Abstract.
Presently, few studies have investigated the role of domestic cats (Felis catus) in the recrudescence of flea-borne rickettsioses in California and the southern United States. In this study, we aimed to investigate the presence of Rickettsia typhi or Rickettisa felis in domestic cats (F. catus) and the fleas (primarily Ctenocephalides felis, the cat flea) associated with these cats in Riverside County, California. Thirty cats and 64 pools of fleas collected from these cats were investigated for rickettsial infections. Three cats and 17 flea pools (from 10 cats) tested positive for rickettsial infections. polymerase chain reaction and DNA sequencing indicated that one of the cats was positive for R. felis infections, whereas two were positive for Candidatus Rickettsia senegalensis infection. In addition, 12 of the flea pools were positive for R. felis, whereas five were positive for Ca. R. senegalensis. By contrast, no cats or their associated fleas tested positive for R. typhi. Finally, eight sera from these cats contained spotted fever group Rickettsia (SFGR) antibodies. The detection of R. felis and SFGR antibodies and the lack of R. typhi and TGR antibodies support R. felis as the main rickettsial species infecting cat fleas. The detection of Ca. R. senegalensis in both fleas and cats also provides additional evidence that cats and their associated fleas are infected with other R. felis–like organisms highlighting the potential risk for human infections with R. felis or R. felis–like organisms.
INTRODUCTION
Starting in 1915, cases of a sporadic type of typhus, different from the classic and more widely recognized epidemic typhus, were documented in California.1–3 It was quickly established that many of these cases referred to as endemic typhus or murine typhus were due to a flea-borne Rickettsia carried by rats.4 Murine typhus continued to be considered a major health concern through the 1940s in California and parts of the southern United States, with 16,332 cases of murine typhus documented in the United States between 1922 and 1939 ranging from 24 in 1922 to 2,942 cases in 1939.2 An estimated 505 cases of murine typhus were diagnosed between 1916 and 1948 in California alone, with most cases of murine typhus reported from the southern counties.1,2 Vector control techniques, which mainly included dichlorodiphenyltrichloroethane dusting, were implemented during this time period, and the number of cases of murine typhus dropped significantly from its peak in the 1940s.5
More recently, cases of murine typhus began to resurface and identification of cases increased in the 1980s and 1990s in parts of southern California and the southern United States.6–10 However, these cases were not attributed to the classical (urban) cycle of murine typhus of the past. Traditionally, transmission of Rickettsia typhi (causative agent of murine typhus) involved rats (Rattus rattus and Rattus norvegicus) and rat fleas (Xenopsylla cheopis), but many of these more recent cases have been attributed to a suburban cycle involving cats (Felis catus), opossums (Didelphis virginiana), and the cat flea (Ctenocephalides felis).7,8,10–12 The cat flea has been shown to harbor R. typhi, and is thought to be the principal vector responsible for the increase in murine typhus cases in the southern United States and California.8,11–15 However, the role of cats as a reservoir for infecting cat fleas with R. typhi in the environment is not well known.
