Abstract.
Aedes aegypti (L.) (Diptera: Culicidae) have a global distribution and are the primary vector of a number of mosquito-borne viruses responsible for epidemics throughout the Americas. As in much of South America, the threat from pathogens including dengue virus (DENV; Flaviviridae, Flavivirus) and chikungunya virus (CHIKV; Togaviridae, Alphavirus) has increased in Argentina in recent years. The complexity of transmission cycles makes predicting the occurrence and intensity of arbovirus outbreaks difficult. To gain a better understanding of the risk of DENV and CHIKV in Argentina and the factors influencing this risk, we evaluated the role of population and temperature in the vector competence and vectorial capacity (VC) of Ae. aegypti from geographically and ecologically distinct locations. Our results demonstrate that intrinsic and extrinsic factors including mosquito population, viral species, and temperature significantly influence both vector competence and overall VC of Ae. aegypti in Argentina, yet also that the magnitude of these influences is highly variable. Specifically, results suggest that CHIKV competence is more dependent on mosquito genetics than is DENV competence, whereas temperature has a greater effect on DENV transmission. In addition, although there is an overall positive correlation between temperature and competence for both viruses, there are exceptions to this for individual virus–population combinations. Together, these data establish large variability in VC for these pathogens among distinct Ae. aegypti populations in Argentina and demonstrate that accurate assessment of arbovirus risk will require nuanced models that fully consider the complexity of interactions between virus, temperature, mosquito genetics, and hosts.
INTRODUCTION
Aedes aegypti (L.) (Diptera: Culicidae), an African native species of mosquito, has expanded across the world’s tropics and is now omnipresent in tropical and subtropical urban centers.1 This distribution, together with a preference for human hosts, frequent blood feeding, and competence for pathogens such as yellow fever virus (YFV; Flaviviridae, Flavivirus), dengue virus (DENV; Flaviviridae, Flavivirus), Zika virus (ZIKV; Flaviviridae, Flavivirus), and chikungunya virus (CHIKV; Togaviridae, Alphavirus), makes Ae. aegypti the most important vector of arthropod-borne viruses (arboviruses) worldwide. Although major eradication programs during the mid-20th century achieved some control in Central and South America, the species was reintroduced into Argentina from Brazil in 1986 and now is regularly responsible for driving major arbovirus epidemics in the region.2
Dengue virus is the most prevalent arbovirus worldwide, including in South America. Although most of the DENV activity has been in Brazil, neighboring countries, including Argentina, have also experienced significant outbreaks over the last decade. Dengue virus reemerged in Argentina in 1997 following more than 80 years without a reported case.3 In 2009, a significant outbreak with more than 27,000 cases occurred in northern Argentina, and in 2016, the largest recorded outbreak of DENV in the country occurred, with more than 41,000 confirmed cases and 79,000 probable cases. Although CHIKV activity has been much less significant than DENV, 2016 also marked the first year that autochthonous transmission of CHIKV was recorded in Argentina.4
Whereas the recent activity in Argentina clearly highlights an expanding threat from arboviruses in the region, the complexity of transmission cycles makes predicting the occurrence and intensity of these outbreaks difficult. Multiple dynamic factors influence arbovirus transmission including extrinsic factors such as temperature, rainfall, and land use, and intrinsic factors such as mosquito genetics and immunity.5 Increasing temperatures accelerate virus replication in mosquitoes and, therefore, have generally been shown to decrease extrinsic incubation periods (EIPs) and increase overall competence.6–8 Fluctuations in temperature and daily extremes may be particularly important and, consequently, experimental studies which have used constant, rather than cycling, temperatures might not accurately represent the influence of temperature on competence.9–12 Despite enhancing competence, higher temperatures can also decrease overall vectorial capacity (VC) in some populations or species because of decreased longevity and/or feeding rates.13,14 A number of studies have demonstrated population-specific competence of mosquitoes for arboviruses,15 including with DENV and Ae. aegypti.16–19 Furthermore, studies have shown that in addition to the independent influence of these factors, specific interactions between mosquito genotype and temperature likely influence transmission in nature.20,21 To gain a better understanding of the risk of DENV and CHIKV in Argentina and the factors influencing this risk, we evaluated the role of population and temperature in the vector competence and VC of Ae. aegypti from geographically and ecologically distinct locations. Our results highlight the relative importance of these factors and the need to consider genetic and environmental interactions when evaluating the regional threat from arboviruses.
MATERIALS AND METHODS
Mosquitoes.
