Abstract.
Timely identification and treatment of malaria transmission “hot spots” is essential to achieve malaria elimination. Here we investigate the relevance of using an Anopheles salivary biomarker to estimate Plasmodium falciparum malaria exposure risk along the Thailand–Myanmar border to guide malaria control. Between May 2013 and December 2014, > 9,000 blood samples collected in a cluster randomized control trial were screened with serological assays to measure the antibody responses to Anopheles salivary antigen (gSG6-P1) and P. falciparum malaria antigens (circumsporozoite protein, merozoite surface protein 119 [MSP-119]). Plasmodium falciparum infections were monitored through passive and active case detection. Seroprevalence to gSG6-P1, MSP-119, and CSP were 71.8% (95% Confidence interval [CI]: 70.9, 72.7), 68.6% (95% CI: 67.7, 69.5), and 8.6% (95% CI: 8.0, 9.2), respectively. Multivariate analysis showed that individuals with the highest Ab response to gSG6-P1 had six times the odds of being positive to CSP antigens (P < 0.001) and two times the odds of P. falciparum infection compared with low gSG6-P1 responders (P = 0.004). Spatial scan statistics revealed the presence of clusters of gSG6-P1 that partially overlapped P. falciparum infections. The gSG6-P1 salivary biomarker represents a good proxy for estimating P. falciparum malaria risk and could serve to implement hot spot–targeted vector control interventions to achieve malaria elimination.
INTRODUCTION
Malaria along the Thailand–Myanmar border (TMB) is characterized by foci of high prevalences of asymptomatic and submicroscopic Plasmodium spp. carriage.1 Over the past several decades malaria caused by Plasmodium falciparum infection has continuously declined in populations living along the TMB that has mainly been attributed to the use of rapid diagnostic tests (RDTs) for early malaria detection and treatment of malaria with highly effective artemisinin-based combination antimalarial therapies and, to a lesser extend to prevention of mosquito bites.2,3 The recent emergence of P. falciparum parasites that are resistant to artemisinin and artemisinin partner drugs along the TMB raised concern about the potential spread of artemisinin resistance to India and Africa and threatens recent gains in the reduction in the burden of malaria in the region and globally.4,5 To contain P. falciparum artemisinin resistance, the Shoklo Malaria Research Unit and partners conducted a mass drug administration (MDA) pilot trial with the aim to eliminate submicroscopic reservoirs of Plasmodium spp. parasites and interrupting malaria transmission.6,7 Along the TMB, local malaria transmission is heterogeneously distributed and the risk of malaria may be confined to geographically small high-risk areas and subsets of the population.3 Identifying and targeting these malaria transmission “hot spots” and “hot-pops” is therefore essential to remove the remaining sources of transmission and to achieve malaria elimination.8
Measuring local malaria transmission poses considerable challenges because of the lack of sensitivity of commonly used entomology methods.9,10 Assessing malaria transmission intensity by determining the entomological inoculation rate (EIR) is challenging considering the low frequency of mosquitoes positive for infection and spatial and temporal variations in mosquito densities and composition necessitate long-term intensive sampling.11 Serological biomarkers using antibodies specific for Plasmodium spp. are increasingly used to estimate changes in malaria transmission in areas of low endemicity.12–16 Measuring antimalarial antibodies to detect malaria transmission offer several advantages (compared with entomological and parasite outcomes) because of the longer duration of specific antibody responses, they are indicative of recent malaria exposure rather than point prevalence.12,17 Recently, new serological biomarkers that measure the intensity of human exposure to mosquito salivary antigens have been identified (reviewed in Doucoure and Drame18). Compared with other serological tools, salivary markers offer great potential for measuring small-scale variations in the exposure to malaria vectors as they provide shorter lived antibody responses.18 In a previous study, we demonstrated that antibodies specific for the salivary biomarker gSG6-P1 are relevant to quantify human–vector contact and estimate the malaria transmission risk at the TMB.19 We showed that the risk of malaria transmission strongly varied in space and time and was influenced by the environment and human behavior. Nevertheless, the relationship between human exposure to Anopheles bites and the risk of being infected by P. falciparum malaria was not elucidated.
The aim of the present study was to address whether Anopheles salivary biomarker can detect small-scale variations in human exposure to P. falciparum malaria in a context of malaria elimination. This information is pivotal in public health programs to improve malaria surveillance and guide vector control programs.
MATERIAL AND METHODS
Study site and survey procedure.
