Abstract.
The western region of Edo state in southern Nigeria is highly endemic for onchocerciasis. Despite years of mass drug administration (MDA) with ivermectin (IVM), reports suggest persistently high prevalence of onchocerciasis, presumably because of poor coverage. In 2016, twice-per-year treatment with IVM (combined with albendazole for lymphatic filariasis in the first round where needed) began in five local government areas (LGAs) of Edo state. We undertook a multistage cluster survey within 3 months after each round of MDA to assess coverage. First-round coverage was poor: among 4,942 people of all ages interviewed from 145 clusters, coverage was 31.1% (95% confidence intervals [CI]: 24.1–38.0%). Most respondents were not offered medicines. To improve coverage in the second round, three LGAs were randomized to receive MDA through a “modified campaign” approach focused on improved supervision and monitoring. The other two LGAs continued with standard MDA as before. A similar survey was conducted after the second round, interviewing 3,362 people in 87 clusters across the five LGAs. Coverage was not statistically different from the first round (40.0% [95% CI: 31.0–49.0%]) and there was no significant difference between the groups (P = 0.7), although the standard MDA group showed improvement over round 1 (P < 0.01). The additional cost per treatment in the modified MDA was 1.6 times that of standard MDA. Compliance was excellent among those offered treatment. We concluded that poor mobilization, medicine distribution, and program penetration led to low coverage. These must be addressed to improve treatment coverage in Edo state.
INTRODUCTION
Nigeria is the most endemic country in the world for onchocerciasis, home to 27% of the nearly 200 million people at risk globally.1 The National Onchocerciasis Control Program is the largest ivermectin (IVM) (Mectizan®; donated by Merck & Co., Kenilworth, NJ) mass drug administration (MDA) program in the world, reporting between 20 and 35 million treatments per year. Preventive chemotherapy is administered by village-based volunteers through community-directed treatment with IVM (CDTI).2,3 Nigeria also bears much of the world’s burden of lymphatic filariasis.4 The Nigerian Federal Ministry of Health (FMoH) aims to eliminate both diseases. Complete geographic coverage and high MDA coverage through CDTI—at least 80% of the total population for onchocerciasis, 65% for lymphatic filariasis—are essential to achieve these goals.
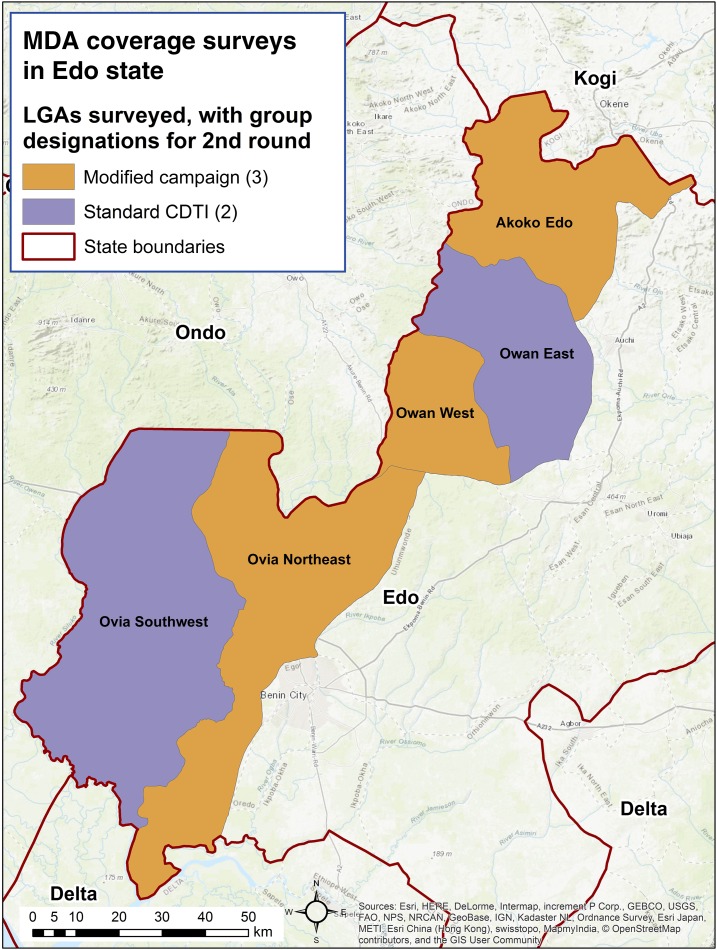
Edo state (population about 4.3 million) is known to be endemic for both diseases, particularly in five local government areas (LGAs) along its western border with neighboring Ondo state. Compared with other parts of Nigeria, this area is notorious for entrenched onchocerciasis prevalence, presumably due to poor MDA coverage.5–8 Precontrol nodule prevalence was 36.4%, and annual treatment began in meso- and hyperendemic villages in 1994 and continuously until 2016.8 The five LGAs in question had nodule rates ranging from 42% to 62% and had microfiladermia as high as 83% in 2008/2009.5 Recent surveys by African Programme for Onchocerciasis Control (APOC) and others have demonstrated persistent microfiladermia and ongoing transmission in this area.9,10 For example, studies by APOC in 2010 showed continued high prevalence in Edo state, with 33.4% of participants positive by skin snip.9 Although reported coverage was always high, these disappointing results were corroborated by internal monitoring that suggested low coverage. In response, the program undertook efforts to find new villages and camps in the region and include them in treatment registers, aiming for LGA-wide coverage. Particular attention was paid to migrant laborers and areas undergoing sporadic, localized civil unrest. Also, to address persistent prevalence of onchocerciasis, twice-per-year treatment was launched in five LGAs in Edo state in 2016 to further Nigeria’s goal of rapidly stopping transmission throughout the country (Figure 1).11,12 There is great interest in examining how MDA coverage will perform under twice-per-year treatment.