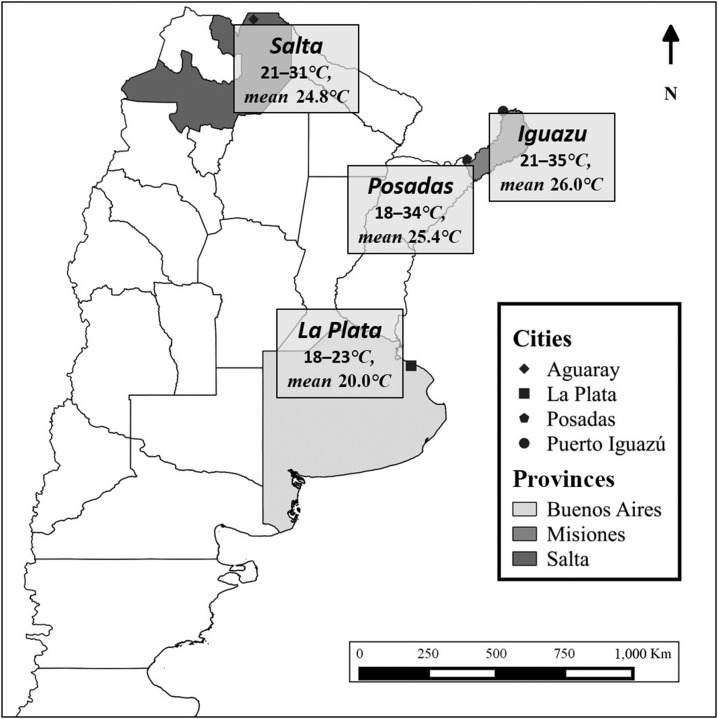
Mosquitoes originated from four geographically and environmentally distinct regions (Figure 1). These included Aguaray (22°14′30″ S 63°44′00″ W) in Salta Province (Salta), La Plata (34°55′07″ S 57°57′15″ W) in Buenos Aires Province (La Plata), Posadas (27°21′42″ S–55°54′15″ W) in Misiones Province (Posadas), and Puerto Iguazú (25°35′49″ S–54°34′42″ W) in Misiones Province (Iguazu). Mosquito eggs were collected on paddle sticks from artificial containers at each of the four study sites in February and March of 2014 and reared to adults in the laboratory of Centro de Estudios Parasitológicos y de Vectores (CEPAVE), CCT-La Plata-CONICET-UNLP. Following identification of adults morphologically and blood feeding, first generation (F1) eggs were collected and sent to the Arbovirus Laboratory at the Wadsworth Center, New York State Department of Health, for colonization and experimentation. Mosquitoes were maintained at 27°C under standard rearing conditions before experimentation.22 All experiments were carried out with F1–F4 mosquitoes.
Figure 1.
Study sites in Argentina where Aedes aegypti populations were obtained. Mosquitoes were trapped as larvae during February of 2014 and temperatures were monitored during the same period. F1–F4 mosquitoes were used for experiments.
Vector competence.
Dengue virus 306 (serotype 2)23 and CHIKV 9107724 stocks were used to infect confluent monolayers of C6/36 cells in T-75 flasks. Dengue virus 306 was originally isolated from the serum of an infected patient in Nicaragua in 2008.23 Chikungunya virus 91077, which belongs to the East Central South African genotype, was originally isolated from the serum of an infected patient in the United States in 2006, following travel to India.24 Following three (CHIKV) or five (DENV) days, cells and supernatant containing freshly propagated viruses were mixed 1:1 with bovine blood (Colorado Serum Co, Denver, CO) to create infectious blood meals. Approximately 400 3- to 7-day-old Ae. aegypti females housed with ∼50 males in 1-L mesh-top cardboard holding cups were offered infectious blood meals in porcine sausage casing for 1 hour following heating to 37°C. Mosquitoes were anesthetized on ice and fully engorged females were saved for subsequent experimentation. Mosquitoes were held in separate environmental chambers at either site-specific or mean cycling hourly temperature regimes based on environmental monitoring at individual sites in February and March of 2014. Temperature ranges for each site were as follows (Supplemental Table 1): Salta (21–31°C, mean 24.8°C, median 24°C), Iguazu (21–35°C, mean 26.0°C, median 25°C), Posadas (18–34°C, mean 25.4°C, median 25.5°C), and La Plata (18–23°C, mean 20.0°C, median 20°C). The mean temperature regimen ranged from 20°C to 30°C with a mean of 24.2°C and a median of 24°C. Infection and dissemination were determined by testing bodies or legs, respectively, from 20 to 30 individuals at 5, 10, 14, and 21 days post-feeding. For infectivity experiments, mosquitoes were fed 3–4 doses with 10-fold virus dilutions ranging from approximately 107–104 pfu/mL blood. Virus was identified by plaque assay on Vero cell culture.25 Rates and infectivity curves were compared among groups with GraphPad Prism 5.0 (La Jolla, CA) using χ2 tests and linear regression analyses.
Vectorial capacity.
Vectorial capacity is defined here as the probability of virus transmission following exposure to an infectious host.
| (1) |
where h is the host feeding rate, p is the probability of daily survival, N is the mean EIP, and b is the vector competence (proportion of exposed mosquitoes developing disseminated infections). Variables h and p were calculated based on experimental assessment of life history traits under the same conditions at CEPAVE.26 Specifically, h is the proportion of mosquitoes acquiring at least two artificial blood meals during their lifetime and p = (100 − [−slope]) of the best-fit linear relationship between mortality and time. N and b were calculated based on vector competence experiments, where b represents the proportion of exposed mosquitoes capable of transmitting (day 21 dissemination rate) and N represents the time point at which 50% of b is reached, which was extrapolated from the linear relationship between time and transmission. Linear regression analyses were completed using GraphPad Prism 5.0.
Mosquito genetics.
Multilocus genotype analysis was completed on 39 mosquitoes (10 from each population except Posadas where only nine were successfully genotyped) using the SNPChip.27 Specifically, specimens were individually genotyped at 24,412 previously identified single nucleotide polymorphic (SNP) sites.27 Statistical analyses were performed on a subset of 6,276 sites found to be polymorphic across the Argentine Ae. aegypti populations examined. We evaluated genetic discontinuities among populations using a multilocus Bayesian clustering method implemented by the software FastSTRUCTURE28 which assigns individuals to genetic clusters based on Hardy–Weinberg expectations with no a priori geographic information.29 We used a standard admixture model with correlated allele frequencies with a burn-in period of 100,000 followed by one million iterations and determined the most likely number of clusters using STRUCTURE HARVESTER30 to implement the method of Evanno et al.31 We also performed a principal component analysis of the 6,276 SNPs in R32 using the script p.pca=prcomp(t(data)).