Four villages, Htoo Pyin Nyar (TPN, 17°14′N, 98°29′E), Tar Au Ta (TOT, 16°36′N, 98°57′E), Ka Nu Hta (KNH, 17°18′N, 98°24′E), and Htee Kaw Taw (HKT, 16°85′N, 98°47′E), with > 10% P. falciparum prevalence by high-volume ultrasensitive quantitative polymerase chain reaction (uPCR) were selected after the engagement of the community.1 A community-based malaria clinic (“malaria post” or MP) was set up in each village to monitor malaria infections with SD Bioline Pf/Pv RDT.20 A census was performed before the surveys and demographic information was collected.19 Two villages (TOT and KNH) were randomly assigned to MDA intervention immediately and two (HKT, TPN) were followed for 9 months (control period) before receiving MDA as described in Landier et al.20 Briefly a 3-day treatment course of dihydroartemisinin–piperaquine and a single 0.25-mg base/kg dose of primaquine was administered orally under supervision every month for 3 months to all participants. Long-lasting impregnated nets (LLIN) were distributed to all households at the start of the study (M0). Seven household surveys were carried in each village every 3 months (month 0, 3, 6, 9, 12, 15, and 18) from May 2013 to December 2014. At each survey, dried blood spots on Whatman filter papers (3MM, 0.34 mm thickness) were collected during surveys of the entire village population for enzyme-linked immunosorbent assay (ELISA). Plasmodium spp. infections were also recorded by uPCR according to methods described previously.1,21
Antigen selection.
The gambiae salivary peptide gSG6-P1 was selected because it is highly antigenic and highly specific to the Anopheles genus, with no relevant cross-reactivity with epitopes from other proteins or vectors of parasites.22,23 The synthetic nature largely ensures high reproducibility of the assay and it induces short-term (up to 2 months) and host-specific humoral response.18 The P. falciparum antigens merozoite surface protein 119 (MSP-119) and CSP were selected for their capacity to detect different life cycle stages of the Plasmodium (sporozoite versus merozoite) and for their difference in immunogenicity and persistence.15,24
Enzyme-linked immunosorbent assay: Human antibody response to P. falciparum antigens.
Serologic testing of human exposure to MSP-119 and CSP antigens was carried out by using ELISA as described in the study by Drakeley et al.,15 but with some modifications (Supplemental Text 1). Individual responses were expressed as the ∆OD value: ∆OD = ODx − ODn. ODx and ODn represent the mean of individual optical density (OD) in two antigen wells and one blank well containing no Pf MSP1-19/CSP peptide, respectively. Specific anti-MSP-119/CSP immunoglobulin G responses were also determined by ELISA in nonmalaria-exposed individuals (negative samples from France: N = 18) to quantify the nonspecific background Ab level and to calculate the cut-off value for seropositivity (mean [∆DOneg] + 3 SD). Procedures for testing human exposure to gSG6-P1 salivary biomarker have previously been described.19
Statistical analysis.
To investigate the association between the intensity of Ab response to Anopheles salivary antigen (gSG6-P1; categorical variable: low, medium, high, very high; based on quartiles) and Ab responses specific for P. falciparum CSP and MSP-119 (binary variable: positive, negative), we used a multivariate multilevel logistic mixed regression model with adjustment for relevant covariates, which represented proxies for social, demographic, or environmental status. At the individual level, covariates included Ab response to gSG6-P1, age group, gender, and MDA treatment (a time-dependent individual binary variable, 0 as long as the individual has not taken any drugs, 1 when individuals have received 1, 2, or 3 doses). Household-level covariates included LLIN use (never, sometimes, and every night). At the village level, the population size at each survey, the temperature, and relative humidity were taken into account in the model (i.e., second order polynomial) and season was defined according to the Thai Meteorological Department. In a second analysis, we investigated the relationship between the intensity of Ab response to Anopheles salivary antigens (four categories) and P. falciparum malaria infections (binary variable: positive and negative) using a multivariate mixed logistic regression adjusted for covariates described previously. Statistical analyses were carried out with Stata version 13.0 (StataCorp LP, College Station, TX). Graphs were constructed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA).
Spatial analysis.
Geographic references (latitude and longitude) were recorded for all households in the study villages. Each house was given an identification code and all study participants could be linked back to their respective houses using the identification code. Scan statistics were used to test for statistically significant clusters of salivary biomarker (gSG6-P1) and P. falciparum malaria infections as measured by uPCR (surveys) and RDT (MP). A discrete space-time Poisson model was used to test for statistically significant clusters for each of these outcomes and for each study village across all screenings (M0–M18).25,26 The scan statistic uses a moving window (a spherical kernel) that centers on each point (house) in the village and calculates the number of cases (P. falciparum infections or biomarker positives) within the window and the number of expected cases within the window, given the population within the window and the distribution of cases and population across the entire village. The window increases in size until half of the village population is contained and then moves to the next point. Likelihood ratios are calculated for each window location, size, and time point and P values are calculated using Monte Carlo simulations for the largest ranking clusters. Scan statistics were calculated using SaTScan software (https://www.satscan.org/). A sensitivity analysis was conducted using ellipsoid rather than spherical windows.