Ctenocephalides felis fleas are arguably the most important ectoparasite of domesticated cats and dogs worldwide.16 In southern California, in addition to parasitizing cats, C. felis fleas are found in abundance on a wide variety of mammals, including opossums collected in areas adjacent to the residences of human typhus cases.13,16 From 2001 to 2016, 738 human cases of flea-borne typhus were reported to the California Department of Public Health, with most cases occurring in Los Angeles and Orange counties; the etiologic agent of these flea-borne rickettsioses is presumed to be caused by infections with R. typhi.17,18
In addition to R. typhi, C. felis fleas have also been shown to harbor Rickettisa felis, another agent of flea-borne rickettsiosis (i.e., flea-borne spotted fever), which may be under-recognized as a cause of rickettsial disease in California.14,15,19,20 The presence of R. felis in C. felis fleas provides another possible etiology for flea-borne rickettsioses seen in the United States and in particular, California. Rickettsia felis was detected for the first time in 1991 in the blood of a Texas patient who had been diagnosed initially with murine typhus.21 Since then, R. felis has been found to be a common cause of rickettsiosis in sub-Saharan Africa and other regions of the world.22–25 Although human R. felis infections have been seen in Central and South America,23–25 R. felis is generally not considered a cause of flea-borne rickettsioses in the United States.22–28 However, reports from California indicate that R. typhi–endemic areas overlap (because of the common flea vectors) with areas where R. felis infections could be endemic.11,15,19,20,29,30 To further complicate matters, the clinical symptomology, consisting of high fever, headache, myalgia, and rash, of R. typhi and R. felis infections in humans is similar.25
Studies conducted in California’s Los Angeles and Orange counties found R. felis to be the predominant Rickettsia spp. detected in pooled C. felis fleas (ca. 28–53%), whereas R. typhi infections were relatively few (ca. 1.3–3%).14,18,20 Rickettsial disease investigations in southern California have shown that opossums carry the highest C. felis flea load of any host animal.11,14,15,19,20 By contrast, C. felis fleas collected off cats from case households in Los Angeles and Orange counties were comparatively fewer in number than those collected from opossums but exhibited similar or higher R. felis infection rates.14,20
Cats are readily parasitized by C. felis, and previous investigations have implicated cats as sources of flea-borne rickettsial disease outbreaks.10,12,31 Rickettsia typhi–seropositive cats have been found in close association with human cases,13,31 and experimental infection of cats with R. felis has been demonstrated.32 However, infection rates of R. felis and R. typhi in cats are largely unknown in southern California. To this end, we herein report the results from a study undertaken in Riverside County, California, which borders Los Angeles and Orange counties (Figure 1), to assess the presence of rickettsial agents and antibodies against both spotted fever group Rickettsia (SFGR) and typhus group Rickettsia (TGR) in cats and the presence of rickettsial agents in C. felis and sticktight fleas (Echidnophaga gallinacea) fleas removed from these cats.
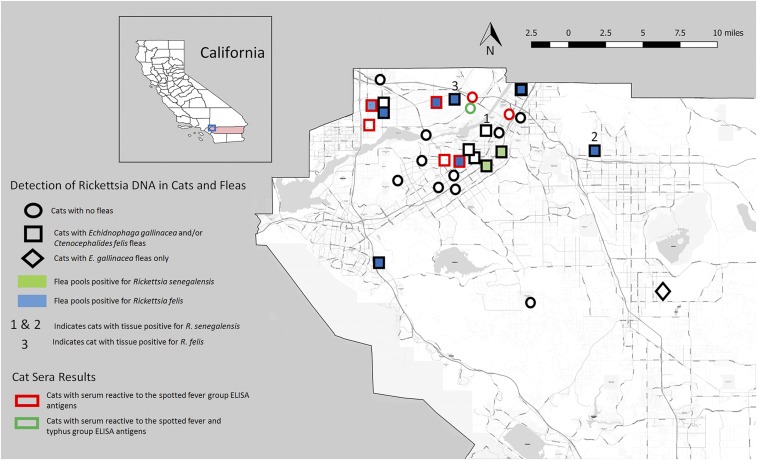
Figure 1.
Map showing sites where cats and cat fleas with rickettsial infections and cats with antibodies to TGR and/or SFGR were found in Riverside County, California.
MATERIALS AND METHODS
Sample collection.
Groups of fleas, serum samples, and tissue samples were collected from 30 randomly chosen unsocialized (also referred to as unowned, feral, stray, or community cats) cats impounded by the Riverside County Department of Animal Services, Riverside, California (33'7827739°N, 116'976688°W), from July 2014 through January 2015 that were scheduled for euthanasia (independent of this study) by shelter staff. Each submitted cat represented a unique geographical location in the county (Figure 1). Cats were euthanized following American Veterinary Medical Association guidelines with sodium pentobarbital.33 Fleas, blood samples, and sections of the ear, liver, lymph node, rump muscle, spleen, lungs, heart, and kidneys were collected and stored at −80°C for testing.
DNA extraction.
Fleas were washed in molecular-grade water and mechanically disrupted using disposable pellet pestles (Fisher Scientific, Pittsburgh, PA). Genomic DNA was extracted using the Prepman Ultra sample preparation kits (Applied Biosystems, Foster City, CA). Genomic DNA from cat tissue was extracted using the DNeasy blood and tissue kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions and using a final elution volume of 100 μL.
qPCR assays.