RESULTS
Vector competence.
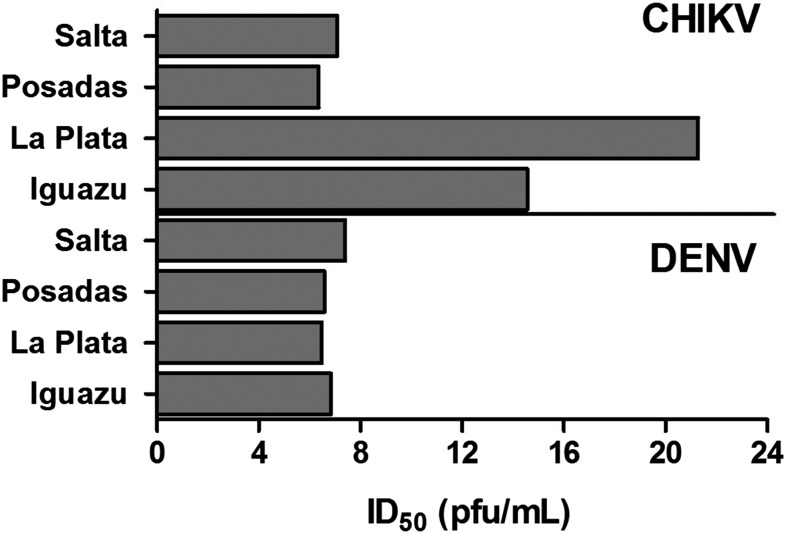
Infectivity was evaluated at multiple doses for both viruses in each population and the does at which 50% of mosquitoes became infected (ID50s) were extrapolated from best-fit linear relationships (linear regression analyses, r2 = 0.85–0.99, P < 0.05). Dengue virus ID50s were similar among populations and species for Posadas, La Plata, and Iguazu, ranging from 6.5 log10 pfu/mL for Posadas Ae. aegypti to 6.9 log10 pfu/mL for Iguazu Ae. aegypti (Figure 2). Infectivity was moderately lower for Salta Ae. aegypti (ID50 = 7.4 log10 pfu/mL). This difference is significant when comparing Salta with all groups except Posadas Ae. aegypti (linear regression analysis, P < 0.05).
Figure 2.
The dose at which 50% of mosquitoes become infected (ID50) with either chikungunya virus (CHIKV) 91077 or dengue virus (DENV) 306. Aedes aegypti were blood fed using three doses of virus, including ∼105, 106, and 107 pfu/mL, and virus-positive mosquito were identified by plaque assay at 14 days post-feeding. Linear regression analyses were used to determine the relationship between dose and infectivity and ID50 values were extrapolated from these linear relationships.
Chikungunya virus infectivity was much more variable than that of DENV, with ID50s ranging from 6.4 log10 pfu/mL (Posadas) to 21.3 log10 pfu/mL (estimated, La Plata; Figure 2). For Salta and Iguazu, infectivity was similar when comparing population and virus (DENV versus CHIKV; linear regression analysis, P > 0.05). La Plata and Iguazu populations, on the other hand, were distinct from each other and all other groups (linear regression analysis, P < 0.05), with extrapolated ID50 values well above the maximum blood meal dose used and/or achievable (Figure 2, > 7.6 log10 pfu/mL).
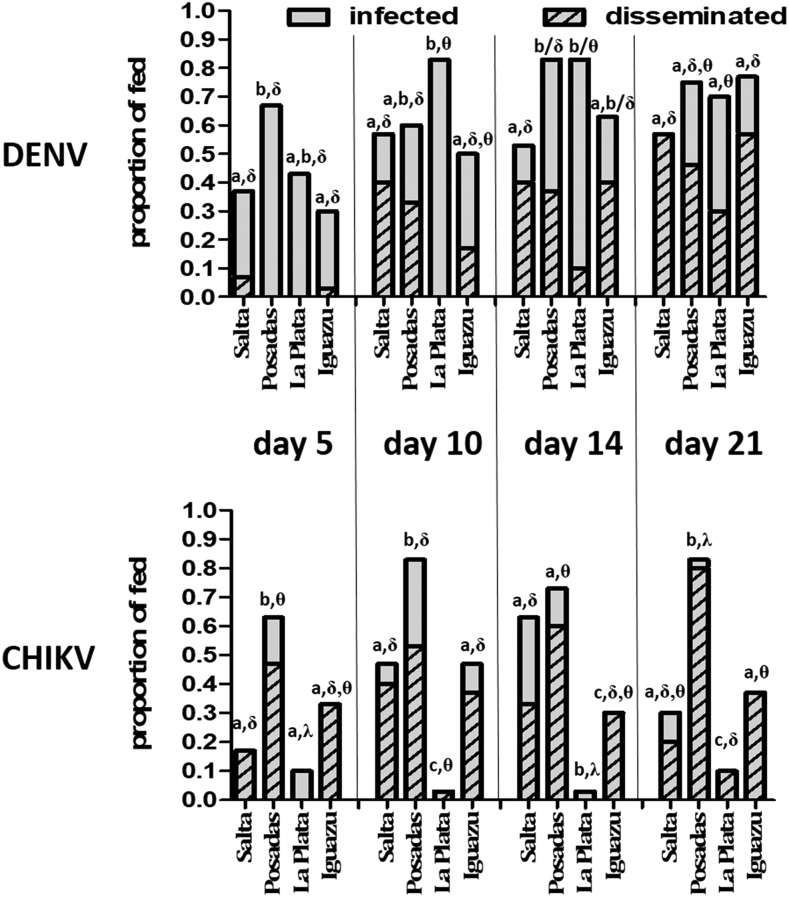
Competence differed among virus, day, population, and temperature regimen. Overall, infection rates at site-specific temperature regimes were high for DENV relative to CHIKV when considered across days (61.8% [DENV] versus 39.5% [CHIKV]; Fisher’s exact test, P < 0.01) or at the last assayed time point (day 21; 69.8% [DENV] versus 40.0% [CHIKV]; Fisher’s exact test, P < 0.01). Dissemination rates of exposed mosquitoes were statistically similar for DENV and CHIKV across all days (31.3% [CHIKV] versus 26.1% [DENV]) but higher for DENV by day 21 (36.8% [CHIKV] versus 47.5% [DENV]; Fisher’s exact test, P = 0.04). This difference at day 21 is wholly attributed to the increased infection rates with DENV, as dissemination among infected individuals was in fact more efficient with CHIKV (79.1% [CHIKV] versus 42.2% [DENV]; Fisher’s exact test, P < 0.01).