Ethical approval.
This study was part of a multicenter cluster randomized control trial conducted in several sites in the Greater Mekong subregion and registered on ClinicalTrials.gov: NCT01872702. Study protocol was reviewed and approved by OxTREC (reference no. 1017–13 and 1015–13).
RESULTS
Study populations and outcomes.
Participants consisted of 2,602 people followed up every 3 months over 18 months and are described in Table 1. The compositions of the four study villages were comparable in age and gender. The overall prevalence of P. falciparum infections over the surveys ranged from 0.88% (17/1,934) in TPN to 3.14% (64/2,036) in KNH. The incidence of P. falciparum recorded at MP ranged from 1 per 1,000 people per year at HKT to 45.5 at TOT. A total of 9,373 and 9,401 dried spots were analyzed for CSP and MSP-119, respectively. Overall, Ab seroprevalence was higher for MSP-119 (range: 23.9–100%) than for CSP (range: 0.4–42%) and increased with age in each village, with the exception of TPN where CSP seroprevalence was lower in subjects > 60 years than in other age groups (Table 1). The seroprevalence to gSG6-P1 ranged from 59% at TPN to 86% at HKT.
Table 1.
Descriptive statistics of participants, by study sites
| Characteristics | Htoo Pyin Nyar | Tar Au Ta | Ka Nu Hta | Htee Kaw Taw | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years), median (range) | 21 (0–66) | 19 (0–80) | 22 (0–73) | 19 (0–94) | ||||
| Female gender, % | 47 | 52 | 46 | 50 | ||||
| Pf prevalence by uPCR % (no. positive/no. tested) | 0.88 (17/1,934) | 2.77 (51/1,843) | 3.14 (64/2,036) | 1.62 (59/3,648) | ||||
| Pf incidence by rapid diagnostic test (cases per 1,000 per year) | 11.4 | 45.5 | 6.6 | 1.0 | ||||
| Antibody prevalence to gSG6-P1 | 59.3 (1,131/1,906) | 68.8 (1,356/1,970) | 61.4 (1,256/2,046) | 86.3 (3,024/3,503) | ||||
| Antibody prevalence to Pf antigen, visits (%) | CSP | MSP-119 | CSP | MSP-119 | CSP | MSP-119 | CSP | MSP-119 |
| All ages | 6.0 (113/1,892) | 66.7 (1,268/1,902) | 14.5 (284/1,958) | 73.7 (1,452/1,969) | 8.7 (179/2,050) | 77.9 (1,591/2,043) | 6.7 (231/3,469) | 61.3 (2,136/3,484) |
| Ages 0–4 years | 0.4 (1/241) | 51.4 (125/243) | 3.2 (10/309) | 28.0 (87/311) | 1.3 (3/225) | 44.0 (99/225) | 1.5 (7/453) | 23.9 (109/456) |
| Ages 5–15 years | 3.6 (20/551) | 63.6 (353/555) | 5.4 (34/627) | 66.4 (420/633) | 4.1 (22/540) | 68.8 (368/535) | 3.4 (431/1,262) | 54.0 (683/1,264) |
| Ages 16–59 years | 8.7 (91/1,051) | 70.9 (748/1,055) | 21.7 (202/932) | 91.8 (857/934) | 11.9 (145/1,215) | 86.9 (1,055/1,214) | 9.9 (165/1,661) | 76.2 (1,273/1,671) |
| Ages > 60 years | 2.0 (1/49) | 85.7 (42/49) | 42.2 (38/90) | 96.7 (88/91) | 12.9 (9/70) | 100.0 (69/69) | 17.2 (16/93) | 76.3 (71/93) |
MSP-119 = merozoite surface protein 119; uPCR = ultrasensitive qPCR.
Seroprevalence in antibody response to Anopheles spp. and P. falciparum antigens.