Flea and cat tissue nucleic acid preparations were initially screened for rickettsial DNA using a genus-specific qPCR assay (Rick17b) targeting the 17-kDa antigen gene.34 DNA from flea and cat samples that tested positive by the Rick17b assay were subsequently tested using a species-specific R. felis assay (Rfel_phosp_MB), targeting the membrane phosphatase gene from R. felis and the R. typhi species-specific qPCR assay (Rtyph), which targets a fragment of the R. typhi OmpB gene.35,36 In addition, flea DNA that tested positive for Rick17b were tested with a Rickettsia asembonensis–specific qPCR assay, which targets a fragment of R. asembonensis ompB.37
Sequencing.
PCR amplification and sequencing of ompB was attempted for a subset of flea DNA preparations. This subset included all preparations that were positive for rickettsial DNA using the Rick17b qPCR assay but negative for R. felis DNA based on the Rfel_phosp_MB qPCR assay. This also included three flea DNA samples positive with both the Rick17b and the Rfel_phosp_MB qPCR assays to confirm the specificity of Rfel_phosp_MB.37 PCR amplification and sequencing of ompB, gltA, and 17-kDa antigen gene fragments were attempted for all cat tissue DNA samples that tested positive for Rickettsia DNA by qPCR, as previously described.37 Sequencing reactions were performed in the forward and reverse directions using the BigDye Terminator v3.1 Reaction Cycle sequencing kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions using an ABI 3500 genetic analyzer (Applied Biosystems). Sequence assembly was performed using CodonCode Aligner version 5.0.1 (CodonCode Corporation, Centerville, MA), and sequences obtained were compared with sequences available through NCBI using MEGA 6 software.38
Serology.
Evidence of previous infection in cats by SFGR and TGR was assessed using group-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assays (ELISAs) based on whole cell antigens Rickettsia conorii str. Morocco (SFGR) and R. typhi str. Wilmington (TGR), as previously described.20,39 Serum samples were diluted 1:100 and screened for antibodies against rickettsiae using both the SFGR and TGR ELISAs. Screen-positive samples (those with net absorbance of ≥ 0.5) were titered by 4-fold serial dilution (100-6,400). Commercial anti-cat IgG (KPL, Gaithersburg, MD) antibodies labeled with horseradish peroxidase were used in both ELISAs.
RESULTS
Thirty sera and 231 tissue samples were collected from 30 cats in Riverside County. Liver, lung, spleen, and heart samples were obtained from all 30 cats, whereas ear, lymph node, rump muscle, and kidney samples were collected from 29, 29, 28, and 25 cats, respectively. Of the 30 cats, 17 harbored fleas with a range of 1–14 fleas per cat. A total of 77 fleas (C. felis and E. gallinacea) were removed from 17 cats (N = 17 of 30 cats, 56.7%) (Figure 1). The fleas were separated into 64 samples, including 55 single C. felis fleas, one pool of 14 C. felis fleas, and eight single E. gallinacea fleas.
Flea survey results for Rickettsia.
A total of 17 of 64 flea samples (26.6%) were positive for rickettsial DNA by the Rick17b qPCR assay with a minimum infection rate of 22.1% (95% exact binomial confidence interval = 13.4–33%). All 17 positive samples were derived from C. felis flea samples (N = 17 of 56 samples [30.4%]), whereas none of the E. gallinacea fleas (N = 8 samples from 3 cats) were positive for rickettsial DNA by the Rick17b qPCR assay. In addition, the 17 flea samples positive for rickettsial DNA were obtained from 10 of the 17 cats (58.8%) harboring fleas (Table 1).
Table 1.
Summary of rickettsial qPCR assay results for fleas from 17 of 30 cats impounded in Riverside County, California
| Flea species | No. of Flea DNA samples* | Rickettsia genus (%; 95 CI) | Rickettsia felis (%; 95 CI) | Rickettsia typhi | Rickettsia asembonensis | No. of cats | No. Rickettsia Pos cats (%; 95 CI) | No. of Rickettsia felis Pos cats (%; 95 CI) |
|---|---|---|---|---|---|---|---|---|
| Ctenocephalides felis | 56 | 17 (30.4; 18.8–44.1) | 12 (21.4; 11.6–34.4) | 0 | 0 | 16 | 10 (62.5; 35.4–84.8) | 8 (50; 23.0–72.2) |
| Echidnophaga gallinacea | 8 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Totals | 64 | 17 (26.6; 16.3–39.1) | 12 (18.8; 10.1–30.5) | 0 | 0 | 17 | 10 (58.8; 32.9–81.6) | 8 (47; 24.7–75.3) |
Sixty-three individual fleas and one pooled sample that contained 14 individual C. felis fleas. Pool was positive for R. felis.
