0–97.5 |
| Red squirrel (Tamiasciurus hudsonicus) | 0/1 (0.0) | 0.0–97.5 | 0/1 (0.0) | 0.0–97.5 | 0/1 (0.0) | 0.0–97.5 | NT | NT |
| Total | 36/266 (13.5) | 9.7–18.2 | 1/265 (0.4) | 0.0–2.1 | 16/264 (6.1) | 3.5–9.7 | 0/219 (0.0) | 0.0–1.7 |
CI = confidence intervals; HI = hemagglutination inhibition; HRTV = heartland virus; NT = not tested; POWN = Powassan virus; PRNT = plaque reduction neutralization test; WNV = West Nile virus.
Serum samples were screened for anti-Flavivirus antibodies by HI test; virus identity was determined by PRNT.
One sample had insufficient volume for POWV and WNV PRNT.
One sample had insufficient volume for WNV PRNT.
Geographic data.
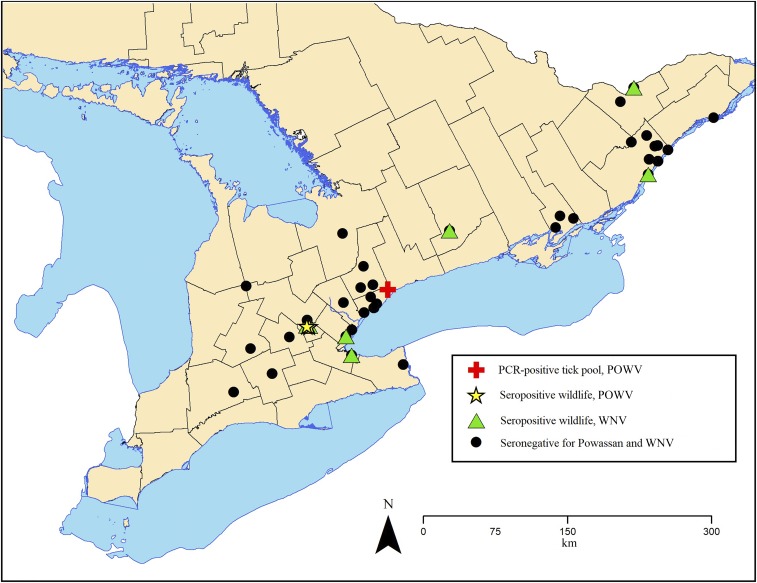
Tissues, blood, and ticks were collected from local wildlife (i.e., species native to the area with relatively small home ranges) and a smaller number of dogs across southern Ontario, Canada, none of which had a reported travel history outside of Ontario. The geographic distribution of wildlife with anti-POWV and -WNV antibodies is depicted in Figure 2. The POWV-positive I. cookei were collected in the Rouge Valley of Toronto, Ontario.
Figure 2.
The distribution of wildlife from which serum samples were tested for antibodies to Powassan virus (POWV) and West Nile virus (WNV) by plaque reduction neutralization test in southern Ontario, Canada, 2015 to 2016. This figure appears in color at www.ajtmh.org.
DISCUSSION
Evidence for the emergence of the DTV lineage of POWV as a public health threat in North America includes the recent increased incidence in human cases in the northeastern United States and southern Ontario, Canada, and infections documented in novel areas (e.g., Connecticut, Minnesota, New Hampshire, and Virginia) (R. Lindsay, unpublished data).18,34,35 The continuing northward expansion of I. scapularis tick populations, facilitated by global climatic change, may alter host–pathogen–vector dynamics and intensify the transmission of tick-borne pathogens such as POWV in Ontario and other northern latitudes.19 These developments underscore the importance of ongoing monitoring for POWV and other tick-borne viruses both within and outside of recognized endemic areas.
