Abstract.
Human cystic echinococcosis (CE) is a chronic, complex and neglected infection causing severe disease in humans. Hepatic CE cysts are detected and classified mainly by using ultrasound. Expert opinion and published data suggest that uncomplicated inactive liver cysts do not require treatment and only need to be monitored over time (“Watch and Wait”). Here we update our findings as published in 2014 on the “Watch and Wait” approach applied to inactive, asymptomatic cysts of the liver to keep the medical community informed. Clinical data of patients who accessed the World Health Organization Collaborating Center for CE at the University of Pavia-San Matteo Hospital Foundation from January 1991 to October 2017 were analyzed. Inclusion criteria were presence of one or more inactive uncomplicated cysts in the liver (CE4 or CE5), without any history of previous treatment, and an ultrasound-based follow-up of at least 24 months. Fifty-three patients with 66 inactive cysts fulfilled the inclusion criteria. Of these, 11 patients are newly described here; 37 were part of our previously described cohort and the follow-up for 17 of them was further extended; and five were excluded from the previously published analysis as their follow-up was too short, but could be included now. Without the need for treatment and without development of complications, 98.5% of cysts remained inactive over time. In only one patient (1.9% of patients), a reactivation of one cyst (1.5% of cysts) was observed.
INTRODUCTION
Cystic echinococcosis (CE) is a chronic, complex, and neglected parasitic zoonosis caused by the larval stage of Echinococcus granulosus sensu lato species complex that may cause severe disease in humans. The number of people affected by CE is estimated to be more than one million worldwide.1 The life cycle of E. granulosus develops between a definitive host (dog or other canids), which harbors the adult tapeworms in the intestine and sheds the parasite eggs with the feces, and an intermediate host (ungulates) where the larval stage develops as fluid-filled cysts in organs and tissues. Humans act as occasional intermediate hosts and acquire the infection through accidental ingestion of E. granulosus eggs. The larval cysts in humans are located in the liver in about 80% of cases but infection of almost any organ has been reported.2,3
In 2010, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) published an expert consensus document on the diagnosis and clinical management of CE in humans.4 According to these recommendations, the decision on the best clinical management option, in case of hepatic localization of uncomplicated cysts, has to be guided by a stage-specific approach, after assessment of the cyst stage on imaging (Figure 1), using ultrasound (US) as the reference technique or magnetic resonance imaging.5 In particular, uncomplicated inactive liver cysts (classified as CE4 and CE5 stages, Figures 1 and 2) do not require any treatment and need to be exclusively monitored over time using US.4
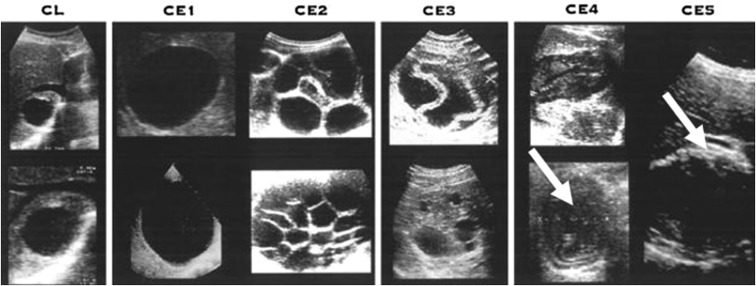
Figure 1.
Sonographic appearance of different liver stages of cystic echinococcosis (CE). Inactive stages (CE4 and CE5) are indicated with white arrows (Brunetti E, Kern P, Vuitton DA; Writing Panel for the World Health Organization Informal Working Group on Echinococcosis, 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114: 1–16).
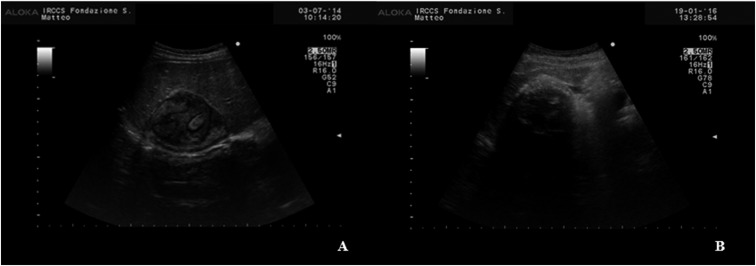
Figure 2.
Ultrasound appearance of CE4 (A) and CE5 (B) cyst of the liver. The “ball of wool” appearance of the hyperechoic folded laminated layer in the iso-hyperechoic cyst content is characteristic of the CE4 stage. Complete or near-complete egg-shell calcification of a cyst with the same content appearance is characteristic of the CE5 stage. CE = cystic echinococcosis.
