Abstract
Paeoniflorin serves important cellular roles, exerting anti-cancer, anti-inflammatory and anti-pulmonary fibrosis effects and possesses immune-modulatory properties. However, the exact role of paeoniflorin in the pathogenesis of osteoarthritis (OA) remains unclear. The aim of the present study was to investigate the effects of paeoniflorin on articular surfaces in vitro. Rat chondrocytes were cultured in vitro and an MTT assay was performed to assess chondrocyte survival. Following treatment with interleukin (IL)-1β and paeoniflorin, the production of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) was examined using reverse transcription-quantitative polymerase chain reaction and western blotting. The interleukin (IL)-1β-induced nuclear factor (NF)-κB pathway activation was also investigated. The results demonstrated that paeoniflorin was able to downregulate the expression of MMP and increase the expression of TIMP-1ntmRNA and protein in IL-1β-induced rat chondrocytes. Furthermore, treating chondrocytes with paeoniflorin blocked the activation of NF-κB. These results suggest that paeoniflorin may serve am anti-catabolic role in the progression of OA and may be an effective preventative treatment for OA.
Keywords: paeoniflorin, osteoarthritis, matrix metalloproteinase, interleukin-1β, nuclear factor NF-κB
Introduction
Osteoarthritis (OA), which is the most common joint disorder, is characterized by the destruction of articular cartilage and osteophyte formation (1). OA has been recognized as a slowly progressing whole-joint soft tissue disease, characterized by synovial inflammation and subchondral bone degradation (2). During the development of OA, the imbalance between synthesis and degradation of extracellular matrix remodeling may lead to erosion of articular cartilage (3). Matrix metalloproteinases (MMPs) are considered to be the most important catabolic enzymes associated with the pathogenesis of OA due to their ability to digest collagen fibers and proteoglycans (4). Tissue inhibitors of metalloproteinases (TIMPs), which inhibit MMP activity, also serve an important role in the catabolic and anabolic processes of cartilage matrix maintenance (5). Additionally, pro-inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6, are able to stimulate the expression of MMP and the subsequent development of OA (6,7).
Paeoniflorin, a pinane monoterpene glucoside, was first isolated from plants in the Ranunculaceae family in the 1960s (8). Recent studies have reported that paeoniflorin exhibits a number of pharmacological activities, including anti-cancer, anti-inflammation, anti-pulmonary fibrosis and anti-spasmodic effects (9–11). In nucleus pulposus cells, paeoniflorin serves an anti-apoptotic role via regulating B-cell lymphoma-2 family and caspase expression (12). At present, the association between paeoniflorin and chondrocytes remains unclear. A recent study demonstrated that paeoniflorin-6′-O-benzene (CP-25), a derivative of paeoniflorin, is able to decrease the production of ILs and TNF-α whilst increasing the expression of transforming growth factor-β in an adjuvant-induced arthritis model (13). Furthermore, CP-25 decreased the expression of receptor activator of nuclear factor (NF)-κB ligand and MMP-9, which suggests that it may have an anti-arthritic effect and so may be used in the treatment of human rheumatoid arthritis (RA). Although MMPs are considered to be important for the pathophysiology of OA, the role of paeoniflorin in OA remains unclear.
The aim of the present study was to assess the protective effects of paeoniflorin on IL-1β-induced chondrocytes and measure the levels of MMP-1, MMP-3, MMP-13 and tissue TIMP-1 in vitro. In addition, the activation of the NF-κB pathway was measured using western blotting.
Materials and methods
Primary cultures of normal rat articular chondrocytes
A total of 24 Sprague-Dawley rats (male:female, 1:1; 4-week old; Animal Center of Zhejiang University, Hangzhou, China) were housed at 25°C with 45-75% relative humidity and food and water at regular intervals with a 12 h light/dark cycle. Rat articular chondrocytes were isolated as previously described (14). Briefly, the rats were sacrificed by intraperitoneal injection with 10% chloral hydrate (4 ml/kg; Sigma Aldrich; Merck KGaA, Darmstadt, Germany). Rat knees were then disinfected with 75% alcohol and transferred into a sterile bench. Following this, knee joints were opened and the articular cartilage were carefully isolated, rinsed three times in PBS, cut into 1-3 mm3 pieces and digested with 0.2% pronase (Sigma Aldrich; Merck KGaA) for 30 min at 37°C followed by 0.1% collagenase (Sigma Aldrich; Merck KGaA) for 4 h at 37°C. Cells were cultured in complete Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing antibiotic-antimycotic solution and 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere containing 5% CO2. The medium was replaced every 2 days. The present study was approved by the University of Zhejiang Institutional Animal Care and Use Committee, Zhejiang University.