Flea samples positive by the genus-specific Rick17b assay were further tested using species-specific qPCR assays. Twelve of the 17 flea nucleic acid preparations that tested positive for rickettsial DNA using the Rick17b qPCR assay also tested positive for R. felis DNA using the Rfel_phosp_MB qPCR assay, indicating that 18.8% of all the flea DNA samples and 21.4% of the C. felis flea DNA samples were positive for R. felis. These 12 samples were obtained from eight of 17 individual cats (47.0%) included in this study or 8 of 16 cats (50%) that harbored C. felis fleas. No flea DNA tested positive using the Rtyph qPCR assay (Figure 1, Table 1). The results from the genus- and species-specific qPCR assays were confirmed by PCR amplification and sequencing of ompB. The 518-bp ompB sequences were generated for eight flea DNA samples. Five single flea samples from two cats that were positive for rickettsial DNA using the Rick17b assay but negative for all other assays generated amplicons that were 100% identical to Candidatus Rickettsia senegalensis PU01-02 KF666470 (Figure 1). The remaining three fleas, which had tested positive by the Rick17b and the Rfel_phosp_MB qPCR assay, generated amplicons that were 100% identical to R. felis CP000053.
Cat tissue results.
A total of six of 231 DNA preparations (2.6%) from tissue samples (one ear, two spleen, and three muscle) obtained from three of 30 cats (10%) were positive using Rick17b genus-specific qPCR assay (Table 2). Samples positive by the Rick17b assay were further tested using species-specific qPCR assays. Two of these samples (muscle and spleen from one cat (#3) also tested positive for R. felis DNA using the Rfel_phosp_MB qPCR assay. No DNA samples were positive using the Rtyph qPCR assay (Table 2). Interestingly, two of the three cats that were positive for rickettsial infection also harbored fleas positive for rickettsial species (Figure 1).
Table 2.
Summary of rickettsial qPCR assay results for cat tissues (ear, liver, lymph node, rump muscle, spleen, lungs, heart, and kidneys) from cats in Riverside County, California
| qPCR assay | Specificity | No. Pos tissue (%; 95 CI) | Total tissue | No. Pos cats (%; 95 CI) | Total cats | Tissue type Pos |
|---|---|---|---|---|---|---|
| Rick17b | Rickettsia spp. | 6 (2.6; 1.0–5.6) | 231 | 3 (10; 2.1–26.5) | 30 | Cat 1: Muscle, spleen; Cat 2: mar, muscle; Cat 3: muscle, spleen |
| Rfel_Phos_MB | Rickettsia felis | 2 (0.8; 0.1–3.1) | 231 | 1 (3.3; 0.1–17.2) | 30 | Cat 3: muscle, spleen |
| Rtyph | Rickettsia typhi | 0 | 231 | 0 | 30 | N/A |
To confirm the identities of the rickettsiae detected by the genus- and species-specific qPCR assays from cat tissues, PCR amplification and sequencing of the 17-kDa, gltA, and ompB genes were attempted for all positive samples. For two cats (#1 and #2), 416-bp sequences of the 17-kDa gene were obtained and found to be 100% identical to Ca. R. senegalensis (KU167052) and Rickettsia sp. cf15 (AY953285). For one of these cats (#2), a 1093-bp length sequence of gltA was also obtained and found to be 100% identical to Ca. R. senegalensis (KF666472). In addition, a 393-bp 17-kDa sequence and a 518-bp ompB sequence from one cat (#3) were found to be 100% identical to R. felis URRWXCal2 (Figure 1).
Serology.