Population significantly influenced Ae. aegypti competence (Figures 2 and 3). For DENV, overall infection rates were significantly higher for Posadas and La Plata relative to Salta and Iguazu when combining days 5, 7, and 14 (Fisher’s exact test, P < 0.05), although differences were not measured on day 21. In general, the opposite relationship was measured for DENV dissemination rates, with the highest rates measured in Salta mosquitoes, followed by Iguazu, Posadas, and La Plata, respectively. Significant differences were measured with individual comparisons on days 7, 14, and 21 and combined dissemination rates were significantly lower for La Plata mosquitoes relative to all other populations (Figure 3; Fisher’s exact test, P < 0.05).
Figure 3.
Vector competence of Argentine Aedes aegypti for dengue and chikungunya viruses (DENV, CHIKV) under population-specific temperature regimes. Like letters (a, b, c) represent statistically equivalent infection rates and like symbols (δ, θ, λ) represent statistically equivalent dissemination rates (Fisher’s exact test, P > 0.05).
Variability in competence among populations was greater for CHIKV. Overall, infection and dissemination were significantly higher in Posadas mosquitoes relative to all other groups (Fisher’s exact test, P < 0.05). Chikungunya virus infection rates for this population were similar to that of DENV. For all other populations, infection rates were generally lower than that of DENV, yet the proportion of infected individuals with disseminated infections was consistently higher in all groups (Figure 3). La Plata mosquitoes were largely refractory to CHIKV infection, with an overall infection rate of just 5.0%, which was significantly lower than that of both the highly infected Posadas mosquitoes (72.5%) and moderately infected Salta and Iguazu mosquitoes (37.5% combined, Figure 3).
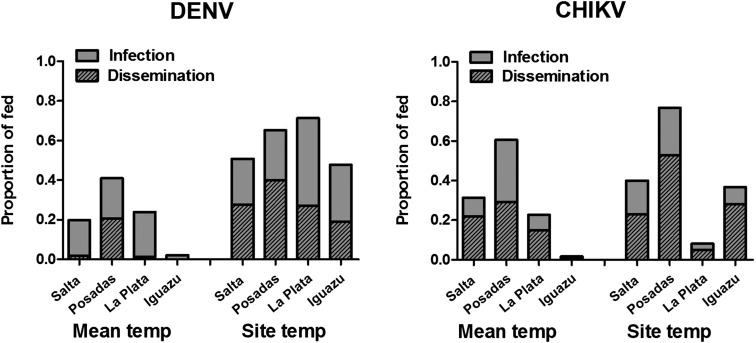
To evaluate the extent to which these differences are attributable to mosquito population and/or temperature, experiments were repeated for all populations at a temperature cycle representing the mean across sites, and competence was compared with experiments performed at site-specific temperature regimes (Figure 4). Temperature significantly affected competence, although the effect was both virus and population specific. For all populations, the effect of temperature was greater for DENV relative to CHIKV, with an average change in infectivity from site-specific to mean temperatures of 37.0% for DENV and 20.0% for CHIKV and an average change in dissemination rates of 23.0% for DENV and 15.0% for CHIKV. The overall decreases in DENV competence were similar for Salta and La Plata mosquitoes (26.0% decrease in dissemination rates), as well as Iguazu and Posadas (19.0% decrease). It is notable that although temperatures were substantially lower in La Plata relative to the mean, DENV competence was still significantly higher at the temperature regimen matching site-specific conditions (Fisher’s exact test, P < 0.05). Temperature effects were more variable for CHIKV. Although similar decreases in dissemination were measured for Posadas and Iguazu mosquitoes (24.0% and 27.0%, respectively), an increase of 10.0% was measured for La Plata mosquitoes and no significant effect was measured for CHIKV in Salta mosquitoes. Overall, results demonstrate that both population and temperature, as well as the interaction of the two, have a significant effect on the competence of Ae. aegypti for both DENV and CHIKV (Table 1). The independent effect of temperature on infection and dissemination is substantially higher for DENV, whereas the independent effect of population on infection and the effect of interactions on both infection and dissemination are substantially higher for CHIKV (Table 1).
Figure 4.