An increasing amount of published data, reporting that spontaneously inactivated cysts remain inactive in most cases during the follow-up, support the rationale of leaving uncomplicated inactive cysts untreated, which was based on observations that a good proportion of cysts become inactive spontaneously and that cysts in general tend to remain stable over time.6–9 The reactivation rate of cysts that become inactive spontaneously appears to be lower (0–6%) compared with that observed in cysts becoming inactive after therapy (25–60%).6–8,10 Reactivation to stage CE3b, in any case, appears to occur in most cases within 2 years from first observation of inactivity.6,7,10
In 2014, we published the first report on the long-term follow-up of spontaneously inactivated hepatic cysts on a clinical rather than epidemiological basis.6 In that report, 28 patients (22% of the potentially eligible patients) could not be included in the cohort because they were visited for the first time in our center less than 2 years prior, thus, not meeting the minimum follow-up length inclusion criterion. The “Watch and Wait” approach spares patients unnecessary treatments, including surgery, with attendant side effects, complications, and costs. As such, it is crucial to gather evidence on its safety in the appropriate cases. To this aim, in this work we update the results published in 2014 by our center, evaluating the results of “Watch and Wait” approach, in terms of safety and usefulness, in patients with spontaneously inactivated hepatic CE cysts visited from 1991 to 2017 in a single referral center in Italy.
MATERIALS AND METHODS
Clinical data of patients who accessed the WHO Collaborating Center for CE at the University of Pavia-San Matteo Hospital Foundation from January 1991 to October 2017 were searched in our database and analyzed. Data regarding demographic information and number, stage, size, and location of hepatic CE cysts were recorded.
Inclusion criteria were the presence of only one or more inactive uncomplicated cysts in the liver (classified as CE4 or CE5 using the WHO-IWGE classification, Figure 2) on US examination, a negative history of any previous treatment and an US-based follow-up of at least 24 months in our center.4,11 A minimum length of 2 years of follow-up was chosen because most reactivations happen within 24 months from first observation of inactivity.6,7,12
Diagnosis of inactive CE cysts was based on US features alone if characteristic US signs were present (Figure 2) or complemented by serology and/or contrast enhanced imaging in other cases. Exclusion criteria were the presence of inactive CE but in extra-hepatic localization, the presence at the same time of cysts in active or transitional stage in any organ, and history of previous treatment. All patients who did not complete a minimum follow-up of 24 months were also excluded. Independently of inclusion or exclusion, patients who did not come to our clinic for more than 12 months as of October 2017 were considered lost to follow-up.
In our center, in compliance with the recommendations of the WHO Expert Opinion document, all patients newly discovered with inactive and asymptomatic CE4-CE5 cysts of the liver are only followed-up with US at 6 month–1 year intervals by an infectious disease clinician with more than 30 years of experience in US and in clinical management of CE (EB) using an US scanner with a 3.5–5 MHz convex probe.
The appearance of daughter vesicles within the cyst (passing from CE4-CE5 to CE3b stage) was considered a reactivation. Cyst super infection, rupture, or appearance of any symptom related to the cyst were considered as complications.
Descriptive statistics were produced for demographic and clinical characteristics. Quantitative variables were expressed as mean and range. Interquartile ranges (IQR: 25th–75th percentile) were calculated for the median follow-up time. Qualitative variables were summarized as counts and percentages. The McNemar’s test was performed to analyze the difference in cyst stage at the diagnosis and last follow-up visit for each patient.
The study protocol was approved by the Ethics Committee of San Matteo Hospital Foundation, Pavia, Italy (no. 20090000286), and it was in accordance with the Helsinki Declaration of 1975, as revised in 2008. Informed written consent on the use of data for scientific research was obtained from all subjects.
RESULTS
From January 1991 to October 2017, 872 patients with CE were evaluated in our center, 126 of them with CE4-CE5 hepatic cysts. One hundred and nine patients had spontaneously inactive CE4–CE5 echinococcal hepatic cysts, and, of these, 53 (29.6%) fulfilled the inclusion criteria. One hundred and twenty-six patients (70.4%) were excluded from the study for the following reasons: lost to follow-up after less than 2 years of observation (N = 102), previous therapy (N = 17), and new diagnosis less than 2 years before the time of the visit (N = 7).
In the study period, 66 of 214 (30.8%) untreated CE4–CE5 cysts in the included 53 individuals were studied.