Sera collected from all 30 cats were tested for the presence of antibodies to both SFG and TG Rickettsia. Of the 30 cats, eight cats (26.7%) were positive for antibodies against SFG antigens, whereas only one cat (3.3%) was positive for antibodies against TG antigens. Of these eight cats, only three cats harbored fleas positive for rickettsial species. The single cat that was positive for antibodies that reacted to the TG antigen was also positive for antibodies against the SFG antigen. Finally, none of the cats with tissue samples positive for rickettsial infection were positive for antibodies against SFG or TG antigens (Figure 1).
DISCUSSION
This study aimed to determine rickettsial infection rates in cats and their fleas in Riverside County, California. The investigation provided evidence that cats and their fleas are infected with two rickettsial species Ca. R. senegalensis and the human pathogen R. felis. Overall, the results indicate that 10% (3/30) of the cats had rickettsial infections, with rickettsial DNA detected in the ear, spleen, and rump muscle. Of the cats with fleas, 47% had at least one flea positive for R. felis and 11.8% of the cats with fleas had at least one flea positive for Ca. R. senegalensis. These results indicate that R. felis infection of C. felis fleas from cats in Riverside County is common and is the predominant Rickettsia detected in these fleas. The results support previous evidence showing R. felis to be the main Rickettsia spp. in C. felis fleas from cats and opossums in California14,15,18–20,40 and C. felis fleas from cats worldwide.32 Reports from South Carolina and California indicate the existence of Ca. R. senegalensis in the United States, and these results, herein further support this.20,41 However, to date, the pathogenicity of Ca. R. senegalensis in humans is unknown. Interestingly, R. asembonensis was not detected in this study. R. asembonensis is commonly found around the world in cat fleas37,42–44 and was recently found in cat fleas from Orange County, albeit at a very low prevalence.20
In addition to providing DNA evidence of rickettsial infections in cats and cat fleas, our study found that antibodies against SFGR were present in 26.7% of cats. Antibodies to TGR were seen in only one of 30 cats (3.3%), but this might be because of cross-reactivity of SFG antibodies to TGR antigens. However, data from previous studies indicate that when fleas infected with R. felis are fed on cats, these cats produced antibodies that reacted to SFGR (R. conorii str. Morocco) and not TGR (R. typhi str. Wilmington) ELISA antigens.20,45 Therefore, the results suggest that the high prevalence of anti-SFGR antibodies is due to infection with SFGR such as R. felis, whereas R. typhi infections in cats appear to be much less common. The high seroprevalence of antibodies against SFGR and low prevalence of antibodies against TGR agree with our findings that C. felis fleas have substantial infection rates with R. felis and Ca. R. senegalensis.
Interestingly, no cats or flea pools were positive for R. typhi, which is consistent with the historically small number of flea-borne rickettsiosis cases reported from Riverside County (N = 2, 2001–2016).1,2,18 Investigations in nearby Los Angeles and Orange counties, where cases of flea-borne rickettsioses occur annually, show high prevalence of TGR antibodies in cats and opossums, suggesting that flea-borne rickettsial infection in southern California could be due to R. typhi alone13,20,26 and that the high prevalence of R. felis in cat fleas does not predispose people to increased risk of infection.40,46
This, however, does not rule out the possibility that some flea-borne rickettsial infections in southern California might be due to R. felis, or R. felis–like organisms. Eremeeva et al. reported cases where C. felis fleas collected off cats and opossums from households in Los Angeles and Orange counties demonstrated the presence of R. felis but not R. typhi,14 and associations of rickettsial disease cases with cats and their C. felis fleas infected only with R. felis have also been observed in Australia.47 Whereas most cases in Texas have been attributed to R. typhi infection, some flea-borne rickettsiosis victims have tested positive for R. felis antibodies,27 as has been reported elsewhere in the world.22–25,48