Assessment of cell viability
The effect of paeoniflorin on chondrocyte proliferation was assessed using an MTT assay. Chondrocytes were seeded in 96-well plates at a density of 5×103 cells/well and incubated with 0, 12.5, 25, 50, 100 and 200 µM paeoniflorin (Sigma Aldrich; Merck KGaA; Fig. 1) for 72 h at 37°C. The absorbance at 570 nm was subsequently measured using a microplate reader and the cell viability was calculated. Paeoniflorin concentrations that did not induce significant cytotoxicity were considered non-cytotoxic and were selected for using in the following experiments.
Figure 1.
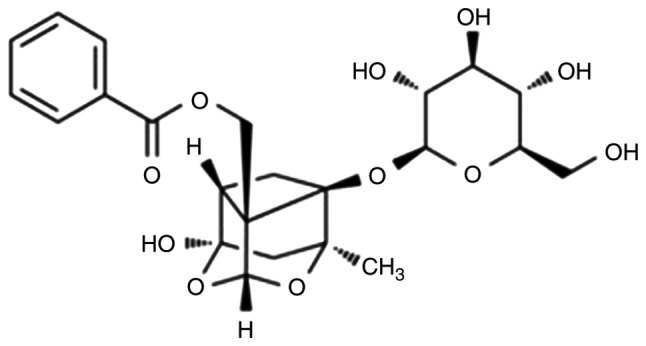
Chemical structure of paeoniflorin.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Isolated articular chondrocytes were seeded in a 6-well plate at a density of 5×105 cells/well. Following 2 days of cultivation at 37°C, cells were cultured in DMEM with 25 or 50 µM of paeoniflorin for 3 h at 37°C, followed by co-incubation with rat recombinant IL-1β (10 ng/ml; Sigma Aldrich; Merck KGaA) for 24 h at 37°C. The monolayer of chondrocytes was harvested and stored at −80°C until use. Total RNA was extracted from chondrocytes using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) as previously described (15). Briefly, 1 µg of total RNA was reverse transcribed in a system containing 10 pmol of random hexanucleotidic primers (Promega Corporation, Madison, WI, USA), 0.5 mM dNTPs and 200 units of Moloney murine leukemia virus reverse transcriptase (Promega Corporation) at 37°C for 1 h. qPCR was performed using the iQTM SYBR Green SuperMix PCR kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's protocol. Thermocycling conditions included 45 cycles of 95°C for 15 sec and 60°C for 30 sec. Annealing temperatures and primers are listed in Table I. GAPDH was used as an internal control and the relative gene expression was calculated using the 2−ΔΔCq method (16).
Table I.
Primers and conditions for reverse transcription-quantitative polymerase chain reaction.
| Gene | Genbank accession | Direction | Primer sequences (5′ to 3′) | Size (bp) | Annealing temp (°C) |
|---|---|---|---|---|---|
| MMP-1 | NM_001134530.1 | Forward | GCTTAGCCTTCCTTTGCTGTTGC | 136 | 60 |
| Reverse | GACGTCTTCACCCAAGTTGTAGTAG | ||||
| MMP3 | NM_133523 | Forward | CTGGGCTATCCGAGGTCATG | 77 | 60 |
| Reverse | TGGACGGTTTCAGGGAGGC | ||||
| MMP13 | NM_133530 | Forward | CAACCCTGTTTACCTACCCACTTAT | 85 | 60 |
| Reverse | CTATGTCTGCCTTAGCTCCTGTC | ||||
| TIMP-1 | NM_053819 | Forward | TCCCTGTTCAGCCATCCCTTG | 96 | 60 |
| Reverse | TCGCTCTGGTAGCCCTTCTC | ||||
| GAPDH | NM_017008.4 | Forward | GAAGGTCGGTGTGAACGGATTTG | 127 | 60 |
| Reverse | CATGTAGACCATGTAGTTGAGGTCA |
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of MMPs.