Included patients.
Of the 53 patients included in this study, 11 (20.8%) were seen for the first time after the date of data analysis of our previously published cohort, 37 (69.8%) were included in the previously published cohort, and 5 (9.4%) patients were excluded from the previously published cohort because their follow-up was still too short, and now are included.6 Of those included in the previous cohort, 17 were further followed in our center, therefore, their follow-up was extended from the previous report. The median follow-up period of patients eligible for inclusion in the cohort was 52 months (IQR: 36.6–90.2). Twenty-two (41.5%) patients completed a follow-up of 5 years. Five patients (9.4%) were followed up for more than 10 years. The demographic characteristics of the patients included in this work are summarized in Table 1.
Table 1.
Demographic characteristics of the patients included in the analysis
| Gender (n, [%]) | All patients 53 (100%) | Males 16 (30.2%) | Females 37 (69.8%) | |||||
| Mean age at diagnosis (years, [range]) | All patients 50 (14–86) | Males 42 (14–78) | Females 54 (19–86) | |||||
| Place of diagnosis (n, [%]) | Our clinic 35 (67.3%) | Other centers 17 (32.7%) | ||||||
| Birth Place (n, [%]) | Italy 41 (77.4%) | Other countries N = 12 (22.6%) | ||||||
| Romania 4 (33.4%) | Morocco 2 (16.7%) | Tunisia 2 (16.7%) | Algeria 1 (8.3%) | Kosovo 1 (8.3%) | Peru 1 (8.3%) | Ukraine 1 (8.3%) | ||
Among the monitored 66 cysts, 41 (62.1%) were classified as CE4 and 25 (37.9%) as CE5. Forty-five (68.2%) cysts were located in the right lobe, 10 (15.2%) in the left lobe, and 11 (16.7%) in the fourth segment. The mean diameter was 52 mm (range 14–94).
During the follow-up time, cysts in 52 (98.1%) patients remained stable, whereas a reactivation (from CE4 to CE3b stage) was observed in one patient (1.9%) after a follow-up period of 24 months. This reactivation was the same described in 2014; no new reactivation events were observed.6 Regarding the studied cysts, 65 (98.5%) remained inactive whereas 1 (1.5%) reactivated. No complications occurred during the follow-up period.
Excluded patients.
One hundred and two patients (81.0%) were excluded from the analysis because they were lost before 2 years of follow-up were completed, seven (5.5%) were still in follow-up in our clinic but were not included because the follow-up period was less than 2 years on October 2017, and 17 (13.5%) were excluded because of previous therapy administered elsewhere despite being staged at diagnosis as CE4-5.
Forty-seven (46.1%) of the 102 patients excluded from the analysis because of loss to follow-up before the completion of 2 years, had already been excluded for the same reason also in our previous report.
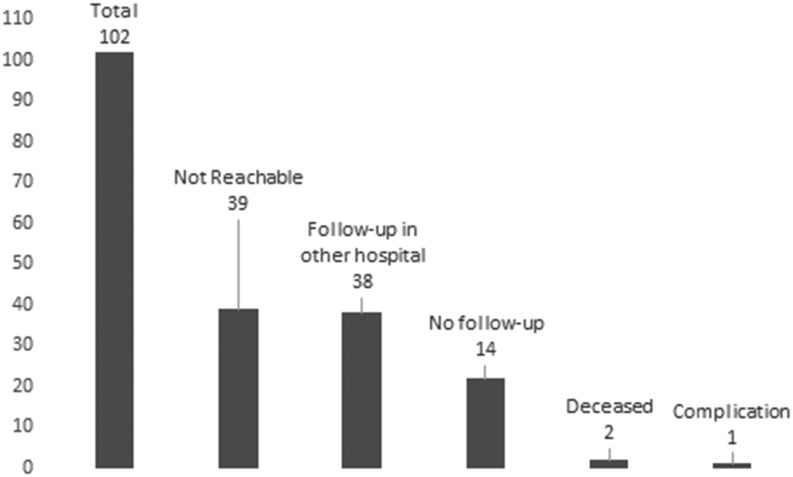
To understand the reason for missing regular visits, we attempted to re-contact, by telephone, all patients excluded from the analysis because of loss to follow-up before the completion of at least 2 years observation. Thirty-nine (38.2%) patients could not be reached over the telephone number provided at the time of the visit (26 of them described in our previous report among excluded patients), 38 (37.3%) were currently followed in another hospital (11 of them described in our previous report among excluded patients) whereas 22 (21.6%) reported being in good clinical condition and decided by themselves to interrupt the follow-up (nine of them described in our previous report among excluded patients), although some expressed the intention to resume follow-up in the near future.