Our results provide additional evidence that cats become infected with R. felis and R. felis–like organisms. Both the prevalence of antibodies to SFGR antigens and the detection of rickettsial DNA in multiple cat tissues indicate that cats in Riverside County are infected with R. felis and/or R. felis–like organisms, and the lack of R. typhi DNA in tissue, along with the low prevalence of antibodies to TGR antigens, suggests that cats could be acting as important vertebrate reservoirs of R. felis and R. felis–like organisms and under-recognized contributors of flea-borne rickettsiosis. Opossums and cats (especially free-roaming ones) in southern California share ecological niches and C. felis fleas, making it imperative for cats to be monitored regularly and treated for fleas to minimize health risks to humans. Municipal animal shelters that engage in Trap-Neuter-Release (TNR) programs, where healthy unsocialized cats are trapped, neutered, and released to neighborhood cat colonies (groups of cats that live together in a territory) cared for by humans to lessen shelter euthanasia rates, should incorporate zoonotic disease reduction practices and prohibit placing TNR cats in areas endemic for flea-borne rickettsiosis or other zoonotic diseases.49–52 Cat colonies have been documented with flea infestation rates as high as 92.5% because flea control products are not routinely applied to these cats.53 Hotspot analysis of flea-borne rickettsial disease cases in Orange County demonstrated significant clustering of rickettsial disease occurrence (0.7–1.5 cases/km2) in areas with high TNR cat density compared with other, less affected areas of the county (Cummings et al., unpublished data). Several flea-borne rickettsiosis outbreaks in Los Angeles and Orange counties have been very focal geographically, occurring in case clusters in families or mobile home communities with high cat densities.54,55 Although exposure to flea-borne rickettsial disease agents is recognized as being highly variable and ecologically complex,12 further research in the eco-epidemiology of flea-borne rickettsiosis is needed to provide a better understanding of the causes of these outbreaks.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States.
REFERENCES
- 1.Meleney HE, French RS, 1945. Endemic typhus fever in southern California. Cal West Med 62: 116–119. [PMC free article] [PubMed] [Google Scholar]
- 2.Meleney HE, 1941. Recent extension of endemic typhus fever in the southern United States. Am J Public Health Nations Health 31: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brill IC, 1917. Typhus exanthematicus in San Francisco. Cal State. J Med 15: 29–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Kligler IJ, Aschner M, Levine S, 1936. Comparative studies of the louse-borne (epidemic) and flea-borne (murine) typhus viruses. Br J Exp Pathol 17: 53–60. [Google Scholar]
- 5.Hill EL, Morlan HB, Utterback BC, Schubert JH, 1951. Evaluation of county-wide DDT dusting operations in murine typhus control (1946 through 1949). Am J Public Health 41: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JR, Schmidtmann ET, Azad AF, 1990. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a Rickettsia-like microorganism. Am J Trop Med Hyg 43: 400–409. [DOI] [PubMed] [Google Scholar]
- 7.Azad AF, 1990. Epidemiology of murine typhus. Annu Rev Entomol 35: 553–569. [DOI] [PubMed] [Google Scholar]
- 8.Civen R, Ngo V, 2008. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis 46: 913–918. [DOI] [PubMed] [Google Scholar]
- 9.Cowan G, 2000. Rickettsial diseases: the typhus group of fevers—a review. Postgrad Med J 76: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schriefer ME, Sacci JB, Jr., Taylor JP, Higgins JA, Azad AF, 1994. Murine typhus: updated roles of multiple urban components and a second typhuslike Rickettsia. J Med Entomol 31: 681–685. [DOI] [PubMed] [Google Scholar]
- 11.Abramowicz KF, Rood MP, Krueger L, Eremeeva ME, 2011. Urban focus of Rickettsia typhi and Rickettsia felis in Los Angeles, California. Vector Borne Zoonotic Dis 11: 979–984. [DOI] [PubMed] [Google Scholar]
- 12.Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM, 1997. Flea-borne rickettsioses: ecologic considerations. Emerg Infect Dis 3: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorvillo FJ, Gondo B, Emmons R, Ryan P, Waterman SH, Tilzer A, Andersen EM, Murray RA, Barr R, 1993. A suburban focus of endemic typhus in Los Angeles County: association with seropositive domestic cats and opossums. Am J Trop Med Hyg 48: 269–273. [DOI] [PubMed] [Google Scholar]
- 14.Eremeeva ME, et al. 2012. Two pathogens and one disease: detection and identification of flea-borne rickettsiae in areas endemic for murine typhus in California. J Med Entomol 49: 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpathy SE, et al. 2009. Detection of Rickettsia felis and Rickettsia typhi in an area of California endemic for murine typhus. Clin Microbiol Infect 15 (Suppl 2): 218–219. [DOI] [PubMed] [Google Scholar]
- 16.Rust MK, Dryden MW, 1997. The biology, ecology, and management of the cat flea. Annu Rev Entomol 42: 451–473. [DOI] [PubMed] [Google Scholar]
- 17.Title 17, California Code of Regulations and Reportable Disease Conditions, Rickettsial Diseases Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/ReportableDiseases.pdf. Accessed November 24, 2017.