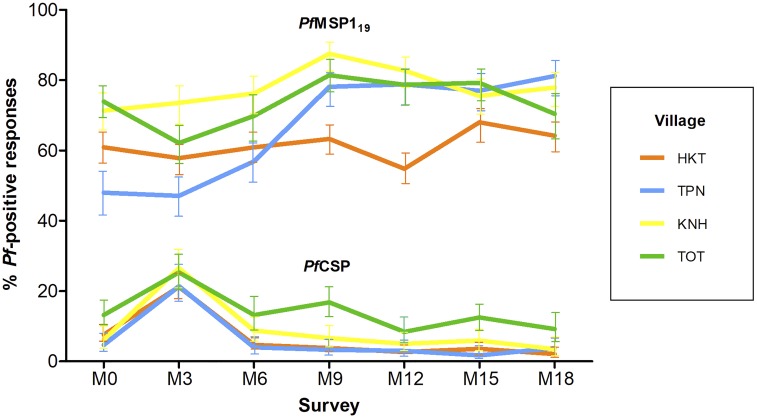
The Ab response to the MSP-119 and CSP antigens varied according to the village and survey (Figure 1). For MSP-119, seroprevalence greatly increased from M3 to M9 in all sites, except at HKT where it remained constant all along the study (≈60%) (Supplemental Table 1). By contrast, seroprevalence to CSP increased by ≈2-fold from M0 to M3 in all sites and then continuously declined until M18 (Supplemental Table 2). The seroprevalence to gSG6-P1 was high (71.8%, 95% Confidence interval [CI]: 70.9, 72.7) and strongly varied across villages and surveys (Supplemental Table 3).
Figure 1.
Seroprevalence data with 95% confidence interval for antibodies against Plasmodium falciparum merozoite surface protein 119 (MSP-119), and P. falciparum sporozoite (CSP), according to villages Ka Nu Hta, Tar Au Ta, Htee Kaw Taw, and Htoo Pyin Nyar (KNH, TOT, HKT, and TPN) and surveys (seven surveys over 18 months). Seropositivity for MSP-119 and CSP was calculated based on cut-off values (ΔOD) > 0.162 and > 0.115, respectively. OD = optical density.
Relationship between P. falciparum malaria and vector exposure.
The mean number of measurements per individual was 3.4 (range, 1–6). Multivariate logistic mixed analyses showed a highly significant and positive dose–response relationship between Ab response to gSG6-P1 and the odds of a positive Ab response against P. falciparum CSP and MSP-119 (Table 2). Individuals with the 25% highest Ab response to gSG6-P1 (i.e., “very high” responders) had 6 times the odds of being positive to CSP antigens (odds ratio [OR]: 5.94, 95% CI: 3.72–9.48, P < 0.001) compared with low gSG6-P1 responders. Increased odds of positive MSP-119 responses were also demonstrated but the magnitude of effect was smaller (OR: 2.91, 95% CI: 1.86–3.67, P < 0.005). Ad hoc multivariate analysis showed that the very high responders to gSG6-P1 were also associated with increased odds of P. falciparum infection after adjustment for other covariates (OR: 2.27, 95% CI: 1.35–3.80, P = 0.002, Table 3).
Table 2.
Multivariate logistic regression mixed model showing the relationship between Plasmodium falciparum antibody responders (CSP, MSP-119) and the intensity of antibody responses to gSG6-P1 salivary antigen and other covariates
| Characteristics | Ab response to CSP | Ab response to MSP-119 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Ab response to gSG6-P1 | ||||||
| Low | 1 | – | – | 1 | – | – |
| Medium | 2.08 | 1.31–3.32 | 0.002 | 1.81 | 1.35–2.44 | < 0.001 |
| High | 3.74 | 2.35–5.95 | < 0.001 | 1.71 | 1.25–2.34 | 0.001 |
| Very high | 5.94 | 3.72–9.48 | < 0.001 | 2.61 | 1.86–3.67 | < 0.001 |
| Age, years | ||||||
| < 5 | 1 | – | – | 1 | – | – |
| 5–15 | 5.05 | 1.89–13.47 | 0.001 | 28.83 | 15.67–52.97 | < 0.001 |
| 16–59 | 32.90 | 12.57–86.12 | < 0.001 | 285.60 | 147.36–553.51 | < 0.001 |
| > 59 | 96.89 | 26.15–359.03 | < 0.001 | 1,629.94 | 345.53–7,688.80 | < 0.001 |
| Gender | ||||||
| Male | 1 | – | – | 1 | – | – |
| Female | 0.70 | 0.46–1.05 | 0.082 | 0.46 | 0.31–0.68 | < 0.001 |
| Village | ||||||
| Htee Kaw Taw | 1 | – | – | 1 | – | – |
| Htoo Pyin Nyar | 0.03 | 0.01–0.10 | < 0.001 | 10.16 | 3.96–26.11 | < 0.001 |
| Ka Nu Hta | 0.05 | 0.02–0.17 | < 0.001 | 22.60 | 8.21–62.15 | < 0.001 |
| Tar Au Ta | 0.78 | 0.34–1.81 | 0.561 | 3.21 | 1.46–7.02 | 0.004 |
| Season | ||||||
| Hot | 1 | – | – | 1 | – | – |
| Cool | 9.48 | 2.69–33.35 | < 0.001 | 0.59 | 0.23–1.48 | 0.268 |
| Rainy | 8.34 | 2.47–28.1 | 0.001 | 0.45 | 0.18–1.09 | 0.079 |
| MDA | ||||||
| No MDA | 1 | – | – | 1 | – | – |
| MDA | 0.49 | 0.33–0.74 | 0.001 | 4.18 | 3.15–5.54 | < 0.001 |
| Sleep under bednet | ||||||
| Never | 1 | – | – | 1 | – | – |
| Some night | 0.72 | 0.16–3.27 | 0.675 | 2.01 | 0.45–9.02 | 0.364 |
| Every night | 0.97 | 0.55–1.72 | 0.912 | 1.80 | 1.02–3.17 | 0.043 |
CI = confidence interval; MDA = mass drug administration; MSP-119 = merozoite surface protein 119. The multilevel model included Ab response to gSG6-P1, age group, gender, and MDA at the individual level, long-lasting impregnated nets use at the household level and season, the population size at each survey, the temperature, and relative humidity at the village level.
