Abstract.
Iron deficiency (ID) and human immunodeficiency virus (HIV) infection frequently coexist. Little data exist on ID in HIV-infected individuals, partly because the iron marker ferritin is altered by inflammation common in HIV infection. We measured iron biomarkers (ferritin, soluble transferrin receptor [sTfR], hepcidin) and red cell indices (hemoglobin, mean corpuscular volume [MCV]) in newly diagnosed, antiretroviral therapy-naive, HIV-infected (N = 138) and uninfected (N = 52) Kenyan adults enrolled in a study of the immune response to malaria. We compared markers between infected and uninfected groups with t test and Wilcoxon Rank–Sum, used Spearman correlation to determine the association between iron and inflammatory markers, and applied logistic regression to determine which markers best predicted anemia. HIV-infected individuals had lower hemoglobin (P < 0.001), lower MCV (P < 0.001), higher sTfR (P = 0.003), and a greater prevalence of ID (sTfR > 8.3 mg/L) than uninfected individuals. Ferritin was elevated in HIV-infected individuals and was more strongly correlated with C-reactive protein (ρ = 0.43, P < 0.001) and hepcidin (ρ = 0.69, P < 0.001) than with hemoglobin. The best predictor of anemia in HIV-infected participants was sTfR, with a one log-unit increase in sTfR associated with a 6-fold increase in the odds of anemia (odds ratio = 6.3, 95% confidence interval: 1.8–21.8). These data suggest a significant burden of ID among treatment-naive HIV-infected Kenyan adults. Soluble transferrin receptor may be a reliable marker of ID in HIV-mediated inflammation.
INTRODUCTION
Iron deficiency (ID) is the most common nutritional deficiency in the world1–3 and a leading cause of disability in 27 countries.4 Iron deficiency in childhood is an established cause of lost developmental potential,5,6 whereas ID in adults limits work productivity and physical energy. Maternal ID is associated with preterm birth, perinatal risk for the mother, and greater risk of subsequent ID in the infant.7
In many regions where ID is most common, HIV infection is also prevalent. Iron deficiency is thought to be prevalent among HIV-infected individuals, but its accurate diagnosis and contribution to anemia in this population are unclear, primarily because of the lack of a reliable biomarker for diagnosis. The iron storage protein ferritin is one of the most frequently measured markers of iron status, with low blood concentrations reflecting dietary ID. However, ferritin is also an acute phase reactant that rises with the chronic inflammation, a hallmark of HIV infection.8 The HIV protein Nef also directly induces the production of ferritin in macrophages.9 As a result, ferritin is often elevated in HIV-infected individuals regardless of dietary iron status. The prevalence of ID is, thus, likely underestimated in HIV-infected populations when ferritin alone is used because this biomarker is reflecting the chronic inflammation of HIV rather than body iron stores.8
Lack of an accurate marker to diagnose ID in the context of HIV prohibits determination of the contribution of ID to anemia in HIV-infected individuals. Anemia is a consistent predictor of hastened disease progression and mortality in HIV-infected children and adults.10–12 Iron deficiency is the most common cause of anemia worldwide and may be a significant, treatable contributor to anemia in HIV, underscoring the necessity of its accurate diagnosis and assessment of its contribution to anemia in this population.
To fully characterize the iron biomarker profile in HIV-infected adults and to assess the effect of inflammation and HIV disease severity on markers of iron status in this group, we recently measured a panel of iron status and inflammatory biomarkers in newly diagnosed, ART-naive, HIV-infected Kenyan adults and compared them with those of HIV-uninfected adults. We also assessed the association of each biomarker with anemia in HIV-infected and uninfected participants. Identification of markers that are resistant to inflammation and also relate to anemia would help HIV practitioners better diagnose dietary ID in the face of chronic inflammation and guide the safe and effective provision of iron therapy to correct ID and anemia in HIV-infected individuals.
METHODS
Enrollment, clinical testing, and specimen collection.
