Abstract.
Murine typhus (MT) is an important cause of febrile illness in endemic areas, and there is an epidemiologic resurgence of this infection currently transpiring in Texas and California. Fatal cases and severe neurological complications are rare. A fatal case of MT in a middle-aged man is reported with a course culminating in multi-organ failure and refractory status epilepticus. An autopsy revealed hemorrhagic pneumonia, acute tubular necrosis, and ischemic necrosis in the liver, adrenals, and brain. We have also reviewed the neurologic complications of MT.
Murine typhus (MT) is a flea-borne infection caused by Rickettsia typhi.1 It is typically a nonspecific febrile illness without complications. Fever, headache, rash, chills, malaise, myalgias, and anorexia are the most common presenting signs and symptoms. Elevated transaminase levels, hyponatremia, thrombocytopenia, and anemia are commonly associated laboratory abnormalities.2,3 A systematic review found 26.1% of patients had complications of organ failure or required hospitalization; however, intensive care unit admission and death were rare.3 Three thousand and forty-eight confirmed or probable cases of MT were reported in Texas from 1985 to 2015, with 11 fatalities.4 However, the specific causes of death were not detailed for these 11 fatal cases. If progress is to be made in preventing complications and death during the course of MT, a better understanding of the pathogenesis of severe illness for this infection is necessary. Herein, we report a case of a middle-aged male with MT who presented with septic shock, renal failure, and status epilepticus, who suffered a fatal course. An autopsy was obtained. The findings were compared with other published autopsy cases of MT and with other neurologic presentations of MT (NPMT).
CASE
The patient was a 46-year-old Caucasian man from suburban San Antonio (TX) with a history of alcohol abuse and cranioplasty after an accident at 12 years of age. He arrived by ambulance after he was found convulsing on the floor of his home. According to his mother with whom he lived, he had been in his usual state of health the day prior presentation. He had not traveled recently and had no known exposure to fleas, ticks, rodents, cats, or other animals.
On arrival, he was convulsing, hypoxemic (oxygen saturation as low as 40%), hypotensive (78/40 mm Hg), and tachycardic (137 bpm). Examination revealed central cyanosis, horizontal nystagmus, rhythmic jerking of all four extremities, and coarse breath sounds. There was no fever, rash, eschar, nor obvious insect bites. The patient was immediately intubated and placed on mechanical ventilation. Initial laboratory investigations showed severe acidosis with elevated lactic acid and depressed bicarbonate levels; severe acute kidney injury with high levels of potassium, phosphorus, blood urea nitrogen, and creatinine; modest effects on the liver, with elevated aspartate transaminase, alanine transaminase, alkaline phosphatase, bilirubin, and ammonia; and evidence of significant endothelial damage with severely low albumin. There was severe thrombocytopenia, moderate anemia, and leukocytosis with 80% neutrophils. Lactate dehydrogenase was moderately elevated (see Table 1). The glucose and creatinine kinase levels and prothrombin time were within normal limits. Blood cultures obtained on days 1, 2, and 5 and were all negative. A urine screen of drugs of abuse was negative. Serum alcohol, acetaminophen, salicylate, and ethylene glycol levels were undetectable. An human immunodefiency virus-1/2 serologic test and RNA polymerase chain reaction were negative. Computed tomography of the head, chest, abdomen, and pelvis showed only splenomegaly and bibasilar atelectasis.
Table 1.
Abnormal laboratory findings of the case patient on admission
| Analyte | Units | Value | Reference range |
|---|---|---|---|
| Venous pH | – | 7.04 | 7.31–7.41 |
| Lactic acid | mmol/L | 9.0 | 0.5–2.2 |
| Sodium | mmol/L | 131 | 135–145 |
| Potassium | mmol/L | 6.1 | 3.5–5.1 |
| Bicarbonate | mmol/L | 14 | 20–29 |
| Blood urea nitrogen | mg/dL | 142 | 7–25 |
| Creatinine | mg/dL | 11 | 0.6–1.3 |
| Aspartate transaminase | U/L | 76 | < 36 |
| Alanine transaminase | U/L | 58 | < 58 |
| Alkaline phosphatase | U/L | 281 | 45–117 |
| Total bilirubin | mg/dL | 2.9 | 0.2–1.2 |
| Albumin | g/dL | 1.9 | 3.2–5.0 |
| Ammonia | μmol/L | 167 | 45–117 |
| Phosphorus | mg/dL | 7.7 | 2.4–4.6 |
| Lactate dehydrogenase | U/L | 406 | 92–240 |
| White blood cell count | K/mm3 | 19.6 | 3.4–10.4 |
| Hemoglobin | g/dL | 11.2 | 12.8–17.1 |
| Platelet count | K/mm3 | 31 | 140–377 |
| International normalized ratio | – | 1.3 | 1.0 |
Septic shock and suspected meningoencephalitis were managed with fluid resuscitation and antimicrobial coverage with vancomycin, cefepime, metronidazole, ampicillin, and acyclovir. An electroencephalogram (EEG) was consistent with nonconvulsive status epilepticus. Epileptic activity remained evident by EEG despite midazolam boluses and infusion, phenytoin, lacosamide, levetiracetam, and continuous ketamine infusion. The patient was, therefore, placed in a pentobarbital-induced coma.