Mean vector competence of Argentine mosquitoes across days 5, 10, 14, and 21 held at either temperature cycles mimicking temperature at sites during trapping (site temp) or the mean hourly temperature cycle across sites (mean temp). Significant differences were measured for overall infection and dissemination rates for both viruses and all populations (Fisher’s exact test, P < 0.05), with the exception of chikungunya virus (CHIKV) in Salta mosquitoes, for which no effect of temperature was measured.
Table 1.
Relative contribution of population and temperature to vector competence of Aedes aegypti in Argentina (two-way analysis of variance) for DENV and CHIKV
| DENV | CHIKV | |||||||
|---|---|---|---|---|---|---|---|---|
| Infection | Dissemination | Infection | Dissemination | |||||
| Population | 25.87% | P < 0.001 | 17.90% | P = 0.004 | 68.25% | P < 0.001 | 5.53% | P = 0.202 |
| Temperature | 55.32% | P < 0.001 | 18.39% | P = 0.028 | 5.89% | P = 0.003 | 6.82% | P = 0.551 |
| Interaction | 1.83% | P = 0.448 | 22.69% | P = 0.013 | 17.88% | P < 0.001 | 37.54% | P = 0.026 |
CHIKV = chikungunya virus; DENV = dengue virus.
Vectorial capacity.
To gain a more comprehensive understanding of the relationship between mosquito population, temperature, and arbovirus transmission, VC, defined here as the probability that an individual mosquito in a population will transmit virus, was estimated for each population at both mean and site-specific temperature regimes. Feeding rates and longevity were evaluated in a separate study using the same populations and temperature regimes,26 and summary data from that study were used to inform our estimates. Although some modest but significant differences in longevity among populations and temperature cycles were identified, linear relationships were similar and this translated to highly similar daily survival rates (0.982–0.987; Table 2). More pronounced differences in the feeding rate were observed. Specifically, the proportion of mosquitoes taking multiple blood meals in their lifetime was found to be highest for Salta (61.0%) and Iguazu (54.0%), followed by La Plata (38.0%) and Posadas (35.0%). Consistent with a positive correlation between temperature and blood feeding, rearing at mean temperature cycles resulted in decreases of 38.0% and 29.0%, respectively, for Salta and Iguazu, similar rates for Posadas (35.0% versus 33.0%), and an increased rate for La Plata (38.0% versus 51.0%, Table 2).
Table 2.
Vectorial capacity of Argentine Aedes aegypti for DENV and CHIKV
| Population | Temp* | h† | p‡ | CHIKV | DENV | ||||
|---|---|---|---|---|---|---|---|---|---|
| b§ | n‖ | VC¶ | b§ | n‖ | VC¶ | ||||
| Salta | Site | 0.61 | 0.984 | 0.40 | 9.6 | 0.21 | 0.57 | 9.9 | 0.30 |
| Mean | 0.23 | 0.983 | 0.36 | 10.0 | 0.07 | 0.07 | 13.6 | 0.01 | |
| Iguazu | Site | 0.54 | 0.982 | 0.37 | 8.0 | 0.17 | 0.57 | 11.2 | 0.25 |
| Mean | 0.25 | 0.983 | 0.05 | 17.8 | 0.01 | ≤ 0.03# | ≥ 21# | ≤ 0.01# | |
| Posadas | Site | 0.35 | 0.983 | 0.80 | 9.3 | 0.24 | 0.46 | 9.7 | 0.14 |
| Mean | 0.33 | 0.985 | 0.44 | 9.3 | 0.13 | 0.3 | 9.1 | 0.09 | |
| La Plata | Site | 0.38 | 0.987 | 0.10 | 13.4 | 0.03 | 0.3 | 15.6 | 0.10 |
| Mean | 0.51 | 0.985 | 0.20 | 8.8 | 0.09 | 0.03 | 10.6 | 0.02 | |
CHIKV = chikungunya virus; DENV = dengue virus; VC = vectorial capacity.
Mosquitoes held at mean temperature cycles or cycles specific to trap location (site).
Proportion of mosquitoes taking at least two blood meals during their lifetime.
Probability of daily survival.
Vector competence = proportion of mosquitoes with disseminated infections by day 21 postinfection.
Extrinsic incubation period in days.
VC = h*b*pn.
Transmission not detected, value indicates limit of detection.
Chikungunya virus EIPs ranged from 8 days (Iguazu) to 13.4 days (La Plata). Dengue virus EIPs were on average 1.8 days longer than that of CHIKV and more population dependent, ranging from 9.7 days (Posadas) to 15.6 days (La Plata). Despite the higher mean and maximum temperatures of Iguazu mosquitoes, the second longest EIP was measured with this population (11.2 days). With the exception of Posadas mosquitoes, for which a decreased mean temperature correlated to a modest decrease in DENV EIP (9.7–9.1 days) and no change in CHIKV EIP (9.3 days for both temperature regimes), temperature was inversely correlated to EIP (Pearson R = −0.894, P = 0.0028). The magnitude of this effect was highly population and virus dependent. For example, decreased temperatures increased EIPs by ∼10 days for Iguazu mosquitoes with both viruses, yet increases of just 0.4 days (CHIKV) and 3.7 days (DENV) were measured with Salta mosquitoes.