- 18.California Department of Public Health , 2018. Human Flea-Borne Typhus Cases in California 2001–2017 Sacramento, California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/Flea-borneTyphusCaseCounts.pdf. Accessed November 24, 2017.
- 19.Abramowicz KF, Wekesa JW, Nwadike CN, Zambrano ML, Karpathy SE, Cecil D, Burns J, Hu R, Eremeeva ME, 2012. Rickettsia felis in cat fleas, Ctenocephalides felis parasitizing opossums, San Bernardino County, California. Med Vet Entomol 26: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maina AN, et al. 2016. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS One 11: e0160604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schriefer ME, Sacci JB, Jr., Dumler JS, Bullen MG, Azad AF, 1994. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol 32: 949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parola P, 2011. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect 17: 996–1000. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Osorio CE, Zavala-Velázquez JE, León JJA, Zavala-Castro JE, 2008. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 14: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zavala-Castro J, Zavala-Velázquez J, Walker D, Pérez-Osorio J, Peniche-Lara G, 2009. Severe human infection with Rickettsia felis associated with hepatitis in Yucatan, Mexico. Int J Med Microbiol 299: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards AL, Jiang J, Omulo S, Dare R, Abdirahman K, Ali A, Sharif SK, Feikin DR, Breiman RF, Njenga MK, 2010. Human infection with Rickettsia felis, Kenya. Emerg Infect Dis 16: 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billeter SA, Metzger ME, 2017. Limited evidence for Rickettsia felis as a cause of zoonotic flea-borne rickettsiosis in southern California. J Med Entomol 54: 4–7. [DOI] [PubMed] [Google Scholar]
- 27.Wiggers RJ, Martin MC, Bouyer D, 2005. Rickettsia felis infection rates in an east Texas population. Tex Med 101: 56–58. [PubMed] [Google Scholar]
- 28.Pieracci EG, Evert N, Drexler NA, Mayes B, Vilcins I, Huang P, Campbell J, Behravesh CB, Paddock CD, 2017. Fatal flea-borne typhus in Texas: a retrospective case series, 1985–2015. Am J Trop Med Hyg 96: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christou C, Psaroulaki A, Antoniou M, Toumazos P, Ioannou I, Mazeris A, Chochlakis D, Tselentis Y, 2010. Rickettsia typhi and Rickettsia felis in Xenopsylla cheopis and Leptopsylla segnis parasitizing rats in Cyprus. Am J Trop Med Hyg 83: 1301–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eremeeva ME, Warashina WR, Sturgeon MM, Buchholz AE, Olmsted GK, Park SY, Effler PV, Karpathy SE, 2008. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis 14: 1613–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthewman L, Kelly P, Hayter D, Downie S, Wray K, Bryson N, Rycroft A, Raoult D, 1997. Domestic cats as indicators of the presence of spotted fever and typhus group rickettsiae. Eur J Epidemiol 13: 109–111. [DOI] [PubMed] [Google Scholar]
- 32.Wedincamp J, Jr., Foil LD, 2000. Infection and seroconversion of cats exposed to cat fleas (Ctenocephalides felis Bouche) infected with Rickettsia felis. J Vector Ecol 25: 123–126. [PubMed] [Google Scholar]
- 33.American Veterinary Medical Association , 2013. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed November 24, 2017.