Figure 1.
Local government areas (LGAs) treating twice per year in 2016, Edo state, Nigeria. This figure appears in color at www.ajtmh.org.
MATERIALS AND METHODS
Study design.
We conducted two coverage surveys within 3 months of completing each round of MDA to minimize recall bias and ensure time to implement needed changes by the next round.13 Both surveys used multistage random sampling, first of clusters, then of households.14,15 We evaluated the outcomes of treatment coverage, historical coverage (previous compliance with MDA), geographic coverage (if anyone in a cluster received treatment), rural/urban differences, knowledge of onchocerciasis/river blindness and lymphatic filariasis (LF), and conduct of MDA (height measurement, location of MDA, health education, etc.). We also asked about ownership and use of long-lasting insecticidal nets, a key factor in LF prevention.
Based on the poor results from the first-round survey, we introduced a set of additional interventions aimed at improving coverage in three randomly selected LGAs. These were compared with two control LGAs in a second study, testing whether increasing the intensity of MDA training and supervision improved coverage and the cost-effectiveness of that approach.
Questionnaires covered treatment compliance, conduct of MDA, knowledge of diseases, and exposure to health education. Other variables like bed net coverage and school attendance were collected for the program. This analysis focuses on coverage and conduct of MDA.
Sampling method.
Nigeria’s file of census enumeration areas (EAs) is a comprehensive, geographically ordered list, developed during the 2006 census, which presumes a population of approximately 200–500 per EA. The EA lists are different from those used by the FMoH or the CDTI program to manage treatment distribution. Each EA has an associated hand-drawn map. We used the EA as the cluster, or first stage of selection. Enumeration areas were selected systematically using a random start.
The second stage of sampling occurred when teams arrived at the EA. Teams would work with a local guide to trace the boundaries of the EA using the maps provided by the census office. While walking along this boundary, teams would enumerate all the households within the EA. A household was defined as a group of people who live together and share cooking arrangements. Once the total number of households was determined, the EA was divided into roughly equal segments of a maximum of 50 households, if necessary. A segment was chosen at random by the local guide by drawing numbered papers from a cup or hat. Teams then used a random number generator to determine the first household to interview, and interviewed a fixed number of households per EA, selected systematically using the number of households in the segment divided by the number to interview. Teams could revisit households a maximum of three times, but absent or nonconsenting households were not replaced. Abandoned households were not included in the enumeration.
All residents of the five LGAs were eligible for the study, including those normally deemed ineligible (e.g., under age five) to confirm that treatment decisions were made accurately by distributors. Only visitors were excluded. Parents could speak for children under age 10 if they wished. The head of household and each household member gave verbal consent to be interviewed.
Sample size calculations.
Following the first round of MDA, which occurred from approximately May through July of 2016, we selected 30 EAs from each LGA (total of 150 EAs). The goal was to develop a statistically robust estimate of coverage for each LGA. The survey required a sample size of 766 using a 95% confidence level and a ±5% margin of error, with a design effect of 2.0 to account for the complex sampling design, and assuming a population size of 100,000. Anticipating a 15% nonresponse rate, this yielded a final sample size of 881 per LGA selected. Sample sizes were calculated using OpenEpi (www.openepi.com); coverage—defined as the proportion of all people who swallowed the medicine—was assumed to be 50%.
Using an average household size of 4.6,16 we needed to survey 191 households. Using a 30-cluster design, we needed approximately seven households per cluster. The total sample size was 4,405.
We used the data from the first round to inform the estimated design effect in our second-round survey. We also had a different aim—comparing groups of LGAs (modified MDA versus standard CDTI) rather than generating an estimate for each LGA. Coverage was assumed to be 50%. To detect a 10-percentage point change in coverage, with 80% power and a 95% confidence level, the survey required a sample size of 389 per group. We used a design effect of four in response to the high level of clustering seen in the first survey.14 Anticipating a 15% nonresponse rate, the final sample size was 1,790 per group (3,580 total). Teams needed to survey 358 households. Given the costs associated with visiting a cluster and the intercluster correlation observed in the first study, we determined that 48 clusters per group and nine households per cluster was the most efficient design.
Evaluation teams.
The evaluation personnel consisted of the survey team (four people—leader, two interviewers, driver) and a representative from The Carter Center, MITOSATH, or the Ministry of Health serving as supervisor. Teams worked with a guide nominated by the village chief to map the boundaries of the EA, count households, and introduce the study to participants. Community-directed distributors (CDDs), the volunteers responsible for treating their own villages, were not allowed to serve as guides to avoid bias.