These population, temperature, and virus-dependent effects on feeding, survival, competence, and EIP resulted in high levels of variability in transmissibility (VC, Table 2), ranging from < 0.01 (DENV, Iguazu, mean temperature cycle) to 0.3 (DENV, Salta, site-specific temperature cycle, Table 2). At site-specific temperature regimes, VC was higher for DENV with Salta, Iguazu, and La Plata mosquitoes, yet higher for CHIKV with Posadas mosquitoes. The lowest risk for transmission was found with La Plata mosquitoes, particularly for CHIKV. The highest transmission risk for DENV was found with Salta mosquitoes and the highest risk for CHIKV was found with Posadas mosquitoes. Changes in VC resulting from changing temperature regimes ranged from 1.6-fold (DENV, Posadas) to 30-fold (DENV, Salta). Despite the increased temperatures of the mean cycle, DENV VC decreased 5-fold for La Plata mosquitoes. The only instance where an increased transmission risk was noted with mean cycling temperatures relative to site-specific regimes was for CHIKV in La Plata mosquitoes.
Mosquito genetics.
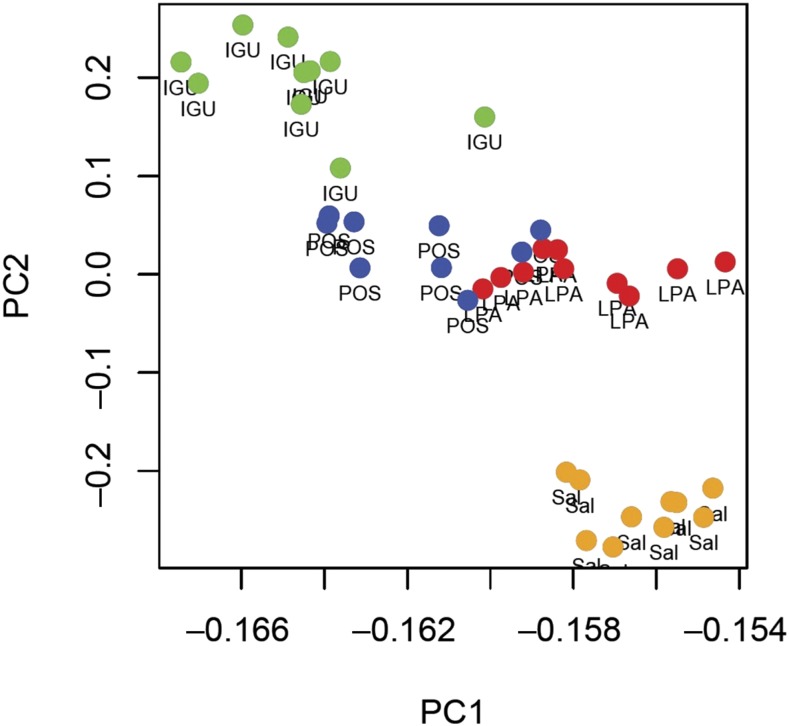
Population structure analysis strongly supported three distinct genetic clusters among the four populations (k = 3), with the most genetically distinct population being Salta (Supplemental Figure 1), the westernmost population. Posadas mosquitoes displayed a mix of genotypes from La Plata and Iguazu. Lower or higher numbers of clusters were less supported and indeed the same pattern was apparent from the principal component analysis (Figure 5), with Posada specimens intermediate between Iguazu and La Plata, and Salta specimens distinct from the others. The first two dimensions of the principal components analysis (PC1 and PC2) explained more than 80% of the observed variation among populations.
Figure 5.
Two-dimensional representation of the Argentine Aedes aegypti genetic variability using PC1 and PC2. Each dot represents an individual mosquito from each of the populations: IGU = Iguazu; LPA = La Plata; POS = Posada; SAL = Salta. This figure appears in color at www.ajtmh.org.
DISCUSSION
Although there are generic virus–mosquito species combinations that facilitate global emergence and spread, there is also a high level of variability in patterns and intensity of transmission of arboviruses by competent species on both regional and local levels. Understanding this variability is critical if we are to accurately predict the risk posed by arboviruses in specific locations. Recent incursions and reemergence of arboviruses including dengue, chikungunya, West Nile, Zika, yellow fever, and Mayaro have demonstrated that the threat from mosquito-borne viruses is increasing in the Americas. Here, we demonstrate that intrinsic and extrinsic factors including mosquito population, viral species, and temperature significantly influence both vector competence and overall VC of Ae. aegypti in Argentina, yet also that the magnitude of these influences is highly variable.
Our experiments with unique populations and location-specific temperature regimes demonstrate that the susceptibility and competence of Argentine mosquitoes are highly variable regionally. Overall, ID50s for both DENV and CHIKV were generally higher than what has been reported in previous studies,33–35 which could be a result of decreased susceptibility for these viral strains in these populations and/or decreased infectivity as a result of lower mean temperatures. Although DENV infectivity was similar among populations and similar to CHIKV infectivity for Salta and Posadas populations, Iguazu and particularly La Plata Ae. aegypti were highly refractory to CHIKV infection. While the mean experiments demonstrate that the decreased La Plata temperatures do contribute significantly to decreased infectivity, even at mean temperatures, this population is significantly more refractory than the other populations. Although autochthonous transmission of CHIKV occurred in Argentina for the first time in 2016, no activity was reported in Buenos Aires Province, which is consistent with the lack of susceptibility identified for this population. The majority of the laboratory-confirmed autochthonous cases (29) were from the province of Salta (http://who.int/csr/don/14-march-2016-chikungunya-argentina/en/). By contrast, in 2016, DENV circulated and caused disease in Buenos Aires.