Western blotting
Rat articular chondrocytes were seeded on a 6-well plate at a density of 5×104 cells/cm2 for 2 days at 37°C. The cells were cultured in DMEM with 25 or 50 µM of paeoniflorin for 3 h at 37°C and followed by co-incubation with 10 ng/ml rat recombinant IL-1β for 24 h at 37°C. Cells were rinsed with ice-cold PBS, lysed using cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA). Protein concentrations were determined by a BCA kit and the protein was boiled at 100°C for 10 min. Western blotting was performed as previously described (17). Briefly, a total of 50 µg protein was loaded per lane. The samples were then separated by 10% SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking with 5% bovine serum albumin. (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, proteins were probed using primary antibodies against MMP-1, MMP-3, MMP-13, TIMP-1 (all Santa Cruz Biotechnology, Inc., Dallas, TX, USA), inhibitor of NF-κB (IκB-α), NF-κB p65 and β-actin (all Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight (Table II). Blots were subsequently incubated with horseradish peroxide-labeled secondary antibodies for 1 h at 37°C, blots were visualized using an enhanced chemiluminescence substrate (Fude Biological Technology, Hangzhou, China) and exposure to Kodak X-Omat film (Kodak, Rochester, NY, USA) according to the manufacturer's protocol. The densitometry of the bands was quantified using the ImageJ software (version 1.45s; National Institutes of Health, Bethesda, MD, USA).
Table II.
Information of antibodies.
| Name | Catalogue numbers | Dilution |
|---|---|---|
| MMP-1 | sc-21731 | 1:1,000 |
| MMP-3 | sc-21732 | 1:1,000 |
| MMP-13 | sc-515284 | 1:1,000 |
| TIMP-1 | sc-21734 | 1:1,000 |
| IκB-α | #9242 | 1:3,000 |
| p65 | #6956 | 1:500 |
| β-actin | #3700 | 1:1,000 |
| Goat anti-Mouse IgG | #31160 | 1:5,000 |
| Goat anti-Rabbit IgG | #31210 | 1:5,000 |
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of MMPs; IκB-α, inhibitor of NF-κB; Ig, immunoglobulin.
Statistical analysis
All experiments were performed in triplicate. Data are presented as the mean + standard deviation and were analyzed using one-way analysis of variance. Statistical analyses were performed using SPSS for Windows software (v19.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of paeoniflorin on cell viability
To investigate whether paeoniflorin is cytotoxic to chondrocytes, cells were treated with varying concentrations of paeoniflorin and an MTT assay was performed. The results revealed that treatment with 12.5-200 µM paeoniflorin was not cytotoxic to chondrocytes (Fig. 2). Referring to previous results from Pubmed, concentrations ranging from 25 to 50 µM were used in the following experiments (18,19).
Figure 2.
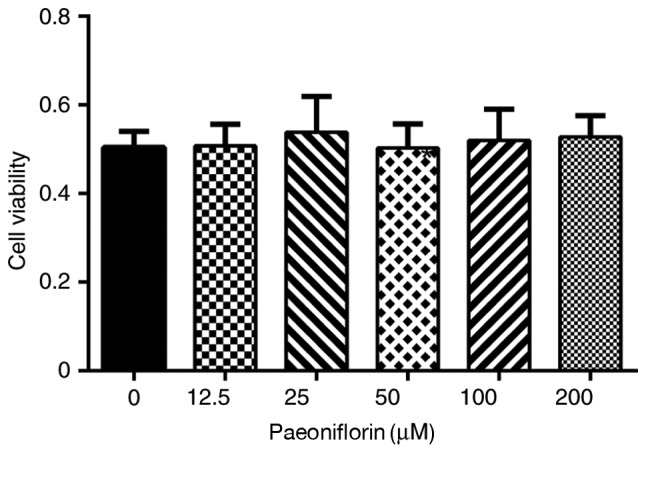
Effects of paeoniflorin on cell viability. Chondrocytes were treated with 0-200 µM paeoniflorin for 72 h and examined using the MTT assay.