A lumbar puncture was performed on day 3. The opening pressure was 17 cm H2O, and cerebrospinal fluid (CSF) studies revealed a lymphocytic pleocytosis (66 nucleated cells/mm3, 46% lymphocytes), elevated protein (170 mg/dL; reference range < 45 mg/dL), and a normal CSF glucose. Aerobic, anaerobic, fungal, and acid-fast bacilli cultures of the CSF were negative, as were serologic tests for West Nile virus and a CSF meningitis/encephalitis polymerase chain reaction panel (FilmArray; Biofire, Salt Lake City, UT). By day 3, the international normalized ratio had increased to 1.8 and the fibrinogen level dropped to 142 mg/dL (reference range 152–145 mg/dL). The white blood cell count increased to 22.5 K/mm3 and the hemoglobin fell to 9.0 mg/dL. Doxycycline was initiated for suspected rickettsial infection. A serum indirect fluorescent antibody serologic test for MT was sent and returned reactive for Rickettsia typhi (immunglobulin M [IgM] 1:4,096; immunoglobulin G [IgG] 1:256). Rocky Mountain spotted fever (RMSF) serology was performed and was also reactive (IgM 1:256; IgG 1:64), titers being 4-fold lower than that for MT.
Renal replacement therapy was started on day 4 because of refractory acidosis and anuria. Despite this the patient developed progressive multi-organ dysfunction with increasing lactic acidosis, hypoxemia, and vasopressor requirements. Because of the progressive deterioration of his condition, the patient was transitioned to comfort care and he died on day 6 of hospitalization.
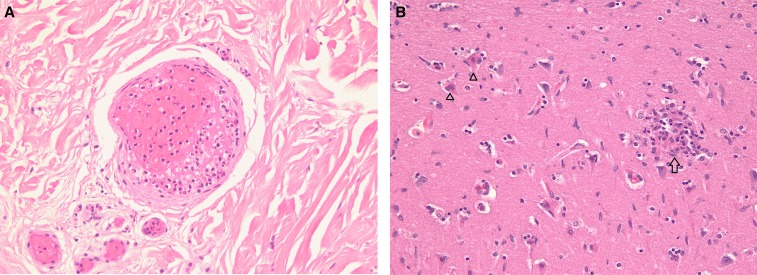
At the time of autopsy, there was a petechial rash of both legs, acute gangrene of the toe tips of both feet, and multiple cutaneous ecchymoses and scleral hemorrhages. Gross examination of the internal organs revealed hemorrhagic pneumonia, splenomegaly (290 g), cerebral edema, and mild cerebellar tonsillar herniation. Microscopic examination revealed multifocal small-vessel thromboses in the lungs, skin, and kidneys; with acute tubular necrosis, cutaneous vasculitis consisting of mononuclear cell inflammation, and small-vessel thrombosis (Figure 1A). The liver showed Stage 3/4 steatohepatitis and zone three hypoxic/ischemic necrosis. The adrenal glands also displayed hypoxic/ischemic necrosis affecting most of the cortex. There was extensive hypoxic/ischemic necrosis of neurons in the “watershed” zone of the bilateral cerebral hemispheres and hippocampus, and scattered foci of perivascular mononuclear cell infiltrates (Figure 1B).
Figure 1.
(A) Dermis with small-vessel thrombosis (hematoxylin and eosin, ×200). (B) Neuronal necrosis (open triangles) with focal perivascular mononuclear cell infiltrate (glial or typhus nodule [open arrow]) (hematoxylin and eosin, ×200). This figure appears in color at www.ajtmh.org.