Interventions in the second round of MDA.
After reviewing the data from the first-round survey, several measures were deployed to some LGAs to see whether they would improve coverage compared with the standard CDTI approach. Local government areas were randomly allocated to study arms. In all LGAs, treatment was delivered by CDDs as usual.
Group one—modified CDTI (“modified”).
Three LGAs were allocated to this arm. These LGAs experienced the following changes, which focused on providing additional funds and resources to local staff:
-
•
Restricted timeframe of MDA, with treatment reports due on a predetermined date.
-
•
Trained two or more front-line health workers (FLHWs) from each primary care health facility in training and supervising CDDs.
-
•
Designed and offered a simple supervision checklist to FLHWs.
-
•
Assigned at most two villages to each FLHW.
-
•
Provided funds for increased supervision by FLHWs.
-
•
Doubled the transport reimbursement for CDDs attending training.
-
•
Applied the Supervisor’s Coverage Tool (SCT), a rapid coverage assessment activity, to identify areas in need of mop-up, and assigned these to specific Ministry of Health staff.17
-
•
Instructed health staff to examine reports immediately upon receipt; any community not reaching 80% coverage was singled out for mop-up activities. Funds were provided for mop-up, to be conducted by the responsible MoH staff person.
Group two—standard CDTI (“standard”).
Two LGAs executed MDA following the same procedures and timelines as previous distributions. Community-directed distributors were trained for 2 days following the standard curriculum. They were provided a transport reimbursement but no other monetary compensation from the program. Mop-up occurred at the discretion of health staff and was not mandated. Front-line health workers supervised five villages on average.
Cost study.
Cost-effectiveness was considered in the second round. Staff from The Carter Center closely tracked expenses in all five LGAs to compare the relative cost per treatment. We tracked the following expenses:
-
•
Transport reimbursements for CDDs
-
•
Per diem for staff and health workers
-
•
Fuel and maintenance costs for vehicles
-
•
Supplies and materials for training and supervision
-
•
Printing costs for additional forms and tools
Cost per treatment was calculated as the estimated number of treatments given in the study arm divided by the total costs for that study arm.
Data collection and management.
Surveys were completed using an electronic data collection tool on Android tablets. Paper forms were used as backups and to organize logistics. Data were submitted every few days at a minimum when teams reached a viable mobile network. We used Excel to clean and manage the data. Many teams chose to enter their data in duplicate; we used the Epi Info data compare procedure to create a composite record for these individuals. If there were conflicts between answers, one record was chosen at random to stand for that individual. Of 464,904 fields compared, 5,360 (1.2%) were discrepant. The vast majority of discrepancies were related to free text fields, multiple-choice responses, scaled responses where neighboring options were selected by each interviewer, or other typing errors such as small differences in age (e.g., entering 55 and 56 for the same person). Differences on key outcome variables were rare.
Data analysis.
Data were analyzed in STATA version 11.2 (StataCorp., College Station, TX) using procedures appropriate for a complex survey design. We applied sampling weights to everyone reflecting their probability of selection at both the LGA group and cluster level. Treatment coverage was defined as the proportion of the total respondent population who took the drug in question, including those normally deemed ineligible for treatment (e.g., those under age five). The target level of coverage is 80% of the total at-risk population.18
To compare results from both rounds, we combined the two datasets but adjusted the sampling design to account for the different selection probabilities; the EA remained the primary sampling unit, the household the secondary sampling unit. Proportions from two rounds were compared using a Pearson χ2 test.
Ethical review.
Approval was also granted by the Edo State Ministry of Health. Both surveys were reviewed by the Institutional Review Board at Emory University and deemed nonresearch. The findings are not generalizable beyond the LGAs in question.
RESULTS
First round.
The survey took place in August 2016. We visited 145 EAs; five were abandoned or inaccessible because of insecurity. Teams interviewed 4,942 respondents, exceeding our minimum sample size of 4,405.
Second round.
The survey took place in January/February 2017. We visited 87 EAs; nine were inaccessible or abandoned. We interviewed 3,362 individuals, which was under our target sample size of 3,580, likely because of the time of year and lack of school holidays, which is evident by the slightly older average age in the second round. Participant characteristics for both surveys are described in Table 1. Differences across LGAs or groups were statistically insignificant.
Table 1.
Description of participants in coverage surveys following each round of mass drug administration in 2016 in Edo state, Nigeria
| Indicator | First round | Second round |
|---|---|---|
| Number of respondents | 4,942 | 3,362 |
| Number of clusters | 145 | 87 |
| Number of households selected | 1,010 | 774 |
| Number of households consented | 978 | 754 |
| Mean total households per cluster | 103 | 72 |
| Mean household size (standard deviation) | 5.1 (2.5) | 4.7 (2.5) |
| Mean age (standard deviation) | 24.8 (19.7) | 27.2 (20.4) |
| Percent ≥ age 5 | 87.7% | 90.6% |
| Percent female | 51.0% | 53.0% |
Treatment coverage and MDA conduct.