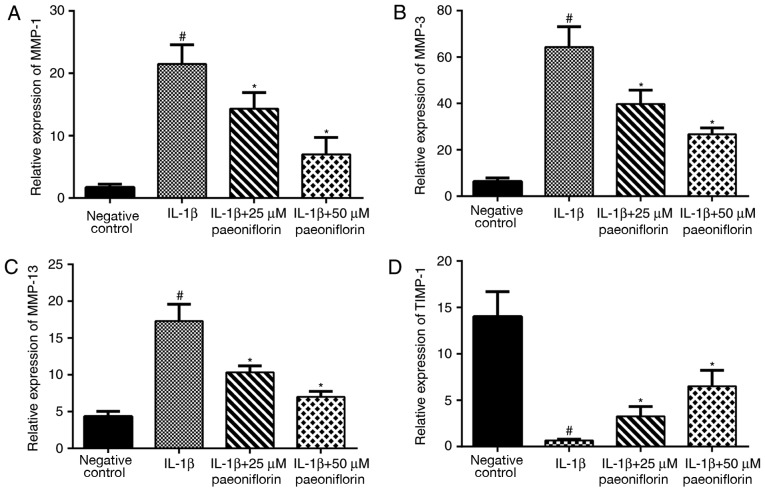
Effect of paeoniflorin on MMP-1, MMP-3, MMP-13 and TIMP-1 mRNA expression
Following treatment with rat recombinant IL-1β (10 ng/ml) and paeoniflorin (25 and 50 µM), the expression of MMP-1, MMP-3, MMP-13 and TIMP-1 were examined using RT-qPCR. Stimulation with IL-1β resulted in a significant upregulation in MMP-1, MMP-3 and MMP-13 expression and a significant decrease in TIMP-1 expression compared with the negative control (P<0.05; Fig. 3). Treatment with 25 or 50 µM paeoniflorin significantly reversed the IL-1β-induced upregulation of MMP-1, MMP-3 and MMP-13 and downregulation of TIMP-1 (P<0.05; Fig. 3).
Figure 3.
Effects of paeoniflorin on the expression of (A) MMP-1, (B) MMP-3, (C) MMP-13 and (D) TIMP-1. Chondrocytes were pretreated with 0, 25 or 50 µM paeoniflorin for 3 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. Gene expression was assessed using reverse transcription-quantitative polymerase chain reaction. *P<0.05 vs. IL-1β alone and #P<0.05 vs. negative control. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of MMP; IL, interleukin.
Effect of paeoniflorin on protein synthesis of MMP-1, MMP-3, MMP-13 and TIMP-1
Following treatment with IL-1β, the expression of MMP-1, MMP-3 and MMP-13 was significantly upregulated (P<0.05; Fig. 4A and B), whereas the expression of TIMP-1 was significantly decreased compared with the negative control group (P<0.05; Fig. 4A and B). Compared with IL-1β treatment alone, the expression of MMP-1, MMP-3 and MMP-13 was significantly decreased (P<0.05; Fig. 4A and B) and the expression of TIMP-1 was significant increased in cells treated with 25 or 50 µM paeoniflorin (P<0.05; Fig. 4A and B).
Figure 4.
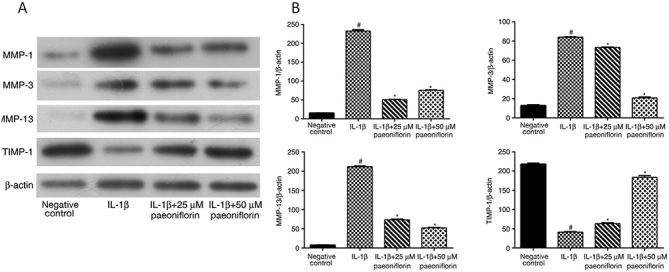
Effects of paeoniflorin on the expression of MMPs and TIMP-1. (A) Western blotting and (B) quantified western blotting results for MMP-1, MMP-3, MMP-13 and TIMP-1. Chondrocytes were pretreated with 0, 25 or 50 µM paeoniflorin for 3 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. Protein expression was assessed using western blotting with β-actin as a control. *P<0.05 vs. IL-1β alone and #P<0.05 vs. negative control. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of MMP; IL, interleukin.
Effect of paeoniflorin on IκB-α degradation and NF-κB activation in IL-1β-treated chondrocytes
Activation of the NF-κB pathway was assessed using western blotting. NF-κB p65 levels were significantly increased and IκB-α levels were significantly decreased in chondrocytes treated with IL-1β alone (P<0.05; Fig. 5A and B). The effects of IL-1β were significantly reversed by treatment with 25 or 50 µM paeoniflorin (P<0.05; Fig. 5A and B).
Figure 5.