This population-specific competence is similar to what has been reported in other studies,16,20 yet our results suggest that CHIKV competence is more dependent on mosquito population than DENV competence, perhaps reflecting the shorter evolutionary history of this virus in the region.36,37 Whereas this suggests that further adaptive evolution of CHIKV in the region could enhance transmission,38 an important caveat of these studies is that a single CHIKV isolate was used and other viral genotypes could be inherently more transmissible.39
Although previous findings of increased temperatures resulting in decreased extrinsic incubation and increased competence are generally supported by our data,6,7,9 results also demonstrate that fluctuations in temperature will have both virus species- and population-specific effects on competence. Overall, DENV competence was much more heavily influenced by temperature than CHIKV was, suggesting that future variability in climate is more likely to have a greater impact on DENV transmission in the region. In addition, there are exceptions to the reported generic relationship between temperature and competence. For instance, competence of La Plata Ae. aegypti for DENV was lower at the mean temperature regimen, despite a 4°C increase in median temperature, and Posadas Ae. aegypti DENV EIP was shorter at the mean temperature regimen, despite lower temperatures. These results, which are supported by previous laboratory studies with both DENV and CHIKV,20,21 demonstrate that the influence of mosquito genetics on competence can at times supersede the effects of temperature and that predictions on the effect of temperature on transmission cannot be accurately made without consideration of this influence. Understanding genetic variability is, therefore, critical to predict how the effects of climate change might impact the intensity and distribution of arbovirus transmission in the region. Consistent with the phenotypic variability in competence and the effect of temperature, our data demonstrate that these populations are indeed genetically distinct. Specifically, La Plata, Iguazu, and Salta Ae. aegypti are highly divergent, whereas Posadas Ae. aegypti comprise a mixture of La Plata and Iguazu genotypes consistent with their geographical location midway between La Plata in the southeast and Iguazu in the northeast.
Studies that exclusively use competence to attempt to inform transmission risk fail to consider what are among the most critical variables determining VC, blood feeding behavior and longevity.40,41 Although assessing these in the laboratory is not likely to provide highly accurate absolute values reflecting natural conditions, relative differences found in the laboratory can accurately inform relative risk. Previous studies with these populations demonstrated that, like competence, life history traits and the influence of temperature on these traits are population dependent.26 Our modeling of transmission risk generally reflected the DENV and CHIKV activity that occurred in Argentina in 2016, yet there are certainly exceptions to this. For instance, a high proportion of DENV cases occurred in Misiones, including in and around Posadas. Although we predicted that this area had a high risk, VC was calculated to be higher for the Salta population, which are derived from a region for which DENV incidence was much lower than that in Misiones. This discrepancy is not necessarily surprising as we are modeling entomological risk without consideration of differences in population density, host behavior, or immune status. The combination of our data with epidemiological models considering these factors42 could provide more accurate forecasts. Furthermore, outbreaks from all four DENV serotypes have been recorded in Argentina in recent years and immunological status could at times have an enhancing or suppressing role in the incidence of apparent DENV disease.43 Last, the rate at which mosquito populations evolve or are displaced in particular regions has not been assessed and could have a large influence on transmission risk. Ultimately, accurate assessment of arbovirus risk will require nuanced models which fully consider the complexity of interactions between virus, temperature, mosquito genetics, and host.
Supplementary Material
Acknowledgments:
We would like to thank the entire staff of the NYS Arbovirus Laboratory insectary staff for assistance with mosquito experiments, and Andrea Gloria-Soria and Jeffery R. Powell from Yale University for mosquito sequencing. We also thank NIH for access to FastSTRUCTURE in Helix.
Note: Supplemental figure and table appear at www.ajtmh.org.
REFERENCES
- 1.Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, Zhao H, Caccone A, Powell JR, 2014. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 68: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcondes CB, Contigiani M, Gleiser RM, 2017. Emergent and reemergent arboviruses in South America and the Caribbean: why so many and why now? J Med Entomol 54: 509–532. [DOI] [PubMed] [Google Scholar]
- 3.Otero M, Solari HG, 2010. Stochastic eco-epidemiological model of dengue disease transmission by Aedes aegypti mosquito. Math Biosci 223: 32–46. [DOI] [PubMed] [Google Scholar]
- 4.Pan American Health Organization , 2017. Platforma de Información en Salud de las Americas. Available at: www.paho.org/data. Accessed January 1, 2018.