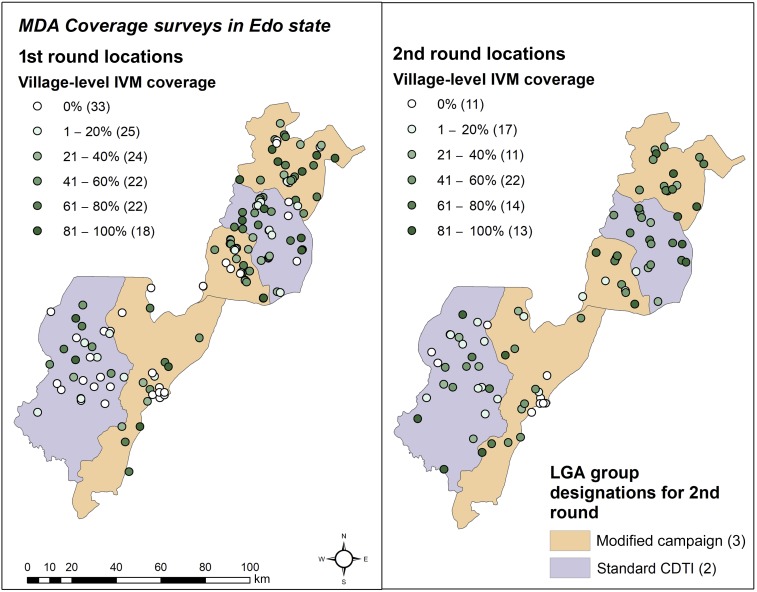
Treatment coverage was generally poor (Table 2). Most participants responded that they had “no opportunity for MDA” or “I was not offered IVM” in each survey, respectively. When untreated EAs were excluded, IVM coverage was estimated to be 45.4% in the first round and 47.2% in the second. Coverage at the EA level ranged from 0% to 100%. The distribution of coverage levels across EAs is shown in Figure 2.
Table 2.
Estimates of treatment coverage and conduct (with 95% CI) in two rounds of MDA in 2016 in Edo state, Nigeria
| Indicator | First round (N = 4,942) | Second round (N = 3,362) |
|---|---|---|
| Took IVM | 31.1% (24.1–38.0%) | 40.0% (31.0–49.0%) |
| Design effect | 5.32 | 5.35 |
| Took IVM, excluding untreated clusters (R1 N = 3,849, R2 N = 3,081) | 45.4% (38.2–52.5%) | 47.2% (39.1–55.3%) |
| Design effect | 4.22 | 4.35 |
| Took ALB (LGAs treating LF only, N = 2,957) | 30.9% (22.2–39.6%) | – |
| Design effect | 5.07 | – |
| Took ALB, excluding untreated clusters (LGAs treating LF only, N = 2,397) | 43.0% (34.3–51.7%) | – |
| Design effect | 3.99 | – |
| Geographic coverage (any treatment in EA) (R1 N = 145, R2 N = 87) | 77% | 90% |
| EAs with coverage more than 80% of total population (R1 N = 145, R2 N = 87) | 12% | 14% |
| Ivermectin coverage in residents of rural EAs (R1 N = 3,356, R2 N = 2,420) | 33.4% (24.2–42.5%) | 45.8% (36.7–54.9%) |
| Ivermectin coverage in residents of urban EAs (R1 N = 1,586, R2 N = 942) | 26.4% (13.0–39.9%) | 34.0% (18.7–49.4%) |
| Received any health education during MDA (ages 5 and above, R1 N = 4,385, R2 N = 3,012) | 33.5% (25.9–41.2%) | 39.1% (30.9–47.3%) |
| Received health education among those treated (R1 N = 1,792, R2 N = 1,510) | 81.6% (74.1–89.1%) | 78.8% (69.5–88.2%) |
| Height measured during MDA | 34.1% (26.4–41.8%) | 36.2% (27.0–45.4%) |
| Height measured among those treated (R1 N = 1,792, R2 N = 1,509) | 82.4% (74.3–90.6%) | 85.5% (78.7–92.2%) |
| Taken IVM before | 34.6% (27.5–41.6%) | 36.3% (26.6–46.0%) |
| Taken ALB before | 33.0% (23.7–42.2%) | – |
ALB = albendazole; EA = enumeration area; IVM = ivermectin; LGA = local government area; MDA = mass drug administration. Italics indicate different denominators than the total study population.
Figure 2.
Geographic distribution and allocation of EAs in different categories of treatment coverage. Global positioning system coordinates were not available for one EA in the first round. Group designations apply to the second round only but are shown in the first for comparison. Note that the plurality of villages was not treated in the first round, whereas in the second 41–60% was the most common level of coverage. EA = enumeration area. This figure appears in color at www.ajtmh.org.
First round: comparisons by LGA.
None of the LGAs reached a level of coverage sufficient for disease control (65%), let alone an elimination (80%) program (Table 3).19,20 Weighted IVM coverage ranged from 16.2% (95% confidence intervals [CI]: 8.1–29.9%) in Ovia Southwest to 53.7% (95% CI: 43.1–64.1%) in Akoko Edo. Albendazole coverage paralleled IVM in most LGAs, but was 5% points lower in Owan East (38.5% versus 33.3%).