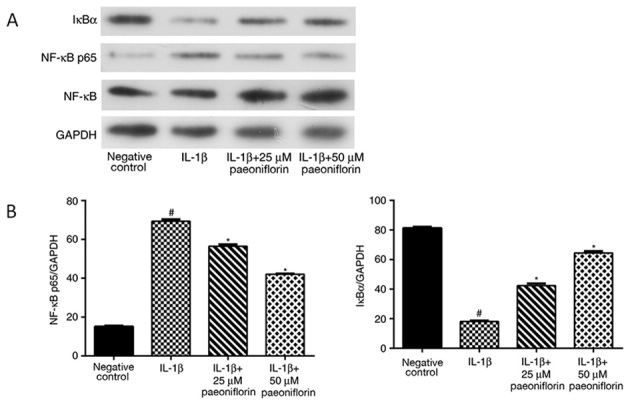
Effects of paeoniflorin on NF-κB and IκB-α expression. (A) Western blotting and (B) quantified western blotting results for NF-κB p65 and IκB-α. Chondrocytes were pretreated with 0, 25 or 50 µM paeoniflorin for 3 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. Protein expression was assessed using western blotting with GAPDH as a control. *P<0.05 vs. IL-1β alone and #P<0.05 vs. negative control. NF-κB, nuclear factor-κB; IκB-α, inhibitor of NF-κB; IL, interleukin.
Discussion
Paeoniflorin is commonly used as a Chinese medicine anti-inflammatory treatment for autoimmune diseases (20–22). At present, little is known about the effects of paeoniflorin on OA. In the present study it was demonstrated that paeoniflorin exhibits a significant chondroprotective effect in vitro.
Previous studies have reported an association between paeoniflorin and arthritis (23,24). Paeoniflorin has been demonstrated to exhibit a potential protective effect against RA in rat models by decreasing the production of IL-1β and TNF-α, thus impeding the progression of arthritis and bone erosion (25). Jia and He (24) reported that paeoniflorin ameliorated RA in rats by decreasing the activity of the NF-κB p65 unit, TNF-α, IL-1β and IL-6, as well as reducing cyclooxygenase-2 protein expression. In human pancreatic cancer cells, paeoniflorin has potential anti-tumor functions, enhancing apoptosis via the inhibition of MMP-9 and extracellular-related kinase signaling (26). A recent study by Zhao et al (27) reported that paeoniflorin is able to reduce the IL-1β-induced upregulation of inflammatory mediators and MMPs in human chondrocytes, which is similar to the results of the present study.
The MMP family, in particular MMP-1, MMP-3 and MMP-13, is known to serve a role in the degeneration of articular cartilage matrix components (28). MMP-1 predominantly destroys fibrillar collagens, while MMP-3 decays extracellular cartilage matrix substrates (29,30). MMP-13, also referred to as collagenase-3, is an enzyme that serves a role in the degradation of type II collagen and is considered to be the principal collagenase associated with restructuring of the collagen matrix (31–33). However, MMP-induced cartilage disintegration may be ameliorated by reducing the expression of endogenous TIMPs (4). As MMPs and TIMP are essential for the pathophysiological progression of OA, the aim of the present study was to assess whether paeoniflorin serves an important role in OA by regulating the expression of MMPs. An in vitro model was produced by cultivating a monolayer of primary rat chondrocytes in a medium containing IL-1β, which is one of the most important pro-inflammatory cytokines released by chondrocytes (34,35). When cells were pre-treated with IL-1β, the expression of MMPs was significantly upregulated at the mRNA and protein levels, whereas TIMP-1 expression decreased compared with the control group. These results were consistent with a previous report (36). However, the IL-1β-induced changes in expression may be attenuated by pretreatment with paeoniflorin (25 and 50 µM), which exerts a protective effect by downregulating the expression of MMPs while upregulating TIMP-1. It is therefore possible that paeoniflorin is able to balance the MMP/TIMP ratio and may be used as a therapeutic treatment for OA.
The results of the present study revealed that NF-κB is associated with paeoniflorin-mediated MMP/TIMP system regulation. The NF-κB signaling pathway regulates the expression of several genes (37,38), including MMP-1, MMP-3 and MMP-13. NF-κB in the cytoplasm maintains an inactive form via binding with IκB (39). When stimulated with IL-1β, NF-κB dimers are activated via a series of signaling pathways, resulting in the phosphorylation and degradation of IκB (37). In the present study, paeoniflorin blocked the activation of NF-κB p65 by protecting IκB-α against degradation. It has previously been reported that paeoniflorin is able to inhibit the nuclear translocation of NF-κB by preventing IκBα phosphorylation in gastric carcinoma cells, which is in agreement with a previous in vitro study by our group (18,40).