DISCUSSION
Fatalities are rare in MT. Even in the era before antibiotics and modern critical care, the mortality rate of MT was less than 4%, with the principal causes of death being pneumonia, nephritis, myocarditis, and “apoplexy” (altered consciousness).5 The present case fatality rate in Texas is 0.4%.4 Nevertheless, the number of cases and the geographic range of MT have increased in Texas and California in the past decade,6,7 so it is increasingly important to recognize this infection to institute timely and appropriate antibiotic treatment, thereby, decreasing the risk of complications and death.
Four other autopsy cases of MT have been reported. Binford and Ecker reported three cases in which they ascribed the cause of death to myocarditis; the brain was examined in two of the cases, with minimal changes.8 Walker and others described a case of an elderly woman who died of MT due to pneumonia, encephalitis, and renal failure9; they observed diffuse alveolar damage, multifocal white matter petechiae, and perivasculitis in the spinal cord and kidneys.
The patient described herein had an extremely rapid progression to multi-organ failure and death. With the brain involvement, pneumonitis, renal failure, and gangrene, this infection behaved like one of the life-threatening rickettsioses, that is, RMSF, epidemic typhus, or scrub typhus. The host factors for more severe rickettsial infection include: age > 40 years old; male gender; underlying liver disease, alcoholism, or chronic lung disease; African origin; and glucose-6-phosphate dehydrogenase (G-6-PD) deficiency.10–12 However, even considering the usual course of RMSF, this patient had a particularly rapid progression of multi-organ dysfunction. “Fulminant” RMSF has been described in patients with G-6-PD deficiency; this results in death within 5 days after the onset as compared with 8–15 days in most fatal cases of RMSF.13 The G-6-PD status of this patient was uncertain, but he did have the former four host risk factors. Although there are no specific data for MT, scrub typhus patients with cirrhosis have about 3-x the rates of renal failure and central nervous system (CNS) involvement, and 9-x the mortality rate compared with those without cirrhosis.14 This patient had grade 3/4 steatohepatitis with splenomegaly suggestive of portal hypertension (spleen weight = 290 g; usual mean spleen weight in males is 139 g15), which was likely the primary risk factor leading to his fulminant course. In cirrhotics, a preexisting hyperdynamic state predisposes to the hypotension, reduced tissue perfusion, and multi-organ failure that arises from sepsis-driven cytokine storm and nitric oxide overproduction.16
Acral gangrene has not previously been reported in MT, but has been observed in RMSF,17 epidemic typhus,18 and scrub typhus.19 The gangrene is due to hypotension, small-vessel occlusion secondary to vasculitis, and disseminated intravascular coagulation.17 However, vasopressor therapy may have also contributed to the acral gangrene in this patient.
Clinically significant NPMT are uncommon and usually manifest in the second week of the illness.20 Headache, a very common symptom in rickettsial infection, does not by itself indicate CNS invasion. Other neurologic signs and symptoms, such as altered mental status, meningismus, focal neurological deficits, or behavioral changes, reveal CNS involvement.21 In an analysis of 1,756 patients with MT, only 69 patients presented with altered consciousness (3.9%), 13 patients had meningism (0.7%), and three patients each presented with ataxia or seizures.3 The most thorough assessment of MT CNS infection derives from a Laotian study of 1,051 patients admitted with a neurological presentation that received a lumbar puncture.22 Twenty-eight patients with NPMT were identified; headache and stiff neck were present in 84% and 69% of patients, respectively, with convulsions in 25%. Hearing loss and photophobia were absent. Meningitis was diagnosed in 68% of the NPMT cases and meningitis with encephalitis was seen in 57%. The average opening pressure was 17 cm H2O with a range 9–40. The mean CSF leukocyte count was 10/mm3 (range 0–605), with an average neutrophil to lymphocyte ratio of 