Powassan virus surveillance in the short term will likely continue to rely on human case reporting and may underestimate POWV activity and distribution, and thus, public health risk. Additional strategies may help in early seasonal detections, better define the geographic range and temporal patterns, and enhance public awareness. Based on the involvement of wildlife in the enzootic POWV maintenance cycles for currently recognized lineages, wildlife-derived samples may provide a useful surveillance tool. Because the established enzootic cycle differs for each POWV lineage, both of which can cause neurologic disease in humans and overlap geographically,1,23,33 tick and wildlife sampling strategies should account for both lineages. Prototype lineage POWV is thought to be maintained primarily between I. cookei and groundhogs. However, evidence from serological surveys and virus isolation studies suggests that additional species, such as striped skunks, raccoons, and eastern gray and red squirrels, may also be involved in natural transmission cycles.10–12,14,16 Deer tick virus lineage circulates between I. scapularis ticks and white-footed mice (Peromyscus leucopus).1
The DTV lineage detected in an I. cookei tick in the present study was unexpected and suggests a deviation from recognized transmission cycles. However, the converse of this atypical virus–tick pairing (i.e., prototype POWV in I. scapularis) has been detected in Ontario (A. Dibernardo, unpublished data). The DTV sequence in the present study was most similar to viruses that originated in Wisconsin and New York State in 1997–1998 and 2007, respectively,25 with which it had 94% identity. The relatively low identity between the Ontario I. cookei sample and these and other DTV in GenBank resulted in a lowered bootstrapping score; however, the sample was consistently amplified with DTV-specific primers by multiple assays and had high-quality sequencing. Within the existing DTV node (Figure 1), one clade contains more central and western North American samples (e.g., Wisconsin and Manitoba) and the other contains more eastern geographical samples (e.g., West Virginia, New York, Massachusetts, and Ontario). The DTV sample sequence in the present study is distinct from these two clades, which may reflect both the current paucity of DTV strain data3 and a likely unique and novel strain in the region. To our knowledge, this is the first detection of DTV in I. cookei.3,23
Powassan virus serosurveillance in Ontario in the 1960s–1980s revealed that groundhogs and striped skunks were infected more often than other species tested,10,12 and groundhogs were commonly infested with I. cookei ticks.20 The present study serves as a follow-up to this early work to further assess the potential involvement of a variety of wildlife and tick species in POWV transmission and to gain insight into current POWV circulation in the region. The results reaffirm past findings that groundhogs were most often infested with I. cookei ticks and that groundhogs, striped skunks, and I. cookei are infected with POWV in Ontario, albeit at a low prevalence. These data were not collected systematically across species or spatiotemporally; therefore, how closely results reflect actual transmission within the study area and across wider regions of Ontario is unknown. A POWV study in white-tailed deer (Odocoileus virginianus) led to the conclusion that just as seroprevalence varies spatiotemporally, the risk of human exposure varies in space and time and, therefore, studies with long-term, systematic sampling in designated regions are needed.36
Experimental infections in groundhogs, Virginia opossums, gray foxes (Urocyon cinereoargenteus), striped skunks, snowshoe hares (Lepus americanus), white-footed mice, and raccoons suggest low levels of POWV virulence in these hosts, evidenced by the lack of clinical signs and transient viremia of low titers.37–39 Although data using currently available detection tools (e.g., RT-PCR) to detect POWV and DTV in wildlife are sparse, these findings, in conjunction with results of the present study, suggest a low likelihood of POWV detection in wild mammal tissues. Therefore, this is not likely to be a productive strategy in helping define POWV activity. However, serosurveys among local, relatively short-lived wildlife species with limited home range sizes (e.g., groundhogs, squirrels, and striped skunks)40,41 may provide valuable insight into POWV distribution. During the 1960s–1980s in Ontario, antibodies to POWV were consistently detected (via HI test) in groundhogs and striped skunks, as well as in eastern gray squirrels, red squirrels, red foxes, and occasionally raccoons.9–12,14,16 Experimental trials, especially for DTV, in groundhogs and striped skunks with current virological detection and quantification methods would provide critical information about the potential roles of these species in natural virus transmission cycles and, thus, usefulness as sentinels.