The underlying mechanism of inflammation in chondrocytes exposed to paeoniflorin remains unclear. Further studies are required to elucidate the precise signal transduction pathway underlying paeoniflorin regulation in inflammatory processes. The present study is limited as rat articular chondrocytes were cultured in a monolayer only. In future studies, a dynamic three-dimensional chondrocyte culture system is strongly recommended (41). In addition, a number of additional catabolic enzymes and inflammatory factors serve key roles in the pathophysiology of OA, including a disintegrin and metalloproteinase with thrombospondin motifs and inducible nitric oxide synthase, which should be investigated in future studies.
In summary, the results of the present study demonstrate that paeoniflorin has a chondroprotective effect in an in vitro model via decreasing the expression of MMP-1, MMP-3 and MMP-13 whilst upregulating TIMP-1. Furthermore, it was revealed that this anti-catabolic effect was exerted by inhibiting the NF-κB pathway. These results suggest that paeoniflorin may be applied as an effective therapeutic for the treatment of OA.
Acknowledgements
Not applicable.
Funding
The present study was subsidized by a grant from the National Natural Science Foundation of China (grant no. 81301584).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PFH and LDW conceived and designed the study. PFH, FFS, LFJ and JPB performed the experiments. PFH and LDW wrote the paper. PFH, FFS, LFJ, JPB and LDW reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
The present study was approved by the University of Zhejiang Institutional Animal Care and Use Committee, Zhejiang University (Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–642. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamimura M, Nakamura Y, Uchiyama S, Ikegami S, Mukaiyama K, Kato H. The pathophysiology and progression of hip osteoarthritis accompanied with joint pain are potentially due to bone alterations-follow-up study of hip oa patients. Open Rheumatol J. 2014;8:46–53. doi: 10.2174/1874312901408010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GN., Jr The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Fron Biosci. 2006;11:3081–3095. doi: 10.2741/2034. [DOI] [PubMed] [Google Scholar]
- 4.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: Role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 5.Naito K, Takahashi M, Kushida K, Suzuki M, Ohishi T, Miura M, Inoue T, Nagano A. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: Comparison with generalized osteoarthritis. Rheumatology (Oxford) 1999;38:510–515. doi: 10.1093/rheumatology/38.6.510. [DOI] [PubMed] [Google Scholar]
- 6.Aida Y, Maeno M, Suzuki N, Shiratsuchi H, Motohashi M, Matsumura H. The effect of IL-1beta on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005;77:3210–3221. doi: 10.1016/j.lfs.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 8.Shibata S, Aimi N, Watanabe M. Paeoniflorin, a monoterpene glucoside of chinese paeony root. Tetrahedron Lett. 1964;5:1991–1995. doi: 10.1016/S0040-4039(00)75129-X. [DOI] [PubMed] [Google Scholar]
- 9.Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q, Tan J, Ma Q. Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer's disease. Mol Med Rep. 2016;13:2247–2252. doi: 10.3892/mmr.2016.4805. [DOI] [PubMed] [Google Scholar]
- 10.Ji Y, Dou YN, Zhao QW, Zhang JZ, Yang Y, Wang T, Xia YF, Dai Y, Wei ZF. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin. 2016;37:794–804. doi: 10.1038/aps.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao J, Yang X, Ding XL, Guo LM, Zhu CH, Ji W, Zhou T, Wu XZ. Paeoniflorin potentiates the inhibitory effects of erlotinib in pancreatic cancer cell lines by reducing ErbB3 phosphorylation. Sci Rep. 2016;6:32809. doi: 10.1038/srep32809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Teng H, Zhu M, Li C, Huang K, Chen BI, Dai Y, Wang J. Paeoniflorin inhibits nucleus pulposus cell apoptosis by regulating the expression of Bcl-2 family proteins and caspase-9 in a rabbit model of intervertebral disc degeneration. Exp Ther Med. 2015;10:257–262. doi: 10.3892/etm.2015.2501. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Chang Y, Jia X, Wei F, Wang C, Sun X, Xu S, Yang X, Zhao Y, Chen J, Wu H, et al. CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage. Sci Rep. 2016;6:26239. doi: 10.1038/srep26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thirion S, Berenbaum F. Culture and phenotyping of chondrocytes in primary culture. Methods Mol Med. 2004;100:1–14. doi: 10.1385/1-59259-810-2:001. [DOI] [PubMed] [Google Scholar]