- 5.Kramer LD, 2016. Complexity of virus-vector interactions. Curr Opin Virol 21: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbaika S, Lutomiah J, Chepkorir E, Mulwa F, Khayeka-Wandabwa C, Tigoi C, Oyoo-Okoth E, Mutisya J, Ng'ang'a Z, Sang R, 2016. Vector competence of Aedes aegypti in transmitting chikungunya virus: effects and implications of extrinsic incubation temperature on dissemination and infection rates. Virol J 13: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards SL, Mores CN, Lord CC, Tabachnick WJ, 2007. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis 7: 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer LD, Hardy JL, Presser SB, 1983. Effect of temperature of extrinsic incubation on the vector competence of Culex tarsalis for western equine enephalomyelitis virus. Am J Trop Med Hyg 32: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 9.Carrington LB, Seifert SN, Armijos MV, Lambrechts L, Scott TW, 2013. Reduction of Aedes aegypti vector competence for dengue virus under large temperature fluctuations. Am J Trop Med Hyg 88: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW, 2011. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci U S A 108: 7460–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danforth ME, Reisen WK, Barker CM, 2016. The impact of cycling temperature on the transmission of West Nile virus. J Med Entomol 53: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD, 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog 4: e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD, 2014. The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol 51: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christofferson RC, Mores CN, 2016. Potential for extrinsic incubation temperature to alter interplay between transmission potential and mortality of dengue-infected Aedes aegypti. Environ Health Insights 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD, 2010. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am J Trop Med Hyg 83: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pongsiri A, Ponlawat A, Thaisomboonsuk B, Jarman RG, Scott TW, Lambrechts L, 2014. Differential susceptibility of two field Aedes aegypti populations to a low infectious dose of dengue virus. PLoS One 9: e92971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behura SK, Gomez-Machorro C, deBruyn B, Lovin DD, Harker BW, Romero-Severson J, Mori A, Severson DW, 2014. Influence of mosquito genotype on transcriptional response to dengue virus infection. Funct Integr Genomics 14: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrechts L, Quillery E, Noël V, Richardson JH, Jarman RG, Scott TW, Chevillon C, 2013. Specificity of resistance to dengue virus isolates is associated with genotypes of the mosquito antiviral gene Dicer-2. Proc Biol Sci 280: 20122437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fansiri T, Fontaine A, Diancourt L, Caro V, Thaisomboonsuk B, Richardson JH, Jarman RG, Ponlawat A, Lambrechts L, 2013. Genetic mapping of specific interactions between Aedes aegypti mosquitoes and dengue viruses. PLoS Genet 9: e1003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge JM, Lourenco-De-Oliveira R, Caro V, Lambrechts L, Failloux AB, 2014. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc Biol Sci 281: pii: 20141078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloria-Soria A, Armstrong PM, Powell JR, Turner PE, 2017. Infection rate of Aedes aegypti mosquitoes with dengue virus depends on the interaction between temperature and mosquito genotype. Proc Biol Sci 284: pii: 20171506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauffman E, Payne A, Franke MA, Schmid MA, Harris E, Kramer LD, 2017. Rearing of Culex spp. and Aedes spp. mosquitoes. Bio Protoc 7: pii: e2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.OhAinle M, et al. 2011. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 3: 114ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, Campbell GL, 2007. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis 13: 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD, 2006. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods 134: 183–187. [DOI] [PubMed] [Google Scholar]
- 26.Muttis EBA, Chuchuy A, Mangudo C, Ciota AT, Kramer LD, 2018. Factors related to Aedes aegypti (Diptera: Culicidae) populations and temperature determine differences on life-history traits with regional implications in disease transmission. J Med Ent, 10.1093/jme/tjy057. [DOI] [PubMed] [Google Scholar]
- 27.Evans BR, Gloria-Soria A, Hou L, McBride C, Bonizzoni M, Zhao H, Powell JR, 2015. A multipurpose, high-throughput single-nucleotide polymorphism chip for the dengue and yellow fever mosquito, Aedes aegypti. G3 (Bethesda) 5: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raj A, Stephens M, Pritchard JK, 2014. fastSTRUCTURE: variational inference of population structure in large SNP data sets. Genetics 197: 573–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard JK, Stephens M, Donnelly P, 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earl D, vonHoldt B, 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4: 359–361. [Google Scholar]
- 31.Evanno G, Regnaut S, Goudet J, 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 32.R Core Team, 2017. R: A Language and Environment for Statistical Computing. Vienna, Australia: R Foundation for Statistical Computing. Available at: http://www.r-project.org. Accessed June 1, 2018.
- 33.Nguyet MN, et al. 2013. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 110: 9072–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubler DJ, Nalem S, Tan R, Saipan H, Saroso JS, 1979. Variation in suseptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am J Trop Med Hyg 28: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong PM, Rico-Hesse R, 2003. Efficiency of dengue serotype 2 virus strains to infect and disseminate in Aedes aegypti. Am J Trop Med Hyg 68: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allicock OM, Lemey P, Tatem AJ, Pybus OG, Bennett SN, Mueller BA, Suchard MA, Foster JE, Rambaut A, Carrington CV, 2012. Phylogeography and population dynamics of dengue viruses in the Americas. Mol Biol Evol 29: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahadeo NSD, et al. 2017. Understanding the evolution and spread of chikungunya virus in the Americas using complete genome sequences. Virus Evol 3: vex010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsetsarkin KA, Weaver SC, 2011. Sequential adaptive mutations enhance efficient vector switching by chikungunya virus and its epidemic emergence. PLoS Pathog 7: e1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC, 2011. Chikungunya virus: evolution and genetic determinants of emergence. Curr Opin Virol 1: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciota AT, Ehrbar DJ, Matacchiero AC, Van Slyke GA, Kramer LD, 2013. The evolution of virulence of West Nile virus in a mosquito vector: implications for arbovirus adaptation and evolution. BMC Evol Biol 13: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black WC, IV, Moore CG, 1996. Chapter 15. Population biology as a tool to study vector-borne diseases. Beaty BJ, Marquardt WC, eds. The Biology of Disease Vectors. San Diego, CA: Elsevier Academic, 187–206. [Google Scholar]
- 42.Ruiz-Moreno D, 2016. Assessing chikungunya risk in a metropolitan area of Argentina through satellite images and mathematical models. BMC Infect Dis 16: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tittarelli E, Lusso SB, Goya S, Rojo GL, Natale MI, Viegas M, Mistchenko AS, Valinotto LE, 2017. Dengue virus 1 outbreak in Buenos Aires, Argentina, 2016. Emerg Infect Dis 23: 1684–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.