Venous blood samples were collected from HIV-infected (N = 138) and uninfected (N = 52) individuals aged 18 years or older at the time of HIV testing at the Bondo Sub-County Hospital, in Bondo township, Western Kenya. All HIV-infected individuals who were seen in the voluntary testing and counseling clinic on enrollment dates between May and October 2012 were offered participation in the parent study that examined the immune response to malaria in HIV.13 Temporally, one HIV-uninfected individual was enrolled for every time three HIV-infected individuals were enrolled to ensure that samples from comparison groups were collected with a similar distribution throughout the malaria season. There was a larger number of HIV-infected individuals enrolled so that outcomes could be compared by CD4 count among HIV-infected individuals. Each HIV-infected participant was antiretroviral naive and was not on any opportunistic infection prophylaxis at the time of blood collection. Individuals with chronic medical conditions, on immunosuppressants, with acute systemic illness including fever (≥ 37.5°C), who were pregnant, or who were taking any antimalarials were excluded.
Patients were assessed for active malaria infection and helminth infection at the time of the collection with commercial rapid diagnostic testing (Carestart HRP2 [Pf], Access Bio, Sommerset, NJ) and Kato Katz examination, respectively. Hemoglobin, mean corpuscular volume (MCV), and CD4 counts were measured in the core clinical laboratory at the Bondo Sub-County Hospital. Anemia was defined according to the World Health Organization definition as hemoglobin < 120 g/L in women and < 130 g/L in men.7 Low MCV was defined as two standard deviations less than the mean based on age and gender (female < 80 fL/cell, male < 81 fL/cell).7 Microcytic anemia was defined as having both low MCV and anemia. Viral load testing was performed by the Centers for Disease Control and Prevention laboratory in Kisumu, Kenya, from dried blood spots (Abbott Laboratories, Abbott Park, IL).14 Plasma samples were stored at −80°C until testing for iron biomarkers at the University of Minnesota.
Plasma iron and inflammatory biomarker measurement.
enzyme-linked immunosorbent assay (ELISA) was used to measure plasma concentrations of ferritin (Ramco Laboratories, Stafford, TX), soluble transferrin receptor ([sTfR], Ramco Laboratories), C-reactive Protein ([CRP] Millipore Corporation, Darmstadt, Germany), and hepcidin (Peninsula Laboratories, San Carlos, CA). Hepcidin concentrations were measured in a subset of participants (95 HIV-infected and 47 HIV-uninfected participants). The prevalence of ID was calculated using two different definitions: 1) ferritin < 15 μg/L15; or 2 sTfR 8.3 mg/L or higher.16
Ethical considerations.
All subjects underwent written informed consent. The Ethics Committee at the Kenya Medical Research Institute and the Institutional Review Board at the University of Minnesota approved all study procedures.
Statistical analysis.
For indicators with a normal distribution (hemoglobin, MCV), a t test was used to compare means between HIV-infected and uninfected individuals. For those with a non-Gaussian distribution (ferritin, sTfR, CRP, and hepcidin), medians were compared using a Wilcoxon rank–sum test. Proportions of conditions including anemia and ID were compared between HIV-infected and uninfected individuals using χ2 test.
To understand the relationship between iron status and HIV, Spearman rank correlation was first used to investigate the association between iron markers and markers of HIV disease severity and inflammation (HIV viral load, CD4 count, and CRP). Logistic regression models to determine biomarkers associated with anemia were generated separately for HIV-infected and HIV-uninfected individuals. For all participants, models were generated using the following covariates: ferritin, sTfR, CRP (each log-transformed), MCV, and helminth infection status. HIV1 viral load and CD4 count was also included in models in HIV-infected subjects. Models were generated in a forward stepwise manner with αcrit of 0.20, i.e., any covariate with a P value of 0.20 or less was retained in the model.
RESULTS
HIV-infected individuals were an average of 4 years older than HIV-uninfected participants at the time of enrollment (Table 1). Of note, the prevalence of two major causes of anemia in this region of Kenya—malaria and intestinal helminth infection—were similar between the groups. Among HIV-infected participants, there was a broad distribution of CD4 counts (Range: 4–1,177), including 30.7% who met the CD4 count threshold for acquired immunodeficiency syndrome of under 200 cells/mL (Table 1).
Table 1.