1. Cerebrospinal fluid protein and lactate were elevated in 46% and 33% of NPMT patients, respectively, and CSF glucose levels were depressed in 24%. The mortality rate in NPMT was 27%, higher than for those with neurologic presentations of scrub typhus or leptospirosis (14% and 13%, respectively), but not as high as pyogenic meningitis (33%). There was no determination of the association of mortality with neurologic/CSF characteristics or with concurrent dysfunction of other organs.22 Neurologic complications, including meningitis, encephalitis, vertigo, dizziness, seizure, and coma, were present in five patients (63%) in the recent Texas series of fatal MT cases4 and are also more common in elderly MT patients.23 Other studies of MT have also reported meningitis, encephalitis, or meningoencephalitis.20,24–36
A hallmark of rickettsial encephalitis is the “typhus nodule” or “glial nodule,” composed of focal perivascular infiltration of the neuropil by macrophages and lymphocytes which form in response to invasion of vascular endothelial cells by the pathogen.21,37 Such “nodules” have been observed in RMSF, epidemic typhus, scrub typhus, and MT37; they were evident in the brain of this patient (Figure 1B). Perivascular hemorrhage or microinfarcts in the brain parenchyma and perivascular infiltrate of the leptomeninges may also be present in rickettsial meningoencephalitis,37 but were not seen in this patient. The intractable seizures observed in this patient were due to the neuronal necrosis, typhus nodules, and severe metabolic derangements that were evident on admission. In addition to the Laotian study, other studies of MT have reported seizures,20,29,38,39 which were ascribed to hyperpyrexia, intracranial hemorrhage, and hypoglycemia.29
Other CNS manifestations that have been reported in MT include: hearing loss40,41; encephalitis with white matter infarctions, and intracerebral and subarachnoid hemorrhages42; cerebellitis with ataxia and tremors43; intracranial hemorrhage29; hemiparesis10; cranial nerve palsies26,44–46; ataxia38,47; behavioral alterations; and neurocognitive deficits20,31,48 and pseudotumor cerebri.20 Nevertheless, in most MT patients, the CNS manifestations resolve after the patient defervesces.49 By contrast, in RMSF, more than half of the patients may have persistent neurologic deficits more than 1 year after the illness.50 In RMSF, the vasculitis may extend to all layers of the blood vessel, leading to necrosis of the intima and the media, with severe thrombotic occlusion and microinfarcts,48,50 a finding which typically does not occur in MT.
Central nervous system manifestations associated with MT are diverse and include severe forms such as the seizures seen in this patient. The course of MT has the potential to be attenuated by the institution of early antibiotic therapy. It is prudent to include MT in the differential diagnosis of patients with neurologic dysfunction in endemic areas.
REFERENCES
- 1.Civen R, Ngo V, 2008. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis 46: 913–918. [DOI] [PubMed] [Google Scholar]
- 2.Afzal Z, Kallumadanda S, Wang F, Hemmige V, Musher D, 2013. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis 23: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsioutis C, Zafeiri M, Avramopoulos A, Prousali E, Miligkos M, Karageorgos SA, 2017. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop 166: 16–24. [DOI] [PubMed] [Google Scholar]
- 4.Pieracci EG, Evert N, Drexler NA, Mayes B, Vilcins I, Huang P, Campbell J, Behravesh CB, Paddock CD, 2017. Fatal flea-borne typhus in Texas: a retrospective case series, 1985–2015. Am J Trop Med Hyg 96: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JN, McAlpine JG, Gill DG, 1939. Endemic typhus. Am J Public Health 24: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray KO, Evert N, Mayes B, Fonken E, Erickson T, Garcia MN, Sidwa T, 2017. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Infect Dis 23: 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.California Department of Health , 2018. Human Flea-Borne Typhus Cases in California Available at: www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/Flea-borneTyphusCaseCounts.pdf. Accessed January 24, 2018.