Table 3.
Multivariate logistic regression mixed model showing the relationship between the Plasmodium falciparum infections and the intensity of antibody responses to gSG6-P1 salivary antigen
| Characteristics | P. falciparum infections | ||
|---|---|---|---|
| OR | 95% Confidence interval | P value | |
| Ab response to gSG6-P1* | |||
| Low | 1 | – | – |
| Medium | 0.88 | 0.49–1.55 | 0.664 |
| High | 1.45 | 0.84–2.52 | 0.178 |
| Very high | 2.27 | 1.35–3.82 | 0.002 |
gSG6-P1 classes were optical density < 0.4125 for low responders, 0.4125 < x < 0.6345 for medium responders, 0.6345 < x < 0.9045 for high responders, and ≥ 0.9045 for very high responders. Analyses were adjusted for temperature, humidity, age, season, and village.
Multivariate logistic analysis also showed that the odds of P. falciparum Ab prevalence strongly increased with age and was lower in females compared with males (Table 2). The seroprevalence of P. falciparum Ab varied according to village (P < 0.001) and season (P < 0.001) but the strength and direction of the association differed according to antigen. The highest seroprevalence to CSP was in HKT whereas KNH showed the highest seroprevalence to MSP-119 (Table 2). The rainy and cool seasons were associated with higher odds of positive CSP responses compared with the hot season (OR: 9.47, 95% CI: 2.69, 33.35, P < 0.001 and OR 8.34, 95% CI: 2.47, 28.11, P = 0.001). Mass drug administration was associated with decreased odds of CSP seroprevalence (OR: 0.49, 95% CI: 0.33, 0.74, P = 0.001) but increased odds of MSP-119 Ab seroprevalence (OR: 4.18, 95% CI: 3.15, 5.54, P < 0.001). Finally, there was no strong evidence of an association between bednet use and the seroprevalence of P. falciparum Ab (Table 2).
Spatial patterns in P. falciparum malaria infections.
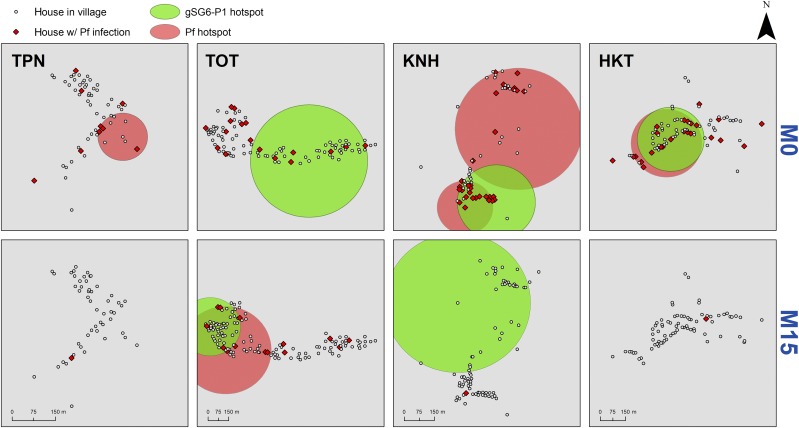
We mapped together the very high responders to Anopheles salivary bites with P. falciparum infections monitored at either surveys or MPs during the rainy season, at baseline (M0, no MDA) and then 15 months after implementation of MDA (M15). At baseline, three villages (KNH, TOT, and HKT) had statistically significant clusters of P. falciparum malaria that partially overlapped with clusters of gSG6-P1 at KNH and HKT (Figure 2). In KNH, there were 34 houses in the salivary response cluster and 65 houses in the two Pf clusters and 28 of these houses overlapped. In HKT, there were 78 houses in the salivary response cluster and also 78 houses in the Pf cluster and 75 of these houses overlapped (96%). Htoo Pyin Nyar showed a detectable hotspot of P. falciparum infections in the middle of the village, with no apparent hotspot of vector exposure (the number of P. falciparum infections were however low). At TOT, P. falciparum infections were dispersed within the village (no clustering) and a wide cluster of vector exposure was seen (P < 0.0001; relative risk = 16.15). P. falciparum infections were drastically reduced by M15 in all villages receiving MDA (Figure 2), except at TOT were remaining pockets of P. falciparum malaria infections and vector exposure persisted in the western part of the village. In this site, there were 64 houses in the salivary response cluster in and 92 in the Pf cluster and 64 of these overlapped. All of the salivary response cluster houses were contained within the Pf cluster (70% of the Pf cluster overlapping with the salivary response cluster).