Two (2.0%) patients died for causes unrelated to CE and one (0.9%) patient reported to have suffered from complications, but it was not possible to clarify the nature and relation to the CE cyst by telephone (these three cases were already described in our previous report) (Figure 3).
Figure 3.
Number and results of telephone interviews of the patients excluded from the analysis because of loss to follow-up before the completion of 2 years of observation.
DISCUSSION
Cystic echinococcosis is a complex, chronic and neglected disease with a wide spectrum of clinical presentations and a chronic evolution requiring many years of follow-up. This is one of the reasons that make prospective randomized clinical trials on this disease difficult, and clinical management recommendations still rely largely on expert opinion.2
According to these recommendations, uncomplicated inactive cysts of the liver should be left untreated and simply monitored by using US, the so-called “Watch and Wait” approach. This indication was originally based on publications reporting that a good proportion of cysts become spontaneously inactive and that cysts in general tend to remain stable over time.9 Additional fieldwork confirmed these observations, and results of two studies focusing on the long-term follow-up of spontaneously inactivated cysts on a clinical rather than epidemiological basis, provided support to this line of conduct.6–8,13–16 Hosch and others analyzed cyst material using light microscopy and ex-vivo magnetic resonance (MR) spectroscopy, and found that in CE4 and CE5 cysts no viable protoscoleces were seen on microscopy and a nonviable metabolic profile was seen on MR spectroscopy.17 Although limited by the small sample size and the lack of information on the spontaneous or induced inactivation of CE4-CE5 cysts, and considering that light microcopy focusing on protoscolices could not provide accurate data on the viability of the germinal layer, this seminal work provides further support to field and clinical observations. Finally, the recent report by Solomon et al.8 shows that albendazole treatment of inactive cysts does not prevent reactivation, further stressing the need to avoid overtreatment of these patients, with its attendant side effect and costs.
Unfortunately, the “Watch and Wait” approach is still poorly followed.16 To add further data on the use of the “Watch and Wait” approach for uncomplicated spontaneously inactivated CE cysts of the liver, we present here an update of a previously described cohort of patients followed-up in a single center by the same physician over more than 25 years.6
In our study, more than 98% of the CE4–CE5 cysts observed for at least 2 years remained stable, without complications, strengthening the conviction that the “Watch and Wait” approach is a safe and useful method for the clinical management of inactive asymptomatic hepatic CE. This avoids overtreatment of patients with CE, with attendant and unjustified risks and costs. Loss to follow-up is unfortunately a common occurrence, and it may be partly because of the fact that our hospital is a referral center and a proportion of patients, after clinical management definition in our hospital, may decide to be followed-up closer to home. It may be that this group of patients lost to follow-up differs from those observed for at least 2 years as far as the incidence of complications is concerned. In an attempt to shed light on this issue, we contacted by phone those patients lost to follow-up before the 2-year observation was achieved, and apart from one case where an uncertain condition was reported that could have been a complication due to CE, none of those who could be contacted reported the development of any complication. Unfortunately, verifying the absence of reactivation, without the concomitant development of symptoms, is only possible if follow-up documents are available, and this information could not be retrieved. It is also possible that complications may occur after an observation of 2 years and, therefore, the patients included in the analysis but lost to follow-up after an observation time > 2 years and not recontacted by phone would have experienced delayed complications. Although this hypothesis cannot be ruled out a priori with the data we retrieved, nearly half of the patients included in our analysis completed a longer follow-up period, reaching the goal of 5 years as recommended by the Expert Consensus document of the WHO,4 and no reactivation or complication occurred.
In more than 25 years of cumulative observations, only one cyst reactivation was observed, and this was the one described in 2014 in the first report of our cohort.6 This points out the need to follow-up these cysts in any case, and to implement a patients information and recall system to reduce the loss to follow-up, which is even more important considering that CE4 cysts that inactivated as the result of therapy, although identical on US, differ in their reactivation rate, which is higher than that of those spontaneously inactivated.6–8,10 The rate of reactivation in our cohort was intermediate between that reported by Stojkovic et al.,10 and by Solomon et al.14 Given that the follow-up periods and the studied populations (untreated patients) from these reports were comparable, the difference could be caused by our smaller sample size.