Table 3.
Treatment coverage of IVM and ALB during the first round of 2016 mass drug administration by local government area
| Akoko Edo | Ovia Northeast | Ovia Southwest | Owan East | Owan West | |
|---|---|---|---|---|---|
| Number interviewed | 1,093 | 895 | 848 | 1,016 | 1,090 |
| Reported IVM coverage (over treatment target) | 80% | 74% | 82% | 92% | 90% |
| Reported IVM coverage (over total population) | 64% | 60% | 66% | 73% | 73% |
| IVM coverage, weighted (95% CI) | 53.9% (43.3–64.2%) | 23.3% (10.4–44.2%) | 16.4% (8.2–30.0%) | 38.5% (28.3–49.8%) | 42.8% (32.3–54.0%) |
| IVM coverage, weighted, treated EAs only (95% CI) | 56.8% (46.6–67.1% | 46.3% (23.1–69.5%) | 33.9% (17.5–50.3%) | 40.1% (29.0–51.3%) | 45.7% (34.9–56.5%) |
| ALB coverage, weighted (95% CI) | 54.23% (43.8–64.7%) | – | 15.4% (7.4–29.3%) | 33.4% (24.3–43.9%) | – |
| Geographic coverage (any treatment) | 93% | 55% | 58% | 90% | 87% |
ALB = albendazole; CI = confidence intervals; EA = enumeration area; IVM = ivermectin. Ovia northeast and Owan west are not endemic for LF. The target coverage is 80% of the population.
Second round: comparisons by group.
Reported coverage in each LGA ranged from 72% to 96% of the treatment target and was comparable with the first round’s reports. Akoko Edo, Ovia Northeast, and Owan West were randomized to the modified CDTI LGAs (N = 1,665). Ovia Southwest and Owan East were randomized to standard CDTI (N = 1,697). The SCT was used in all 34 wards of these LGAs; only 13 (38.2%) of the wards had satisfactory treatment coverage (above 80% of respondents saying they took treatment) and FLHWs were directed to follow-up MDA in the other 21 wards. Although funds were provided, mop-up was not directly monitored by TCC staff. The full-scale coverage survey began after mop-up concluded.
Most respondents in the second-round survey were not offered IVM (Table 4). Of those offered, most complied (95.1% of those offered; 95% CI: 92.1–98.1%). Weighted coverage (taking IVM) was 39.2% (95% CI: 28.2–51.3%) in the modified LGAs and 42.2% (32.1–52.9%) in CDTI LGAs. There was no statistically significant difference between the groups (P = 0.7054). Geographic coverage was 84% in modified LGAs and 93% in standard LGAs.
Table 4.
Treatment coverage of IVM during the second round of 2016 mass drug administration by group
| Modified campaign | Standard CDTI | |
|---|---|---|
| Number interviewed | 1,665 | 1,697 |
| Reported coverage (over treatment target) | 80% | 87% |
| Reported coverage (over total population) | 64% | 69% |
| Offered IVM, weighted (95% CI) | 41.8% (29.2–54.4%) | 42.8% (31.6–52.7%) |
| IVM coverage, weighted (95% CI) | 39.2% (27.4–50.9%) | 42.2% (31.6–52.7%) |
| IVM coverage, weighted, treated EAs only (95% CI) | 39.8% (32.2–47,4%) | 49.4% (42.4–56.4%) |
| Geographic coverage (any treatment) | 84% | 93% |
CDTI = community-directed treatment with IVM; CI = confidence intervals; EA = enumeration area; IVM = ivermectin. Neither group reached the target coverage of 80% of the total population.
The standard group had significantly lower coverage than the modified group in the first round (22.5% versus 36.7%, P = 0.0463). Difference-in-difference analysis showed that the standard group made a significant improvement by the second round (P < 0.01), whereas the modified group made only modest improvements in IVM coverage (standard: 42.2%, modified 39.2%).
Location and delivery of MDA.
More than 93% of respondents in both studies reported receiving MDA at home, delivered by CDDs. Treatments provided by health workers were a distant second, responsible for treatment of 14% (round 1) and 8% (round 2) of participants. A few participants in each study reported receiving treatment in a central part of town, at a religious institution, at school, or at a health facility.
Cost per treatment.
The cost per treatment was approximately US$0.08 in CDTI LGAs and US$0.13 in modified LGAs due to the increase in transport reimbursements and supervision costs. However, because of the larger overall population in the modified group, the cost per treatment was only 1.6 times greater. Per community, the modified arm’s costs were $53.19 (N = 686) compared with CDTI’s $38.45 (N = 330) or 1.4 times greater.