Clinical characteristics of study participants
| HIV-uninfected | HIV-infected | P value* | |
|---|---|---|---|
| N | 52 | 138 | – |
| Age, years, mean (SD) | 28.4 (11.5) | 32.2 (10.3) | 0.03 |
| Female gender, n (%) | 27 (51.9) | 84 (60.9) | 0.26 |
| Malaria-infected, n (%) | 3 (5.8) | 11 (8.0) | 0.60 |
| Helminth infection, n (%) | 9 (17.6)† | 31 (22.6)‡ | 0.46 |
| CD4 count (cells/mL), median (IQR§) | – | 301 (180–476)‡ | – |
| CD4 count < 200 n (%) | – | 42 (30.7)‡ | – |
| HIV-1 viral load (copies, mL), median (IQR§) | – | 50,370 (14,546–198,155)‡ | – |
SD = standard deviation.
P value comparing HIV-uninfected to HIV-infected participants from t test for means and χ2 for proportions.
N = 51.
N = 137.
IQR = interquartile range: 25th percentile, 75th percentile.
The mean hemoglobin was lower and prevalence of anemia higher in HIV-infected compared with HIV-uninfected participants (Table 2). HIV-infected individuals also had a significantly lower MCV and higher prevalence of microcytic anemia when compared with HIV-uninfected individuals. Despite the lower MCV concentrations and greater prevalence of microcytic anemia, median ferritin concentrations were significantly higher in HIV-infected individuals, likely reflecting the greater inflammation present in this group, as evidenced by this group’s significantly higher CRP concentrations (Table 2). Using ferritin to define ID, we found no significant difference in the prevalence of ID between HIV-infected and HIV-uninfected individuals (HIV negative: 26.9% ID versus HIV positive: 20.3% ID, P = 0.33, Table 2). However, median sTfR concentrations was significantly higher in HIV-infected versus uninfected participants, and according to the sTfR definition, the prevalence of ID was significantly higher in HIV-infected individuals (Table 2) and more in accordance with the MCV and microcytic anemia findings. Median hepcidin was not significantly different between the groups.
Table 2.
Iron and inflammatory markers by HIV status
| HIV-uninfected | HIV-infected | P value* | |
|---|---|---|---|
| N | 52 | 138 | |
| Hemoglobin, g/L, mean (SD)†‡ | 140 (23) | 111 (27) | < 0.001 |
| Anemia, n (%)†§ | 10 (19.6) | 86 (65.2) | < 0.001 |
| Mean cell volume (fL/cell), mean (SD)†‡ | 85.1 (9.4) | 76.8 (12.7) | < 0.001 |
| Microcytic anemia, n (%)†§‖ | 5 (9.8) | 62 (47.0) | < 0.001 |
| Ferritin, μg/L, median (IQR)¶ | 27.6 (12.0–65.7) | 51.5 (17.8–166.1) | 0.007 |
| C-reactive protein, mg/L median (IQR)¶ | 0.5 (0.3–1.1) | 4.7 (0.9–26.1) | < 0.001 |
| Soluble transferrin receptor, mg/L, median (IQR)¶ | 4.2 (2.9–5.1) | 4.8 (3.5–7.4) | 0.003 |
| Hepcidin, ng/mL, median (IQR)¶# | 9.1 (0.7–19.8) | 10.2 (2.0–19.8) | 0.30 |
| ID by ferritin criteria, n (%)** | 14 (26.9) | 28 (20.3) | 0.33 |
| ID by sTfR criteria, n (%)†† | 3 (5.8) | 26 (18.8) | 0.03 |
ID = iron deficiency.
P value determined by t test for means, Wilcoxon rank–sum test for medians, and χ2 test for categorical outcomes.
N = 51 for HIV-uninfected and 132 for HIV-infected.
SD = standard deviation.
Anemia defined as < 12 g/dL in women and < 13 g/dL in men.
Microcytosis defines as < 80 fL/cell in women and < 81 fL/cell in men.
IQR = interquartile range: 25th percentile, and 75th percentile.
N = 47 for HIV-uninfected and 95 for HIV-infected.
ID = ferritin < 15 μg/L.
ID = sTfR ≥ 8.3 mg/L.