Figure 2.
Micogeographical clusters of Pf malaria infections and vector exposure in the study villages at baseline (M0) and then after the implementation of the mass drug adminsitration (MDA) pilot trial (M15). The large red (Plasmodium falciparum infections as measured by either ultrasensitive qPCR or rapid diagnostic test) and green (very high responder to gSG6-P1) circles are the statistically significant hotspots detected by spatial scan statistics (P < 0.001). Pf infections are shown in red diamonds. Before MDA (M0), three villages Tar Au Ta, Ka Nu Hta, and Htee Kaw Taw (TOT, KNH, and HKT) had multiple clusters of gSG6-P1 responders (Relative Risk [RR] = 16.15, 9.29, 8.83, and, P < 0.0001, respectively) that partially overlapped with clusters of P. falciparum infections at KNH (RR = 30.20, P < 0.0001) and HKT (RR = 19.46, P < 0.0001). After MDA (M15 conducted at the end of the rainy season), P. falciparum infections almost disappeared in all villages except at TOT where a cluster of P. falciparum infections was detected in the western part of the village (RR = 77.23, P < 0.0001) that mostly overlapped with the cluster of gSG6-P1 (RR = 5.86, P < 0.0001).
All hotspot houses detected with spherical windows were also detected using ellipsoid windows, with slight variations in hotspot window shape. We then retained the spherical windows for final visualization.
DISCUSSION
In the present study, we used antibody responses to demonstrate a strong and significant relationship between Anopheles exposure and P. falciparum malaria in areas of low endemicity along the TMB. This association was shown for antibody responses to two different P. falciparum antigens (MSP-119 and CSP) and confirmed by the observation that very high responders to gSG6-P1 were also at higher risk of P. falciparum infections. This was further supported by spatial statistics showing that P. falciparum clusters partially overlap the gSG6-P1 clusters before and after the use of MDA for malaria elimination. This confirms previous results in the same villages showing a significant correlation between EIR and Ab responses to gSG6-P1 salivary antigen.19 Altogether, these findings suggest that anti-gSG6-P1 could be used to estimate population vulnerability to malaria vector bites and hence be used as a proxy to estimate malaria transmission risk as part of malaria surveillance and elimination.
The spatial mapping data showed that P. falciparum infections clustered in specific parts of the study villages, which were partially geographically correlated to vector mosquito exposure. Although it is likely that some P. falciparum malaria are imported in the study villages by migration and population movement,27 our findings indicate that heterogeneity in P. falciparum malaria is positively associated with heterogeneous mosquito-biting behavior, hence reflecting “focal” transmission risk. Spatial autocorrelation was less obvious over time probably because of the successive introduction of MDA that eliminated > 95% P. falciparum reservoir.20 Despite that, the salivary biomarker was successful to identify remaining sources of P. falciparum transmission at TOT, where low coverage (75%) of MDA was reported.20 Absence of spatial autocorrelation between vector exposure and P. falciparum exposure in some surveys suggest also that a nonnegligible part of malaria transmission probably occurred outside the villages.