DISCUSSION
This study describes the treatment coverages obtained after the first use of semiannual IVM treatment in Nigeria to address entrenched onchocerciasis, as has been successfully carried out elsewhere in Africa and beyond.21–23 These results strongly suggest that low coverage may be to blame for continued high onchocerciasis prevalence in this region. We unsuccessfully tried to improve treatment coverage in the second round through increased supervision and transport reimbursement. Although overall coverage improved slightly from the first round to the second, this difference was not statistically significant. Geographic coverage did, however, appear to improve (Figure 2; Table 4). The costs of this extra effort were higher but still within range of other programs’ costs per treatment.24–27
Our interventions targeted the delivery of drugs rather than their supply and coordination within the health system. The study does not address the old and intense debate regarding payment of CDDs as a means to improve performance.28,29 Increasing the transport reimbursement—without increasing the number or quality of CDDs—did not seem to improve coverage. Perhaps this measure was deployed too late to generate more or better CDDs and insufficient to overcome other weaknesses in the program.
Though treatment coverage was low, compliance was very high. Refusals were generally below 2% in both rounds. Coverage was highly clustered within communities (intracluster correlation coefficients for IVM coverage were 0.72 in the first round and 0.54 in the second), indicating that upstream variables such as drug supply or the number and quality of drug distributors, be they health workers or volunteers, largely determine the outcomes of this treatment program.14,30 It also suggests that treatment is based on convenience or preference, rather than diligence toward universal coverage of the whole community31; IVM coverage among those living in treated EAs was only 45.4% (38.2–52.5%) in the first survey and 47.2% (39.1–55.3%) in the second. There was no statistical difference in coverage between rural (38.4%, 95% CI: 31.7–45.5%) and urban (30.8%, 95% CI: 21.4–42.1%) EAs (P = 0.25). Although we did not conduct a risk-factor analysis, we noted that having previously taken IVM was protective against refusal (round 1 odds ratio [OR]: 0.65, round 2 OR: 0.12), but these results were not statistically significant. Among the very few who refused treatment, most did so because of fear or worry rather than personal experience of adverse events. Overall, these results suggest that mobilization of people and resources for MDA is inadequate. Such a situation creates pockets of systematically unreached people, either through oversight or their own choices.32 Taken together, these data implicate the health system and drug distribution chain rather than widespread rejection of IVM by the population.
Reported coverage (calculated from treatment records), which never dropped below 70% of the target in either round (about 60% of the total population), was grossly overstated, consistent with others’ findings in many settings.33–37 This could be because of incomplete enumeration of targets and communities, inaccurate and outdated census information, inefficient drug distribution, or pressure to meet the program’s goals resulting in deliberate inflation.35,38–41 Political pressure both to achieve targets and to increase the population size can also distort reported coverage.
There were several limitations in these studies. Our teams were unable to reach a few insecure or inaccessible areas. We also fell short of our planned sample size in the second survey; however, we exceeded the minimum sample size of 1,556 in both groups. Errors in counting households by one team in the first survey initially led to overly optimistic weighted estimates of coverage; to counteract these errors, we used the average cluster size from the other clusters, resulting in consistently lower estimates overall. These estimates, although biased, more closely track the unweighted estimates. Such errors were not observed in the second survey. We found the SCT valuable for directing a mop-up but did not have time or resources to revisit every community and ensure mop-up actually occurred. Although the mop-up was assigned, whether it was carried out was left to the discretion of government personnel and CDDs, and was not assiduously traced. This emphasizes the importance of targeted, repeated supervision in a weak program, as well as greater accountability in delivering and tracking interventions both when trying new approaches and more generally. We did not look for gaps in the upstream drug delivery system and MDA planning and oversight, so we could not identify all the possible reasons for poor coverage. We did not examine drug inventories or reports in detail, which could also help identify issues. Furthermore, we do not have reliable figures on the ratio of population to active, engaged CDDs, nor whether the CDDs responsible for these communities participated in the relevant trainings. Without this information, we cannot yet determine whether increased supervision will make a difference in treatment coverage.
These five LGAs represent the first to be treated twice in 1 year for onchocerciasis in Nigeria to our knowledge, a strategy implemented to address poor impact despite years of MDA. The five LGAs are part of a cross-border transmission zone shared with Ondo state, which remains on annual MDA. This discordance could limit progress toward disease elimination even if coverage improves in Edo state due to any continued transmission in Ondo. Although coverage was less than ideal, the program did not collapse when twice-per-year treatment was launched; indeed, coverage in the standard CDTI arm showed significant improvement in the two-round scheme. Staff and volunteers were able to handle an additional MDA at similar levels of service as before. Treating more frequently could provide more opportunities to reach people who would otherwise have been missed, and more chances to see if changes within the system succeed.
Coverage surveys should be repeated after additional work is carried out to correct the problems identified. These include 1) increased recruitment and training of CDDs, 2) better advertising of MDA; 3) better engagement and oversight of endemic communities, and 4) investigation of supply and distribution issues within the health system. Although more must be carried out to eliminate onchocerciasis in this area, we are confident that the program can be strengthened to reach the coverage necessary for accomplishing this important goal.
Acknowledgments:
We would like to thank Katherine Gass, Scott Nash, Gregory Noland, Andrew Nute, Franca Olamiju and MITOSATH, Lindsay Rakers, Hiwote Solomon, and Paul Weiss for their assistance at various points in the preparation and analysis of these surveys. We must also acknowledge the hard work of the data collection teams and the graciousness of the respondents for sharing their time and experiences.