- 8.Binford CH, Ecker HD, 1947. Endemic (murine) typhus: report of autopsy findings in three cases. Am J Clin Pathol 17: 797–806. [DOI] [PubMed] [Google Scholar]
- 9.Walker DH, Parks FM, Betz TG, Taylor JP, Muehlberger JW, 1989. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am J Clin Pathol 91: 720–724. [DOI] [PubMed] [Google Scholar]
- 10.Angelakis E, Botelho E, Socolovschi C, Sobas CR, Piketty C, Parola P, Raoult D, 2010. Murine typhus as a cause of fever in travelers from Tunisia and Mediterranean areas. J Travel Med 17: 310–315. [DOI] [PubMed] [Google Scholar]
- 11.Regan JJ, et al. 2015. Risk factors for fatal outcome from rocky mountain spotted fever in a highly endemic area—Arizona, 2002–2011. Clin Infect Dis 60: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Sousa R, et al. 2008. Host- and microbe-related risk factors for and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. J Infect Dis 198: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker DH, Hawkins HK, Hudson P, 1983. Fulminant Rocky Mountain spotted fever. Its pathologic characteristics associated with glucose-6-phosphate dehydrogenase deficiency. Arch Pathol Lab Med 107: 121–125. [PubMed] [Google Scholar]
- 14.Kim IH, Lee HB, Hwang JH, Kwon KS, Lee CS, 2010. Scrub typhus in patients with liver cirrhosis: a preliminary study. Clin Microbiol Infect 16: 419–424. [DOI] [PubMed] [Google Scholar]
- 15.Molina DK, DiMaio VJ, 2012. Normal organ weights in men: part II-the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol 33: 368–372. [DOI] [PubMed] [Google Scholar]
- 16.Ascione T, Di Flumeri G, Boccia G, De Caro F, 2017. Infections in patients affected by liver cirrhosis: an update. Infez Med 25: 91–97. [PubMed] [Google Scholar]
- 17.Kirkland KB, Marcom PK, Sexton DJ, Dumler JS, Walker DH, 1993. Rocky Mountain spotted fever complicated by gangrene: report of six cases and review. Clin Infect Dis 16: 629–634. [DOI] [PubMed] [Google Scholar]
- 18.Cowan G, 2000. Rickettsial diseases: the typhus group of fevers—a review. Postgrad Med J 76: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishna MR, Vasuki B, Nagaraju K, 2015. Scrub typhus: audit of an outbreak. Indian J Pediatr 82: 537–540. [DOI] [PubMed] [Google Scholar]
- 20.Carr SB, Bergamo DF, Emmanuel PJ, Ferreira JA, 2014. Murine typhus as a cause of cognitive impairment: case report and a review of the literature. Pediatr Neurol 50: 265–268. [DOI] [PubMed] [Google Scholar]
- 21.Drevets DA, Leenen PJ, Greenfield RA, 2004. Invasion of the central nervous system by intracellular bacteria. Clin Microbiol Rev 17: 323–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dittrich S, et al. 2015. Orientia, rickettsia, and leptospira pathogens as causes of CNS infections in Laos: a prospective study. Lancet Glob Health 3: e104–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsioutis C, Chaliotis G, Kokkini S, Doukakis S, Tselentis Y, Psaroulaki A, Gikas A, 2014. Murine typhus in elderly patients: a prospective study of 49 patients. Scand J Infect Dis 46: 779–782. [DOI] [PubMed] [Google Scholar]
- 24.Woo ML, Leung JW, French GL, 1988. Rickettsial infection presenting as culture-negative meningitis. Postgrad Med J 64: 614–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masalha R, Merkin-Zaborsky H, Matar M, Zirkin HJ, Wirguin I, Herishanu YO, 1998. Murine typhus presenting as subacute meningoencephalitis. J Neurol 245: 665–668. [DOI] [PubMed] [Google Scholar]
- 26.Simon NG, Cremer PD, Graves SR, 2011. Murine typhus returns to New South Wales: a case of isolated meningoencephalitis with raised intracranial pressure. Med J Aust 194: 652–654. [DOI] [PubMed] [Google Scholar]
- 27.Galanakis E, Gikas A, Bitsori M, Sbyrakis S, 2002. Rickettsia typhi infection presenting as subacute meningitis. J Child Neurol 17: 156–157. [DOI] [PubMed] [Google Scholar]
- 28.Silpapojakul K, Ukkachoke C, Krisanapan S, Silpapojakul K, 1991. Rickettsial meningitis and encephalitis. Arch Intern Med 151: 1753–1757. [PubMed] [Google Scholar]
- 29.Silpapojakul K, Chayakul P, Krisanapan S, Silpapojakul K, 1993. Murine typhus in Thailand: clinical features, diagnosis and treatment. Q J Med 86: 43–47. [PubMed] [Google Scholar]