The testing of wildlife-derived tick samples, similar to humans and ticks in Lyme disease surveillance,42 may provide valuable information on POWV epidemiology. The observation of I. cookei on different wildlife species in the present study suggests that this tick may opportunistically feed on different host species. Some of these species may enter groundhog burrows (e.g., skunks, foxes, and dogs), which could facilitate POWV spread and maintenance.20 The finding of I. cookei on a Canada goose was surprising because this tick species is rarely observed on birds.23 However, Canada geese spend more time on the ground than many bird species and often congregate in peri-urban areas (in the present case, a zoo), which may allow for artificially concentrated cohabitation of some mammalian and avian wildlife species. Ixodes scapularis, the recognized vector for the DTV lineage of POWV, is known as a more promiscuous feeder that uses a wider variety of mammals than I. cookei, including white-footed mice, white-tailed deer and, more importantly, humans.1,3 Passive tick surveillance for Lyme disease in Ontario revealed that I. scapularis ticks bite humans more often than other known POWV tick vectors (i.e., I. cookei and I. marxi).42 Currently, DTV is widespread among I. scapularis in states bordering Ontario, including New York, where infection rates (i.e., maximum likelihood estimate/100 ticks) of I. scapularis ranged from 1.05 to 3.84, and Wisconsin, where the prevalence of DTV in I. scapularis was 1.3%.23,43 The present study included a small sample of I. scapularis, from which there was no evidence of DTV or prototype POWV. However, I. scapularis is an important target for future research on these viruses in Ontario and surrounding areas, as this tick species is projected to continue its spread and establishment in northern latitudes.19,44
West Nile virus is an endemic, mosquito-borne virus in much of North America. The virus was first detected in Ontario in 2001, and has since been detected seasonally.45–47 West Nile virus–neutralizing antibodies were detected in approximately 18% of groundhogs, 14% of striped skunks, and 4% of raccoons in the present study. Among these three species, only raccoons have been evaluated experimentally for amplifying host status; peak viremia titers below the threshold of infection for Culex pipiens suggest raccoons play a limited role in virus transmission.48 West Nile virus–positive mosquito pools and human cases were reported within Ontario in 2015 and 2016,46 during which time these seropositive mammals were likely infected based on their age and average life expectancy.40,41 These data are consistent with prior serosurveys in other regions, such as Colorado, where multiple mammalian species, including fox squirrels (Sciurus niger), raccoons, striped skunks, and Virginia opossums, were seropositive for WNV.49 Samples that screened Flavivirus positive by HI but were PRNT negative for POWV and WNV may reflect false positives because of nonspecific reactivity or inhibitors in the sera. St. Louis encephalitis virus is very rarely detected in Ontario (A. Dibernardo, unpublished data) and is not considered a probable cause of the reactivity in the assay.
Heartland virus is a tick-borne pathogen that infects a large variety of mammalian hosts, including raccoons, white-tailed deer, and Virginia opossums, all of which live in Ontario. The recognized vector is the lone star tick, Amblyomma americanum, and the distribution of HRTV includes the Midwestern and eastern United States, with a human fatality in Oklahoma in 2014.32,50 The virus has not been detected in Ontario to date. Despite the lack of antibodies to HRTV among wildlife in the present study, the potential for range expansion of A. americanum ticks and the local abundance of candidate host species (e.g., white-tailed deer and raccoons) warrant the continued vigilance for this virus in Ontario, Canada.32,42 For example, an A. americanum tick was recently observed on a dog with no reported travel outside of Ontario (K. Smith, unpublished data), and this species was among the four most common tick species submitted by the public in Ontario from 2008 to 2012.42
The present study reveals rare detections of anti-POWV antibodies in wildlife and viral RNA in ticks, which suggest low levels of virus circulation in some regions of Ontario. A recent fatal human case of POWV in Kingston, Ontario (R. Lindsay, unpublished data), and increased DTV lineage incidence in humans in the northeastern United States underscore the risk that this virus represents to humans in the region. The clinical signs of infection with prototype POWV and DTV lineages in humans may resemble those of other arthropod-borne viruses; consequently, increased awareness of these viruses is crucial.18 Continued surveillance and investigations into host–vector dynamics of POWV in Ontario and other regions are needed to better understand transmission cycles and to monitor for POWV epidemiological patterns associated with environmental changes.
Acknowledgments:
Chris Early and Jamie Heale (University of Guelph) provided expertise in wild mammal behavior and tick identification, respectively, and Beverly Stevenson and Kevin Middel (Ontario Ministry of Natural Resources and Forestry) permitted access to trapped skunks and raccoons. Local veterinary clinics and Nuisance Wildlife Control, Inc. contributed tick and wildlife samples, respectively. Doug Campbell, Lenny Shirose, David Cristo, and Erin Harkness (Canadian Wildlife Health Cooperative) provided logistical support and Tami Sauder, Aleksandra Cetera, Janessa Price, and Alyson Raschkowan (University of Guelph) provided field and laboratory assistance.