The biological mechanisms at the basis of progression to spontaneous inactivation or reactivation are still unknown, and biomarkers of this dichotomous evolution are not yet available. Serology, as currently available, also does not help in predicting reactivation6 Contrary to other parasitic diseases where immunity compromise can induce reactivation or worse, disease progression, virtually no published data exist about the influence of immunosuppression on CE but, from our experience, this seems to not be a major risk factor.18 Other than CE, our patient whose cyst reactivated was healthy, with no history of trauma, immunosuppressive drugs administration, or other comorbidities.
In conclusion, in the absence of large, prospective, studies that can provide definitive recommendations for the clinical management of these patients, and before data from a larger sample size may be analyzed from international databases such as the European Register of CE, our data still support the WHO-IWGE recommendations for the clinical management of inactive and asymptomatic echinococcal cysts of the liver.19
Acknowledgment:
We thank Samuel Goblirsch for helpful criticism and copyediting the text.
REFERENCES
- 1.WHO Echinococcosis WHO/OIE Manual on Echinococcosis , 2001. Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. World Organization for Animal Health (Office International des Epizooties) and World Health Organization. Available at: http://www.who.int/echinococcosis/resources/929044522X/en/. Accessed January 10, 2018.
- 2.Craig PS, Budke CM, Schantz PM, Li T, Qiu J, Yang Y, Zeyhle E, Rogan MT, Ito A, 2007. Human echinococcosis: a neglected disease? Trop Med Health 35: 283–292. [Google Scholar]
- 3.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A, 2003. Hydatid disease from head to toe. Radiographics 23: 475–494. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE , 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114: 1–16. [DOI] [PubMed] [Google Scholar]
- 5.Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W, 2012. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis 6: e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccoli L, Tamarozzi F, Cattaneo F, Mariconti M, Filice C, Bruno A, Brunetti E, 2014. Long-term sonographic and serological follow-up of inactive echinococcal cysts of the liver: hints for a “watch-and-wait” approach. PLoS Negl Trop Dis 8: e3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojkovic M, Rosenberger KD, Steudle F, Junghanss T, 2016. Watch and wait management of inactive cystic echinococcosis–does the path to inactivity matter–analysis of a prospective patient cohort. PLoS Negl Trop Dis 10: e0005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon N, Kachani M, Zeyhle E, Macpherson CNL, 2017. The natural history of cystic echinococcosis in untreated and albendazole-treated patients. Acta Trop 171: 52–57. [DOI] [PubMed] [Google Scholar]
- 9.Junghanss T, Da Silva AM, Horton J, Chiodini PL, Brunetti E, 2008. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg 79: 301–311. [PubMed] [Google Scholar]
- 10.Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, Nicolaidou P, Cobanoglu N, Junghanss T, 2009. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis 3: e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Informal Working Group , 2003. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop 85: 253–261. [DOI] [PubMed] [Google Scholar]
- 12.Rinaldi F, De Silvestri A, Tamarozzi F, Cattaneo F, Lissandrin R, Brunetti E, 2014. Medical treatment versus “watch and wait” in the clinical management of CE3b echinococcal cysts of the liver. BMC Infect Dis 14: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrieu E, et al. 2011. Programme for ultrasound diagnoses and treatment with albendazole of cystic echinococcosis in asymptomatic carriers: 10 years of follow-up of cases. Acta Trop 117: 1–5. [DOI] [PubMed] [Google Scholar]
- 14.Solomon N, et al. 2018. Cystic echinococcosis in Turkana, Kenya: 30 years of imaging in an endemic region. Acta Trop 178: 182–189. [DOI] [PubMed] [Google Scholar]
- 15.Tamarozzi F, et al. 2017. Prevalence and risk factors for human cystic echinococcosis in the Cusco region of the Peruvian highlands diagnosed using focused abdominal ultrasound. Am J Trop Med Hyg 96: 1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chebli H, et al. 2017. Human cystic echinococcosis in Morocco: ultrasound screening in the Mid Atlas through an Italian-Moroccan partnership. PLoS Negl Trop Dis 11: e0005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosch W, Junghanss T, Stojkovic M, Brunetti E, Heye T, Kauffmann GW, Hull WE, 2008. Metabolic viability assessment of cystic echinococcosis using high-field 1H MRS of cyst contents. NMR Biomed 21: 734–754. [DOI] [PubMed] [Google Scholar]
- 18.Evering T, Weiss LM, 2006. The immunology of parasite infections in immunocompromised hosts. Parasite Immunol 28: 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi P, et al. 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasit Vectors 9: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]