REFERENCES
- 1.World Health Organization , 2017. 681 Progress report on the elimination of human onchocerciasis, 2016–2017. Wkly Epidemiol Rec 92: 681–694. [PubMed] [Google Scholar]
- 2.Katabarwa MN, Richards FO, Jr., 2001. Community-directed health (CDH) workers enhance the performance and sustainability of CDH programmes: experience from ivermectin distribution in Uganda. Ann Trop Med Parasitol 95: 275–286. [DOI] [PubMed] [Google Scholar]
- 3.Katabarwa MN, Habomugisha P, Eyamba A, Byamukama E, Nwane P, Arinaitwe A, Musigire J, Tushemereirwe R, Khainza A, 2016. Community-directed interventions are practical and effective in low-resource communities: experience of ivermectin treatment for onchocerciasis control in Cameroon and Uganda, 2004–2010. Int Health 8: 116–123. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, Kamath A, 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 3: e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinbo FO, Okaka CE, 2010. Hyperendemicity of onchocerciasis in Ovia northeast local government area, Edo state, Nigeria. Malays J Med Sci 17: 20–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Akinbo FO, Okaka CE, 2010. Hyperendemicity of onchocerciasis in Ovia northeast local government area, Edo state, Nigeria. East Afr J Public Health 7: 84–86. [DOI] [PubMed] [Google Scholar]
- 7.Wogu M, Okaka C, 2008. Prevalence and socio-economic effects of onchocerciasis in Okpuje, Owan West local government area, Edo state, Nigeria. Int J Biomed Health Sci 4: 113–118. [Google Scholar]
- 8.Tekle AH, Zoure HG, Noma M, Boussinesq M, Coffeng LE, Stolk WA, Remme JH, 2016. Progress towards onchocerciasis elimination in the participating countries of the African programme for onchocerciasis control: epidemiological evaluation results. Infect Dis Poverty 5: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.APOC , WHO , 2010. Year 2010 Progress Report. Ouagadougou, Burkina Faso: World Health Organization, 33. [Google Scholar]
- 10.Otubanjo OA, Adeoye GO, Ibidapo CA, Akinsanya B, Okeke P, Atalabi T, Adejai ET, Braide E, 2008. Adverse reactions from community directed treatment with ivermectin (CDTI) for onchocerciasis and loiasis in Ondo state, Nigeria. Rev Biol Trop 56: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 11.Katabarwa M, Richards F, 2014. Twice-yearly ivermectin for onchocerciasis: the time is now. Lancet Infect Dis 14: 373–374. [DOI] [PubMed] [Google Scholar]
- 12.Cupp EW, Cupp MS, 2005. Short report: impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. Am J Trop Med Hyg 73: 1159–1161. [PubMed] [Google Scholar]
- 13.Budge PJ, Sognikin E, Akosa A, Mathieu EM, Deming M, 2016. Accuracy of coverage survey recall following an integrated mass drug administration for lymphatic filariasis, schistosomiasis, and soil-transmitted helminthiasis. PLoS Negl Trop Dis 10: e0004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker MC, Krotki K, Sankara DP, Trofimovich L, Zoerhoff KL, Courtney L, Chowdhury D, Linehan M, 2013. Measuring treatment coverage for neglected tropical disease control programs: analysis of a survey design. Am J Epidemiol 178: 268–275. [DOI] [PubMed] [Google Scholar]
- 15.Cromwell EA, Ngondi J, McFarland D, King JD, Emerson PM, 2012. Methods for estimating population coverage of mass distribution programmes: a review of practices in relation to trachoma control. Trans R Soc Trop Med Hyg 106: 588–595. [DOI] [PubMed] [Google Scholar]
- 16.Commission NP, International I, 2014. Nigeria Demographic and Health Survey 2013. Abuja, Nigeria and Rockville, MD: National Population Commission, Federal Republic of Nigeria and ICF International. [Google Scholar]
- 17.Center NTDS , 2016. Supervisor’s Coverage Tool Available at: http://www.ntdsupport.org/resources/supervisors-coverage-tool. Accessed December 6, 2017.