REFERENCES
- 1.Ebel GD, 2010. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 55: 95–110. [DOI] [PubMed] [Google Scholar]
- 2.McLean DM, Donohue WL, 1959. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 80: 708–711. [PMC free article] [PubMed] [Google Scholar]
- 3.Hermance ME, Thangamani S, 2017. Powassan virus: an emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis 17: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebel GD, Kramer LD, 2004. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 71: 268–271. [PubMed] [Google Scholar]
- 5.Hinten SR, et al. 2008. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis 8: 733–740. [DOI] [PubMed] [Google Scholar]
- 6.Artsob H, 1989. Powassan encephalitis. Monath TP, ed. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press, 29–45. [Google Scholar]
- 7.Keane DP, Little PB, Wilkie BN, Artsob H, Thorsen J, 1988. Agents of equine viral encephalomyeltis—correlation of serum and cerebrospinal fluid antibiotics. Can J Vet Res 52: 229–235. [PMC free article] [PubMed] [Google Scholar]
- 8.Little PB, Thorsen J, Moore W, Weninger N, 1985. Powassan virus encephalitis—a review and experimental studies in the horse and rabbit. Vet Pathol 22: 500–507. [DOI] [PubMed] [Google Scholar]
- 9.Artsob H, Spence L, Surgeoner G, McCreadie J, Thorsen J, Thng C, Lampotang V, 1984. Isolation of Francisella tularensis and Powassan virus from ticks (Acari, Ixodidae) in Ontario, Canada. J Med Entomol 21: 165–168. [DOI] [PubMed] [Google Scholar]
- 10.Artsob H, Spence L, Thng C, Lampotang V, Johnston D, Macinnes C, Matejka F, Voigt D, Watt I, 1986. Arbovirus infections in several Ontario mammals, 1975–1980. Can J Vet Res 50: 42–46. [PMC free article] [PubMed] [Google Scholar]
- 11.McLean DM, Best JM, Mahaling S, Chernesk MA, Wilson WE, 1964. Powassan virus: summer infection cycle, 1964. Can Med Assoc J 91: 1360–1362. [PMC free article] [PubMed] [Google Scholar]
- 12.McLean DM, Cobb C, Gooderham SE, Smart CA, Wilson AG, Wilson WE, 1967. Powassan virus: persistence of virus activity during 1966. Can Med Assoc J 96: 660–664. [PMC free article] [PubMed] [Google Scholar]
- 13.McLean DM, Crawford MA, Ladyman SR, Peers RR, Purvingo KW, 1970. California encephaitis and Powassan virus activity in British Columbia, 1969. Am J Med Entomol 92: 266–272. [DOI] [PubMed] [Google Scholar]
- 14.McLean DM, Devos A, Quantz EJ, 1964. Powassan virus: field investigations during the summer of 1963. Am J Trop Med Hyg 13: 747–753. [DOI] [PubMed] [Google Scholar]
- 15.McLean DM, Larke RP, 1963. Powassan and Silverwater viruses: ecology of two Ontario arboviruses. Can Med Assoc J 88: 182–185. [PMC free article] [PubMed] [Google Scholar]
- 16.Whitney E, Jamnback H, Means RG, Watthews TH, 1968. Arthropod-borne-virus survey in St. Lawrence County, New York. Am J Trop Med Hyg 17: 645–650. [DOI] [PubMed] [Google Scholar]
- 17.Doughty CT, Yawetz S, Lyons J, 2017. Emerging causes of arbovirus encephalitis in North America: Powassan, chikungunya, and Zika viruses. Curr Neurol Neurosci Rep 17: 12. [DOI] [PubMed] [Google Scholar]
- 18.Piantadosi A, et al. 2016. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 62: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden NH, Maarouf A, Barker IK, Bigras-Poulin M, Lindsay LR, Morshed MG, O’Callaghan CJ, Ramay F, Waltner-Toews D, Charron DF, 2006. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int J Paritisol 36: 63–70. [DOI] [PubMed] [Google Scholar]
- 20.Farkas MJ, Surgeoner GA, 1990. Incidence of Ixodes cookei (Acari, Ixodidae) on groundhogs, Marmota monax, in southwestern Ontario. Proc Entomol Soc Ont 121: 105–110. [Google Scholar]
- 21.Johnson HN, 1987. Isolation of Powassan virus from a spotted skunk in California. J Wildl Dis 23: 152–153. [DOI] [PubMed] [Google Scholar]
- 22.Root JJ, Oesterle PT, Nemeth NM, Klenk K, Gould DH, McLean RG, Clark L, Hall JS, 2006. Experimental infection of fox squirrels (Sciurus niger) with West Nile virus. Am J Trop Med Hyg 75: 697–701. [PubMed] [Google Scholar]
- 23.Dupuis AP, Peters RJ, Prusinski MA, Falco RC, Ostfeld RS, Kramer LD, 2013. Isolation of deer tick virus (Powassan virus, Lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors 6: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindquist EE, Galloway TD, Artsob H, Lindsay LR, Drebot M, Wood H, Robbins RG, 2016. A Handbook to the Ticks of Canada (Ixodida: Ixodidae, Argasidae). Ottawa, Ontario, Canada: Biological Survey of Canada. [Google Scholar]