- 30.Chaliotis G, Kritsotakis EI, Psaroulaki A, Tselenti Y, Gikas A, 2012. Murine typhus in central Greece: epidemiological, clinical, laboratory, and therapeutic-response features of 90 cases. Int J Infect Dis 16: e591–e596. [DOI] [PubMed] [Google Scholar]
- 31.Lew HL, Lane B, Zeiner H, 2005. Neuroimaging and clinical manifestations of bilateral temporal encephalopathy secondary to murine typhus infection. Am J Phys Med Rehabil 84: 310–311. [DOI] [PubMed] [Google Scholar]
- 32.Aouam A, Toumi A, Brahim HB, Loussaief C, Jelliti B, Romdhane FB, Yahia SB, Khairallah M, Chakroun M, 2015. Epidemiological, clinical and laboratory features of murine typhus in central Tunisia. Med Mal Infect 45: 124–127. [DOI] [PubMed] [Google Scholar]
- 33.Fergie JE, Purcell K, Wanat D, 2000. Murine typhus in south Texas children. Pediatr Infect Dis J 19: 535–538. [DOI] [PubMed] [Google Scholar]
- 34.Chang K, et al. 2012. Murine typhus in southern Taiwan during 1992–2009. Am J Trop Med Hyg 87: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernández-Cabrera M, Angel-Moreno A, Santana E, Bolaños M, Francès A, Martín-Sánchez AM, Pérez-Arellano JL, 2004. Murine typhus with renal involvement in Canary Islands, Spain. Emerg Infect Dis 10: 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallejo‐Maroto I, García‐Morillo S, Wittel MB, Stiefel P, Miranda M, Pamies E, Aparicio R, Carneado J, 2002. Aseptic meningitis as a delayed neurologic complication of murine typhus. Clin Microbiol Infect 8: 826–827. [DOI] [PubMed] [Google Scholar]
- 37.Walker DH, Dumler JS, 1997. Rickettsial infections. Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, eds. Pathology of Infectious Diseases. Stamford, CT: Appleton & Lange, 789–800. [Google Scholar]
- 38.Dumler JS, Taylor JP, Walker DH, 1991. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA 266: 1365–1370. [PubMed] [Google Scholar]
- 39.Mas Bernard A, 1960. Focal epilepsy, sole revealing clinical manifestation of murine rickettsiosis. Apropos of 2 cases developing in parallel. Presse Med 68: 1656. [PubMed] [Google Scholar]
- 40.Tsiachris D, Deutsch M, Vassilopoulos D, Zafiropoulou R, Archimandritis AJ, 2008. Sensorineural hearing loss complicating severe rickettsial diseases: report of two cases. J Infect 56: 74–76. [DOI] [PubMed] [Google Scholar]
- 41.Lin SY, Wang YL, Lin HF, Chen TC, Chen YH, Lu PL, 2010. Reversible hearing impairment: delayed complication of murine typhus or adverse reaction to azithromycin? J Med Microbiol 59: 602–606. [DOI] [PubMed] [Google Scholar]
- 42.Xu Z, Zhu X, Lu Q, Li X, Hu Y, 2015. Misdiagnosed murine typhus in a patient with multiple cerebral infarctions and hemorrhage: a case report. BMC Neurol 15: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernabeu-Wittel M, Pachón J, Alarcón A, López-Cortés LF, Viciana P, Jiménez-Mejías ME, Villanueva JL, Torronteras R, Caballero-Granado FJ, 1999. Murine typhus as a common cause of fever of intermediate duration: a 17-year study in the south of Spain. Arch Intern Med 159: 872–876. [DOI] [PubMed] [Google Scholar]
- 44.Vander T, Medvedovsky M, Valdman S, Herishanu Y, 2003. Facial paralysis and meningitis caused by Rickettsia typhi infection. Scand J Infect Dis 35: 887–888. [DOI] [PubMed] [Google Scholar]
- 45.Moy WL, Ooi ST, 2015. Abducens nerve palsy and meningitis by Rickettsia typhi. Am J Trop Med Hyg 92: 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu CH, Hsieh LP, 2014. Murine typhus with presentation of unilateral abducens nerve palsy: a case report. J Intern Med Taiwan 25: 36–40. [Google Scholar]
- 47.Whiteford SF, Taylor JP, Dumler JS, 2001. Clinical, laboratory, and epidemiologic features of murine typhus in 97 Texas children. Arch Pediatr Adolesc Med 155: 396–400. [DOI] [PubMed] [Google Scholar]
- 48.Samra Y, Shaked Y, Maier MK, 1989. Delayed neurologic display in murine typhus: report of two cases. Arch Intern Med 149: 949–951. [PubMed] [Google Scholar]
- 49.Stuart BM, Pullen RL, 1945. Endemic (murine) typhus fever: clinical observations of 180 cases. Ann Intern Med 23: 520–536. [Google Scholar]
- 50.Rosenblum MJ, Masland RL, Harrell GT, 1952. Residual effects of rickettsial disease on the central nervous system: results of neurologic examinations and electroencephalograms following Rocky Mountain spotted fever. AMA Arch Intern Med 90: 444–455. [DOI] [PubMed] [Google Scholar]