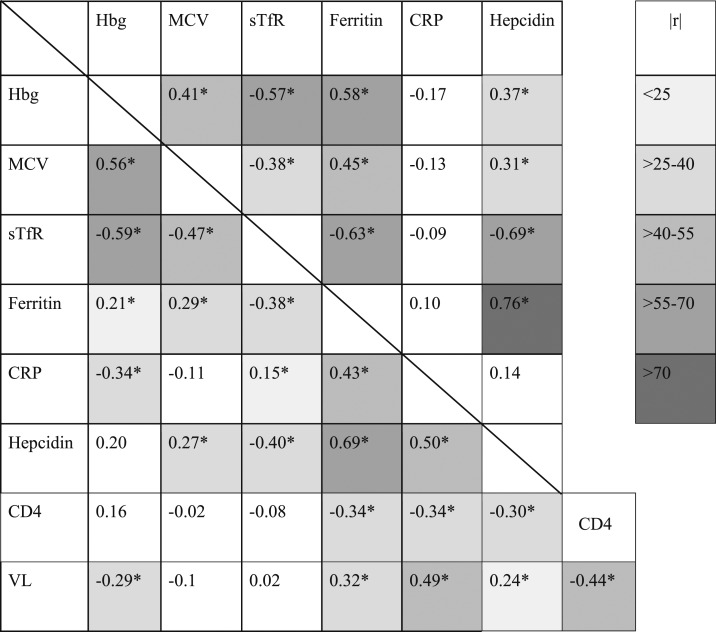
Among HIV-uninfected individuals, sTfR (ρ = −0.57, P < 0.01) and ferritin (ρ = 0.59, P < 0.01) were most strongly correlated with hemoglobin (Table 3), and the direction of these associations reflected the expected relationship between iron status and hemoglobin. There was also a strong inverse association between ferritin levels and sTfR levels (Table 3, ρ = −0.63, P < 0.01), as expected in the absence of inflammation. Of note, CRP was not significantly correlated with any iron marker in HIV-negative participants. However, among HIV-infected individuals MCV (ρ = 0.56, P < 0.01) and sTfR (ρ = −0.59, P < 0.01) were strongly associated with hemoglobin, whereas ferritin was less strongly associated with hemoglobin than was seen in HIV-uninfected individuals and in the opposite direction (Table 3, ρ = 0.21, P < 0.05). The correlation between ferritin and sTfR was also weaker in HIV-infected individuals (Table 3, ρ = −0.38, P < 0.01). C-reactive protein was significantly and strongly correlated with ferritin and hepcidin, with a much weaker correlation between CRP and sTFR, and had no significant correlation between CRP and MCV (Table 3).
Table 3.
Spearman rank correlation analysis of hemoglobin, MCV, and iron homeostasis markers
MCV = mean cell volume. Values shown are Spearman rho calculation. HIV-uninfected shown above and to the right of the diagonal line. HIV-infected shown to the bottom and left of diagonal line, * indicates a correlation with a P value of less than 0.05. Greyscale highlighting indicates the strength of the correlation based on the rho value for all relationships with a significant correlation. The legend on the right shows the highlighting associated with the rho values shown on the table. This table appears in color at www.ajtmh.org.
Using logistic regression to model predictors of anemia and a criterion for inclusion in the model (αcrit of 0.20), we found that ferritin, CRP, sTfR, and helminth status met the inclusion criterion among HIV-uninfected individuals, but only ferritin and CRP reached a statistically significant relationship with anemia (P < 0.5). A one log-unit decline in ferritin (log-transformed) was statistically significantly associated with a 92% increase in the odds of anemia (odds ratio [OR] = 0.08, 95% confidence interval [CI]: 0.01–0.8; P = 0.03). Higher CRP was associated with increased odds of anemia (CRP [log-transformed]: OR = 3.8, 95% CI: 1.0–13.7; P = 0.04). The relationship between ferritin and anemia was maintained when controlling for the other covariates.
In HIV-infected individuals, sTfR, CRP, MCV, viral load, and ferritin all met the inclusion criterion for the anemia model, but only sTFR, CRP, and MCV were statistically significantly related to the odds of anemia in HIV-infected participants. Higher sTfR, higher CRP, and lower MCV were associated with increased odds of anemia (sTfR [log-transformed]: OR = 6.3, 95% CI: 1.8–21.8; P < 0.001; CRP (log-transformed): OR = 1.5, 95% CI: 1.1–2.2, P = 0.003; MCV: OR = 0.94, 95% CI: 0.90–0.98, P = 0.008) when controlling for other covariates. Ferritin, on its own or when controlling for other iron biomarkers, was not statistically associated with anemia in the setting of HIV infection and failed to meet the inclusion criterion for the logistic regression model. Of note, helminth infection status was not statistically significantly associated with anemia in HIV-positive or HIV-negative individuals, and its inclusion in the models did not affect relationships of ferritin and sTfR to anemia in either group.