- 25.Ebel GD, Foppa I, Spielman A, Telford SR, 1999. A focus of deer tick virus transmission in the northcentral United States. Emerg Infect Dis 5: 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jukes TH, Cantor CR, 1969. Evolution of protein molecules. Munro H, ed. Mammalian Protein Metabolism. New York, NY: Academic Press, 21–132. [Google Scholar]
- 27.Kumar S, Stecher G, Tamura K, 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin MA, et al. 2007. Clustal W and clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 29.Clarke DH, Casals J, 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg 7: 561–573. [DOI] [PubMed] [Google Scholar]
- 30.Bailey TN, 1971. Biology of striped skunks on a southwestern Lake Erie marsh. Am Midl Nat 85: 196–207. [Google Scholar]
- 31.Blitvich BJ, Marlenee NL, Hall RA, Calisher CH, Bowen RA, Roehrig JT, Komar N, Langevin SA, Beaty BJ, 2003. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in multiple avian species. J Clin Microbiol 41: 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosco-Lauth AM, et al. 2015. Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012–2013. Am J Trop Med Hyg 92: 1163–1167.25870419 [Google Scholar]
- 33.Tavakoli NP, Wang H, Dupuis M, Hull R, Ebel GD, Gilmore EJ, Faust PL, 2009. Brief report: fatal case of deer tick virus encephalitis. N Engl J Med 360: 2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavanaugh CE, Muscat PL, Telford SR, III, Goethert H, Pendlebury W, Elias SP, Robich R, Welch M, Lubelczyk CB, Smith RP, 2017. Fatal deer tick virus infection in Maine. Clin Infect Dis 65: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 35.Tutolo JW, Staples JE, Sosa L, Bennett N, 2017. Powassan virus disease in an infant—Connecticut, 2016. MMWR Morb Mortal Wkly Rep 66: 408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nofchissey RA, et al. 2013. Seroprevalence of Powassan virus in New England deer, 1979–2010. Am J Trop Med Hyg 88: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokernot RH, Radivoje B, Anderson RJ, 1969. Susceptibility of wild and domestic mammals to four arboviruses. Am J Vet Res 30: 2197–2203. [PubMed] [Google Scholar]
- 38.Mlera L, Meade-White K, Saturday G, Scott D, Bloom ME, 2017. Modeling Powassan virus infection in Peromyscus leucopus, a natural host. PLoS Negl Trop Dis 11: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarnke RL, Yuill TM, 1981. Powassan virus infection in snowshoe hares (Lepus americanus). J Wildl Dis 17: 303–310. [DOI] [PubMed] [Google Scholar]
- 40.Casey GA, Webster WA, 1975. Age and sex determination of striped skunks (Mephitis mephitis) from Ontario, Manitoba, and Quebec. Can J Zool 53: 223–226. [DOI] [PubMed] [Google Scholar]
- 41.Grizzell RA, 1955. A study of the southern woodchuck, Marmota monax monax. Am Midl Nat 53: 257–293. [Google Scholar]
- 42.Nelder MP, Russell C, Lindsay LR, Dhar B, Patel SN, Johnson S, Moore S, Kristjanson E, Li Y, Ralevski F, 2014. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One 9: e105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD, 2008. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg 79: 971–973. [PMC free article] [PubMed] [Google Scholar]
- 44.Brownstein JS, Holford TR, Fish D, 2005. Effect of climate change on Lyme disease risk in North America. EcoHealth 2: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drebot MA, Lindsay R, Barker IK, Buck PA, Fearon M, Hunter F, Sockett P, Artsob H, 2003. West Nile virus surveillance and diagnostics: a Canadian perspective. Can J Infect Dis 14: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Public Health Ontario , 2017. West Nile Virus Surveillance Available at: https://www.publichealthontario.ca/en/DataAndAnalytics/Pages/WNV.aspx. Accessed July 20, 2017.
- 47.Thompson M, Berke O, 2017. Evaluation of the control of West Nile virus in Ontario: did risk patterns change from 2005 to 2012? Zoonoses Public Health 64: 100–105. [DOI] [PubMed] [Google Scholar]
- 48.Root JJ, Bentler KT, Nemeth NM, Gidlewski T, Spraker TR, Franklin AB, 2010. Experimental infection of raccoons (Procyon lotor) with West Nile virus. Am J Trop Med Hyg 83: 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Root JJ, 2013. West Nile virus associations in wild mammals: a synthesis. Arch Virol 158: 735–752. [DOI] [PubMed] [Google Scholar]
- 50.Vasconcelos PFC, Calisher CH, 2016. Emergence of human arboviral diseases in the Americas, 2000–2016. Vector Borne Zoonotic Dis 16: 295–301. [DOI] [PubMed] [Google Scholar]