- 34.Jiang J, Stromdahl EY, Richards AL, 2012. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis 12: 175–182. [DOI] [PubMed] [Google Scholar]
- 35.Henry KM, Jiang J, Rozmajzl PJ, Azad AF, Macaluso KR, Richards AL, 2007. Development of quantitative real-time PCR assays to detect Rickettsia typhi and Rickettsia felis, the causative agents of murine typhus and flea-borne spotted fever. Mol Cell Probes 21: 17–23. [DOI] [PubMed] [Google Scholar]
- 36.Leulmi H, Socolovschi C, Laudisoit A, Houemenou G, Davoust B, Bitam I, Raoult D, Parola P, 2014. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis 8: e3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, et al. 2013. Molecular detection of Rickettsia felis and Candidatus rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis 13: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graf PC, Chretien JP, Ung L, Gaydos JC, Richards AL, 2008. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin Infect Dis 46: 70–77. [DOI] [PubMed] [Google Scholar]
- 40.Billeter SA, Diniz PP, Jett LA, Wournell AL, Kjemtrup AM, Padgett KA, Yoshimizu MH, Metzger ME, Barr MC, 2016. Detection of Rickettsia species in fleas collected from cats in regions endemic and nonendemic for flea-borne rickettsioses in California. Vector Borne Zoonotic Dis 16: 151–156. [DOI] [PubMed] [Google Scholar]
- 41.Reeves WK, Nelder MP, Korecki JA, 2005. Bartonella and Rickettsia in fleas and lice from mammals in south Carolina, USA. J Vector Ecol 30: 310–315. [PubMed] [Google Scholar]
- 42.Odhiambo AM, Maina AN, Taylor ML, Jiang J, Richards AL, 2014. Development and validation of a quantitative real-time polymerase chain reaction assay specific for the detection of Rickettsia felis and not Rickettsia felis-like organisms. Vector Borne Zoonotic Dis 14: 476–481. [DOI] [PubMed] [Google Scholar]
- 43.Parola P, Sanogo OY, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez JP, Miller RS, Telford SR, 3rd, Wongsrichanalai C, Raoult D, 2003. Identification of Rickettsia spp. and Bartonella spp. from the Thai-Myanmar border. Ann N Y Acad Sci 990: 173–181. [DOI] [PubMed] [Google Scholar]
- 44.Oteo JA, Portillo A, Portero F, Zavala-Castro J, Venzal JM, Labruna MB, 2014. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors 7: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang R, Raoult D, 2003. Antigenic classification of Rickettsia felis by using monoclonal and polyclonal antibodies. Clin Diagn Lab Immunol 10: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawley JR, Shaw SE, Lappin MR, 2007. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 9: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams M, Izzard L, Graves SR, Stenos J, Kelly JJ, 2011. First probable Australian cases of human infection with Rickettsia felis (cat-flea typhus). Med J Aust 194: 41–43. [DOI] [PubMed] [Google Scholar]
- 48.Lim MY, Brady H, Hambling T, Sexton K, Tompkins D, Slaney D, 2012. Rickettsia felis infections, New Zealand. Emerg Infect Dis 18: 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerhold RW, Jessup DA, 2013. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health 60: 189–195. [DOI] [PubMed] [Google Scholar]
- 50.Persichetti MF, Solano-Gallego L, Serrano L, Altet L, Reale S, Masucci M, Pennisi MG, 2016. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors 9: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravicini S, Pastor J, Hawley J, Brewer M, Castro-Lopez J, Beall M, Lappin MR, 2016. Prevalence of selected infectious disease agents in stray cats in Catalonia, Spain. JFMS Open Rep 2: 2055116916634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy JK, Crawford PC, 2004. Humane strategies for controlling feral cat populations. J Am Vet Med Assoc 225: 1354–1360. [DOI] [PubMed] [Google Scholar]
- 53.Akucewich LH, Philman K, Clark A, Gillespie J, Kunkle G, Nicklin CF, Greiner EC, 2002. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol 109: 129–139. [DOI] [PubMed] [Google Scholar]
- 54.Green JS, Singh J, Cheung M, Adler FC, Ashouri N, 2011. A cluster of pediatric endemic typhus cases in Orange County, California. Pediatr Infect Dis J 30: 163–165. [DOI] [PubMed] [Google Scholar]
- 55.Croker C, Foo C, 2015. Multi-agency response to a flea-borne typhus outbreak. Los Angels County Department of Public Health, Acute Communicable Disease Control, 2015 Special Studies Report, 65–71. Available at: http://publichealth.lacounty.gov/acd/reports/2015SpecialStudiesReport.pdf. Accessed November 24, 2017.