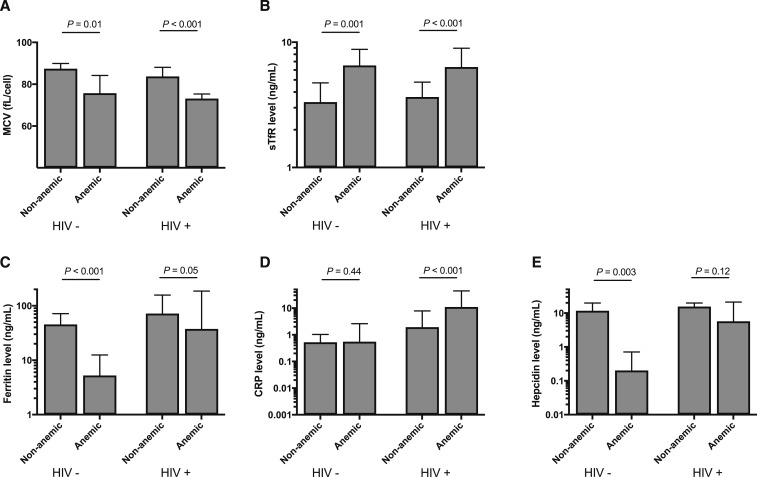
When we stratified by anemia status, differences in relationships between biomarkers in the HIV-uninfected versus infected groups emerged and mirrored the logistic regression findings (Figure 1). In the absence of HIV, iron biomarkers and their relation to anemia reflected the expected relationships with dietary ID—ferritin, hepcidin, and MCV were significantly lower among anemic individuals, whereas sTfR was higher. C-reactive protein was low and not different between anemic and non-anemic, HIV-uninfected individuals. By contrast, among HIV-infected individuals, CRP was significantly higher among anemic participants. Ferritin, a marker affected by inflammation, was correspondingly higher among anemic, HIV-infected individuals when compared with anemic uninfected individuals (P < 0.001), but sTfR and MCV both retained their expected relationship to ID, being significantly higher and lower, respectively, among anemic versus non-anemic HIV-infected participants. Hepcidin concentration was not different between anemic versus non-anemic HIV-infected individuals, perhaps reflecting the dual and opposing pulls on the marker of inflammation, which results in higher hepcidin concentrations, and dietary ID that leads to lower hepcidin concentrations.
Figure 1.
Iron status markers by anemia and HIV status. Bars represent mean with 95% confidence interval (mean cell volume [MCV]) and median with interquartile range (soluble transferrin receptor [sTfR], ferritin, C-reactive protein [CRP] and hepcidin). Significance between groups was evaluated using student’s T test (MCV) or Wilcoxon Rank–Sum testing (sTfR, ferritin, CRP, and hepcidin).
DISCUSSION
Currently, little is known on the burden of ID among HIV-infected adults living in sub-Saharan Africa or whether ID contributes to anemia in this population. Our study demonstrates that ID is prevalent in HIV-infected adults and that higher sTfR and lower MCV are significantly associated with anemia in this newly diagnosed, HIV-positive population. This finding suggests that correcting ID, as defined by an elevated sTfR concentration or low MCV, may ameliorate anemia, an established predictor of HIV progression and mortality.