Our study showed that the risk of exposure to malaria and the vector vary in space (village) and time (season). The reasons for higher malaria risk in particular locations within villages are presently unknown but we assume that this may reflect different human behavior and socioeconomic conditions, environment (presence of mosquito breeding habitats), vector control practices, etc as demonstrated by others.3 As expected, a clear age-dependent response in malaria-specific immune responses was seen, which confirms exposure-driven acquisition of antibody responses.13,28,29 This trend was more pronounced with MSP-119 than CSP, probably due to the stronger immunogenic property of this antigen. Interestingly, the higher odds of P. falciparum seropositivity to CSP and MSP in older age group correlates well with the age-dependent response to Anopheles exposure observed previously in the area.19 We showed that females were also at a lower risk of P. falciparum malaria exposure than males confirming the predominance of P. falciparum infections in adult males along the TMB due to different behavior, movement, and occupation.2
Other risk factors associated with sero-reactivity to P. falciparum infections was MDA, but the strength and direction of the association differed according to antigen (CSP or MSP-119). The observed differences between antigens may be because of differences in immunogenic properties (e.g., longevity of antibody responses) or differences in exposure of life cycle–specific antigens to the immune system (immunogenicity of antigens); sporozoite antigens, such as CSP, are exposed to the immune system for a shorter duration than blood-stage merozoite antigens, such as MSP-119.30,31 Finally, potential cross-reactivity between P. falciparum and Plasmodium vivax antigens cannot be ruled out. In areas co-endemic for P. falciparum and P. vivax, homologous antigens may elicit cross-reactive antibodies32 and MSP-119 shares ∼50% amino acid identity between the two species. Cross-reactivity may also have contributed to the maintenance of specific Ab responses to MSP in areas where P. vivax persists after MDA.20
Finally, our study revealed that frequent bednet use (“self-reporting”) was not associated with a reduction of P. falciparum malaria exposure, which is consistent with previous findings, showing an absence of correlation between net use and the Ab response to Anopheles bites.19 Along the TMB, malaria vectors bite preferentially outdoors and in the early evening when people are not protected by insecticide treated nets.33,34 This finding suggests that more appropriate personal protection tools (e.g., repellents, treated clothes) should be delivered to people at risk of malaria to strengthen malaria control and elimination efforts. Our results also confirm the great potential of using salivary markers to evaluate the efficacy of vector control interventions, where malaria prevalence and intensity of transmission become low.35 The development of factory-made rapid diagnostic device for detection of human exposure to Anopheles bites would be useful to replace labor-intensive ELISA and be routinely incorporated into elimination programs.
CONCLUSION
In communities living in areas of low seasonal malaria transmission, where reservoirs of submicroscopic infections have been identified, the biomarker of exposure to vector correlates well with the risk of P. falciparum infections. In this study, the relationship between the vector exposure (biomarker) and risk of malaria carriage (Ab) is complex, partly because of MDA and because of the complexities of malaria ecology in the region. Biomarker could be a useful tool to measure the impact of targeted vector control measures deployed as part of an elimination effort that addresses the parasite reservoirs in the “hot spots” villages. These vector control measures are important because LLIN may provide little protective efficacy against Anopheles vectors in this region.
Supplementary Material
Acknowledgments:
We thank villagers of KNH, TOT, HKT, and TPN for their kind support and collaboration; field and laboratory staff from SMRU-TCE research project (Jordi Lantier, Chiara Andolina, Prapan, Billion, Khin Maung Lwin, Myo Chit Min, Taw Nay Soh, and Poe Wah) and STOP-VEC (B. Fustec and Kitti) for their commitment to this project. We thank the Thailand International Development Cooperation Agency (TICA) of the Ministry of Foreign Affairs for providing financial support to the IRD-KU international research program “STOP-VEC.”
Note: Supplemental text and tables appear at www.ajtmh.org.
Disclaimer: The funding sources listed here did not have any role in the analysis or preparation of the data in this manuscript, nor was any payment received by these or other funding sources for this study. Its contents are solely the responsibility of the authors.