- 18.WHO , 2016. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human onchocerciasis: Criteria and Procedures. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 19.Plaisier AP, van Oortmarssen GJ, Habbema JD, Remme J, Alley ES, 1990. ONCHOSIM: a model and computer simulation program for the transmission and control of onchocerciasis. Comput Methods Programs Biomed 31: 43–56. [DOI] [PubMed] [Google Scholar]
- 20.Winnen M, Plaisier AP, Alley ES, Nagelkerke NJ, van Oortmarssen G, Boatin BA, Habbema JD, 2002. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull World Health Organ 80: 384–391. [PMC free article] [PubMed] [Google Scholar]
- 21.Cupp EW, Sauerbrey M, Richards F, 2011. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop 120 (Suppl 1): S100–S108. [DOI] [PubMed] [Google Scholar]
- 22.Higazi TB, et al. 2013. Interruption of Onchocerca volvulus transmission in the Abu Hamed focus, Sudan. Am J Trop Med Hyg 89: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarroug IM, et al. 2016. The first confirmed elimination of an onchocerciasis focus in Africa: Abu Hamed, Sudan. Am J Trop Med Hyg 95: 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick C, Fleming FM, Madin-Warburton M, Schneider T, Meheus F, Asiedu K, Solomon AW, Montresor A, Biswas G, 2016. Benchmarking the cost per person of mass treatment for selected neglected tropical diseases: an approach based on literature review and meta-regression with web-based software application. PLoS Negl Trop Dis 10: e0005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner HC, Osei-Atweneboana MY, Walker M, Tettevi EJ, Churcher TS, Asiedu O, Biritwum NK, Basanez MG, 2013. The cost of annual versus biannual community-directed treatment of onchocerciasis with ivermectin: Ghana as a case study. PLoS Negl Trop Dis 7: e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans D, McFarland D, Adamani W, Eigege A, Miri E, Schulz J, Pede E, Umbugadu C, Ogbu-Pearse P, Richards FO, 2011. Cost-effectiveness of triple drug administration (TDA) with praziquantel, ivermectin and albendazole for the prevention of neglected tropical diseases in Nigeria. Ann Trop Med Parasitol 105: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady MA, Hooper PJ, Ottesen EA, 2006. Projected benefits from integrating NTD programs in sub-Saharan Africa. Trends Parasitol 22: 285–291. [DOI] [PubMed] [Google Scholar]
- 28.Downs PW, Bardin LE, McFarland DA, 2014. Modeling the dynamics of incentives in community drug distribution programs. Trends Parasitol 30: 317–319. [DOI] [PubMed] [Google Scholar]
- 29.Fleming FM, Matovu F, Hansen KS, Webster JP, 2016. A mixed methods approach to evaluating community drug distributor performance in the control of neglected tropical diseases. Parasit Vectors 9: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katabarwa M, Habomugisha P, Eyamba A, Agunyo S, Mentou C, 2010. Monitoring ivermectin distributors involved in integrated health care services through community-directed interventions—a comparison of Cameroon and Uganda experiences over a period of three years (2004–2006). Trop Med Int Health 15: 216–223. [DOI] [PubMed] [Google Scholar]
- 31.Chami GF, Kontoleon AA, Bulte E, Fenwick A, Kabatereine NB, Tukahebwa EM, Dunne DW, 2017. Community-directed mass drug administration is undermined by status seeking in friendship networks and inadequate trust in health advice networks. Soc Sci Med 183: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanji S, et al. 2015. Relationship between oral declaration on adherence to ivermectin treatment and parasitological indicators of onchocerciasis in an area of persistent transmission despite a decade of mass drug administration in Cameroon. Parasit Vectors 8: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cromwell EA, Ngondi J, Gatpan G, Becknell S, Kur L, McFarland D, King JD, Emerson PM, 2009. Estimation of population coverage for antibiotic distribution for trachoma control: a comparison of methods. Int Health 1: 182–189. [DOI] [PubMed] [Google Scholar]
- 34.Worrell C, Mathieu E, 2012. Drug coverage surveys for neglected tropical diseases: 10 years of field experience. Am J Trop Med Hyg 87: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cromwell EA, King JD, McPherson S, Jip FN, Patterson AE, Mosher AW, Evans DS, Emerson PM, 2013. Monitoring of mass distribution interventions for trachoma in Plateau State, Nigeria. PLoS Negl Trop Dis 7: e1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huhn GD, et al. 2006. Vaccination coverage survey versus administrative data in the assessment of mass yellow fever immunization in internally displaced persons—Liberia, 2004. Vaccine 24: 730–737. [DOI] [PubMed] [Google Scholar]
- 37.Zuber PL, Yameogo KR, Yameogo A, Otten MW, Jr., 2003. Use of administrative data to estimate mass vaccination campaign coverage, Burkina Faso, 1999. J Infect Dis 187 (Suppl 1): S86–S90. [DOI] [PubMed] [Google Scholar]
- 38.Chami GF, Kontoleon AA, Bulte E, Fenwick A, Kabatereine NB, Tukahebwa EM, Dunne DW, 2016. Profiling nonrecipients of mass drug administration for schistosomiasis and hookworm infections: a comprehensive analysis of praziquantel and albendazole coverage in community-directed treatment in Uganda. Clin Infect Dis 62: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okpara EM, Mnaemeka AM, Iyioku UU, Udoh UV, 2015. Distribution and utilisation of ivermectin (Mectizan): a chemotherapeutic approach to the control of onchocerciasis in old Ohaozara Lga, Ebonyi state, eastern Nigeria. J Egypt Soc Parasitol 45: 663–670. [PubMed] [Google Scholar]
- 40.Tuhebwe D, Bagonza J, Kiracho EE, Yeka A, Elliott AM, Nuwaha F, 2015. Uptake of mass drug administration programme for schistosomiasis control in Koome Islands, central Uganda. PLoS One 10: e0123673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rakers LJ, Emukah E, Onyenama J, Amah G, Ukairo N, Enyinnaya U, Miri E, Richards F, 2009. Sustainability of ivermectin distribution programmes. Lancet 374: 785–786. [DOI] [PubMed] [Google Scholar]