In our population from western Kenya, nearly half of the HIV-infected individuals suffered from microcytic anemia, a rate four times higher than HIV-uninfected individuals. HIV-infected study participants also had a significantly higher elevation in plasma sTfR concentrations and higher prevalence of ID based on sTfR concentrations than uninfected individuals. These differences in MCV and sTfR were not apparent when using ferritin alone, as this indicator was elevated along with CRP in HIV-infected individuals, consistent with HIV-mediated immune activation and inflammation and also with the anemia of chronic disease that is common in individuals with HIV. We additionally found that ID, based on sTfR and MCV, is an important contributor to anemia in HIV-infected adults, but this contribution is obscured if using ferritin alone. Overall, these data suggest that in the setting of untreated HIV, ferritin should not be primarily relied on for the diagnosis of ID. This observation is supported by the existing literature on the impact of acute inflammation on most iron homeostasis biomarkers including not only ferritin, but also serum iron, total iron binding capacity, and transferrin concentration.17 Early examinations of the impact of iron status on HIV disease outcomes has relied heavily on these markers and may reflect the degree of inflammation rather than true iron status.18
The strongest predictor of hemoglobin among HIV-infected individuals in our study was sTfR, suggesting that this marker may be a good candidate for iron status evaluation in HIV-infected individuals. We estimated the prevalence of ID with sTfR using a threshold (8.3 mg/L) based on previous observations in inflammatory joint and bowel diseases.16,17 The relatively low prevalence of ID by sTfR criteria alone when compared with the prevalence of ferritin-diagnosed ID and microcytic anemia among HIV-uninfected individuals, suggests a cutoff of 8.3 mg/L may not be sensitive enough in our population. Among HIV-uninfected individuals with ID based on a ferritin value lower than 15 μg/L, the median (p25–p75) sTfR was 5.5 mg/L (4.4–8.0 mg/L). Furthermore, the high prevalence of ID using ferritin among HIV-negative individuals in our study suggests that this is still a useful tool in evaluating iron status among adults in sub-Saharan Africa. Indeed, ferritin is more sensitive in mild ID.19 Before sTfR can be more widely used for the diagnosis of ID in this population, a validated cutoff for the diagnosis of ID using this marker should be evaluated with adults living in sub-Saharan Africa, where HIV is highly prevalent before making this biomarker a tool for ID diagnosis. Although dried blood spot methods have been developed, measuring sTfR requires an ELISA assay or other automated method, which precludes its immediate implementation as a widely used screening tool in sub-Saharan Africa and other resource-limited settings.
Despite the apparent high prevalence of ID among HIV-infected individuals in Kenya and its significant contribution to anemia, additional research weighing the risks versus benefits of iron treatment of the correction of anemia in this population is needed. In untreated HIV, elevated hepcidin levels and ferritin levels, as seen in our study and previous research in other populations, are suggestive of increased iron sequestration in macrophages.20 Paired with the impaired intracellular immunity in HIV, iron supplementation interventions must be evaluated for safety. Elevated ferritin levels may predict tuberculosis susceptibility21 and mouse models have demonstrated increased Salmonella burden in mice with increased intracellular iron stores.22 Furthermore, the increased risk of malaria with iron supplementation is well established in the absence of malaria control and prevention efforts.23 Accordingly, aggressive iron repletion may lead to increased susceptibility to infections with an already high burden in this population, including tuberculosis, Salmonella, and malaria.23–26
However, the finding that ID is an important contributor to anemia in this population is a potentially significant counterbalance to this infection risk and raises the question of whether correcting anemia with safe iron replenishment might lead to improved health. Anemia is a consistent predictor of HIV disease progression and mortality. Whether the cost of iron replenishment in terms of potential subsequent infection is too great is unclear, but not knowing where this delicate balance points lies emphasizes the urgent need for future trials aimed at determining the safety of iron in HIV-infected adult populations and establishing the mechanism for any increased risk of infection.
Finally, our cohort is drawn from HIV-infected individuals before treatment. There should be additional studies among individuals on antiretroviral therapy to determine if iron status improves and if ferritin is a more reliable marker of ID with the treatment of HIV. If ART alone is sufficient to improve iron status, this would be another strong argument for early initiation of therapy in limited-resource settings, particularly areas with an already high burden of ID. Overall, our data brings to light a significant public health issue that significantly impairs the productivity and wellness of HIV-infected individuals. There should be particular concern about the implications of our findings on high-risk populations including pregnant women and children living with HIV.
Acknowledgments:
This work is published with the permission of the office the Director of the Kenya Medical Research Institute.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.World Health Organization Vitamin and Mineral Nutrition Information System (VMNIS): Micronutrient Database Geneva, Switzerland: WHO. Available at: http://www.who.int/vmnis/database/en/. Accessed December 4, 2015.