REFERENCES
- 1.Imwong M, et al. 2015. The epidemiology of subclinical malaria infections in south-east Asia: findings from cross-sectional surveys in Thailand–Myanmar border areas, Cambodia, and Vietnam. Malar J 14: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrara VI, et al. 2013. Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai–Myanmar border, 1999–2011: an observational study. PLoS Med 10: e1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker DM, et al. 2015. Microgeography and molecular epidemiology of malaria at the Thailand–Myanmar border in the malaria pre-elimination phase. Malar J 14: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phyo AP, et al. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379: 1960–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phyo AP, et al. 2016. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai–Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis 63: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thu AM, Phyo AP, Landier J, Parker DM, Nosten FH, 2017. Combating multidrug-resistant Plasmodium falciparum malaria. FEBS J 284: 2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landier J, Parker DM, Thu AM, Carrara VI, Lwin KM, Bonnington CA, Pukrittayakamee S, Delmas G, Nosten FH, 2016. The role of early detection and treatment in malaria elimination. Malar J 15: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R, 2012. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 9: e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mbogo CM, et al. 2003. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg 68: 734–742. [PubMed] [Google Scholar]
- 10.Imwong M, Nakeesathit S, Day NP, White NJ, 2011. A review of mixed malaria species infections in anopheline mosquitoes. Malar J 10: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly-Hope LA, McKenzie FE, 2009. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, McCarthy J, Vallely A, Drakeley C, 2010. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longley RJ, et al. 2017. Asymptomatic Plasmodium vivax infections induce robust IgG responses to multiple blood-stage proteins in a low-transmission region of western Thailand. Malar J 16: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helb DA, et al. 2015. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci U S A 112: E4438–E4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drakeley CJ, et al. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102: 5108–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ataíde R, et al. 2017. Declining transmission and immunity to malaria and emerging artemisinin resistance in Thailand: a longitudinal study. J Infect Dis 216: 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott SR, Fowkes FJ, Richards JS, Reiling L, Drew DR, Beeson JG, 2014. Research priorities for the development and implementation of serological tools for malaria surveillance. F1000Prime Rep 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doucoure S, Drame PM, 2015. Salivary biomarkers in the control of mosquito-borne diseases. Insects 6: 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ya-Umphan P, Cerqueira D, Parker DM, Cottrell G, Poinsignon A, Remoue F, Brengues C, Chareonviriyaphap T, Nosten F, Corbel V, 2017. Use of an Anopheles salivary biomarker to assess malaria transmission risk along the Thailand–Myanmar border. J Infect Dis 215: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landier J, et al. 2017. Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: a pilot trial in four villages of eastern Myanmar. Wellcome Open Res 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imwong M, Hanchana S, Malleret B, Renia L, Day NP, Dondorp A, Nosten F, Snounou G, White NJ, 2014. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol 52: 3303–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poinsignon A, et al. 2008. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One 3: e2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poinsignon A, Remoue F, Rossignol M, Cornelie S, Courtin D, Grebaut P, Garcia A, Simondon F, 2008. Human IgG antibody response to glossina saliva: an epidemiologic marker of exposure to glossina bites. Am J Trop Med Hyg 78: 750–753. [PubMed] [Google Scholar]
- 24.Ambrosino E, et al. 2010. A multiplex assay for the simultaneous detection of antibodies against 15 Plasmodium falciparum and Anopheles gambiae saliva antigens. Malar J 9: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulldorff MaIMSI, Inc. , 2009. SaTScan Version 9.1.1: Software for the Spatial and Space-Time Scan Statistics Available at: http://www.satscan.org.
- 26.Kulldorf M, 1997. A spatial scan statistic. Commun Stat Theory Methods 26: 1481–1496. [Google Scholar]
- 27.Parker DM, Landier J, von Seidlein L, Dondorp A, White L, Hanboonkunupakarn B, Maude RJ, Nosten FH, 2016. Limitations of malaria reactive case detection in an area of low and unstable transmission on the Myanmar–Thailand border. Malar J 15: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biggs J, et al. 2017. Serology reveals heterogeneity of Plasmodium falciparum transmission in northeastern South Africa: implications for malaria elimination. Malar J 16: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkhof K, et al. 2016. Geographical patterns of malaria transmission based on serological markers for falciparum and vivax malaria in Ratanakiri, Cambodia. Malar J 15: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White MT, Griffin JT, Akpogheneta O, Conway DJ, Koram KA, Riley EM, Ghani AC, 2014. Dynamics of the antibody response to Plasmodium falciparum infection in African children. J Infect Dis 210: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 31.Fowkes FJ, et al. 2012. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis 206: 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagao Y, Kimura-Sato M, Chavalitshewinkoon-Petmitr P, Thongrungkiat S, Wilairatana P, Ishida T, Tan-Ariya P, de Souza JB, Krudsood S, Looareesuwan S, 2008. Suppression of Plasmodium falciparum by serum collected from a case of Plasmodium vivax infection. Malar J 7: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwansomboon N, Chaumeau V, Kittiphanakun P, Cerqueira D, Corbel V, Chareonviriyaphap T, 2017. Vector bionomics and malaria transmission along the Thailand–Myanmar border: a baseline entomological survey. J Vector Ecol 42: 84–93. [DOI] [PubMed] [Google Scholar]
- 34.Sriwichai P, Samung Y, Sumruayphol S, Kiattibutr K, Kumpitak C, Payakkapol A, Kaewkungwal J, Yan G, Cui L, Sattabongkot J, 2016. Natural human Plasmodium infections in major Anopheles mosquitoes in western Thailand. Parasit Vectors 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Londono-Renteria B, et al. 2015. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors 8: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.