- 2.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B, 2009. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12: 444–454. [DOI] [PubMed] [Google Scholar]
- 3.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group , 2007. Developmental potential in the first 5 years for children in developing countries. Lancet 369: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators , 2016. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engle PL, Black MM, Behrman JR, Cabral de Mello M, Gertler PJ, Kapiriri L, Martorell R, Young ME; International Child Development Steering Group , 2007. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet 369: 229–242. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T, 2006. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64: S34–S43; discussion S72–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO , 2001. Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 8.WHO/Centers for Disease Control and Prevention , 2007. Assessing the Iron Status of Populations: Including Literature Reviews: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 9.Swingler S, Zhou J, Swingler C, Dauphin A, Greenough T, Jolicoeur P, Stevenson M, 2008. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe 4: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belperio PS, Rhew DC, 2004. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med 116 (Suppl 7A): 27S–43S. [DOI] [PubMed] [Google Scholar]
- 11.Clark TD, Mmiro F, Ndugwa C, Perry RT, Jackson JB, Melikian G, Semba RD, 2002. Risk factors and cumulative incidence of anaemia among human immunodeficiency virus-infected children in Uganda. Ann Trop Paediatr 22: 11–17. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, dArminio Monforte A, Ledergerber B, Lundgren JD, 1999. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS 13: 943–950. [DOI] [PubMed] [Google Scholar]
- 13.Frosch AE, Odumade OA, Taylor JJ, Ireland K, Ayodo G, Ondigo B, Narum DL, Vulule J, John CC, 2017. Decrease in numbers of naive and resting B cells in HIV-infected Kenyan adults leads to a proportional increase in total and Plasmodium falciparum-specific atypical memory B cells. J Immunol 198: 4629–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marconi A, Balestrieri M, Comastri G, Pulvirenti FR, Gennari W, Tagliazucchi S, Pecorari M, Borghi V, Marri D, Zazzi M, 2009. Evaluation of the Abbott Real-Time HIV-1 quantitative assay with dried blood spot specimens. Clin Microbiol Infect 15: 93–97. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization , 2011. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations: Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: World Health Organization.
- 16.Ramco Laboratories Inc , 2016. An In Vitro Enzyme Immunoassay for Quantifying Human Transferrin Receptor in Serum or Plasma as an Aid in the Diagnosis of Iron Deficiency Anemia, Particularly in the Presence of Other Disease States. St. Ingbert, Germany: Ramco Laboratories Inc. [Google Scholar]
- 17.Chiari MM, Bagnoli R, De Luca PD, Monti M, Rampoldi E, Cunietti E, 1995. Influence of acute inflammation on iron and nutritional status indexes in older inpatients. J Am Geriatr Soc 43: 767–771. [DOI] [PubMed] [Google Scholar]
- 18.McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, Jeffries D, Awasana AA, Whittlex AA, Prentice A, 2007. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr 46: 498–507. [DOI] [PubMed] [Google Scholar]
- 19.Choi JW, 2005. Sensitivity, specificity, and predictive value of serum soluble transferrin receptor at different stages of iron deficiency. Ann Clin Lab Sci 35: 435–439. [PubMed] [Google Scholar]
- 20.Armitage AE, et al. 2014. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci USA 111: 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermid JM, Hennig BJ, van der Sande M, Hill AV, Whittle HC, Jaye A, Prentice AM, 2013. Host iron redistribution as a risk factor for incident tuberculosis in HIV infection: an 11-year retrospective cohort study. BMC Infect Dis 13: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DK, et al. 2014. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med 20: 419–424. [DOI] [PubMed] [Google Scholar]
- 23.Sazawal S, et al. 2006. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367: 133–143. [DOI] [PubMed] [Google Scholar]
- 24.Kortman GA, Boleij A, Swinkels DW, Tjalsma H, 2012. Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One 7: e29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lounis N, Truffot-Pernot C, Grosset J, Gordeuk VR, Boelaert JR, 2001. Iron and Mycobacterium tuberculosis infection. J Clin Virol 20: 123–126. [DOI] [PubMed] [Google Scholar]
- 26.Murray MJ, Murray AB, Murray MB, Murray CJ, 1978. The adverse effect of iron repletion on the course of certain infections. Br Med J 2: 1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]