- 15.Hu PF, Chen WP, Tang JL, Bao JP, Wu LD. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother Res. 2011;25:878–885. doi: 10.1002/ptr.3359. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Hu PF, Chen WP, Tang JL, Bao JP, Wu LD. Apelin plays a catabolic role on articular cartilage: In vivo and in vitro studies. Int J Mol Med. 2010;26:357–363. [PubMed] [Google Scholar]
- 18.Liu H, Wang J, Wang J, Wang P, Xue Y. Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 2015;1618:149–158. doi: 10.1016/j.brainres.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Liu W. Paeoniflorin inhibits proliferation and promotes apoptosis of multiple myeloma cells via its effects on microRNA29b and matrix metalloproteinase2. Mol Med Rep. 2016;14:2143–2149. doi: 10.3892/mmr.2016.5498. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Lin X, Li H, Yuan J, Peng Y, Dong L, Dai S. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed Pharmacother. 2016;84:1899–1905. doi: 10.1016/j.biopha.2016.10.097. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Du P, Wang J. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways. Mol Med Rep. 2015;12:3937–3943. doi: 10.3892/mmr.2015.3870. [DOI] [PubMed] [Google Scholar]
- 22.He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front Pharmacol. 2011;2:10. doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li PP, Liu DD, Liu YJ, Song SS, Wang QT, Chang Y, Wu YJ, Chen JY, Zhao WD, Zhang LL, Wei W. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. J Ethnopharmacol. 2012;141:290–300. doi: 10.1016/j.jep.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Jia Z, He J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp Ther Med. 2016;11:655–659. doi: 10.3892/etm.2015.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D, Chen J, Zhu H, Xiong XG, Liang QH, Zhang Y, Zhang Y, Wang Y, Yang B, Huang X. UPLC-PDA determination of paeoniflorin in rat plasma following the oral administration of radix paeoniae alba and its effects on rats with collagen-induced arthritis. Exp Ther Med. 2014;7:209–217. doi: 10.3892/etm.2013.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang N, Cui H, Han F, Zhang L, Huang T, Zhou Y, Zhou J. Paeoniflorin inhibits human pancreatic cancer cell apoptosis via suppression of MMP-9 and ERK signaling. Oncol Lett. 2016;12:1471–1476. doi: 10.3892/ol.2016.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Chang Q, Huang T, Huang C. Paeoniflorin inhibits IL1β-induced expression of inflammatory mediators in human osteoarthritic chondrocyte. Mol Med Rep. 2018;17:3306–3311. doi: 10.3892/mmr.2017.8222. [DOI] [PubMed] [Google Scholar]
- 28.Burrage PS, Brinckerhoff CE. Molecular targets in osteoarthritis: Metalloproteinases and their inhibitors. Curr Drug Targets. 2007;8:293–303. doi: 10.2174/138945007779940098. [DOI] [PubMed] [Google Scholar]
- 29.Jo H, Park JS, Kim EM, Jung MY, Lee SH, Seong SC, Park SC, Kim HJ, Lee MC. The in vitro effects of dehydroepiandrosterone on human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11:585–594. doi: 10.1016/S1063-4584(03)00094-3. [DOI] [PubMed] [Google Scholar]
- 30.Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, DeGroot J. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–698. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: The clue to intervertebral disc degeneration? Spine (Phila Pa 1976) 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 32.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL, Qian LH, Qian DW, Duan JA. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem. 2011;18:977–1001. doi: 10.2174/092986711794940905. [DOI] [PubMed] [Google Scholar]
- 34.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 35.Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D694–D703. doi: 10.2741/Martel. [DOI] [PubMed] [Google Scholar]
- 36.Wu DQ, Zhong HM, Ding QH, Ba L. Protective effects of biochanin A on articular cartilage: In vitro and in vivo studies. BMC Complement Altern Med. 2014;14:444. doi: 10.1186/1472-6882-14-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–2584. doi: 10.1016/j.biocel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Ono R, Kaisho T, Tanaka T. PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm. Sci Rep. 2015;5:18327. doi: 10.1038/srep18327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Li W, Wang T, Shu Y, Liu P. Paeoniflorin suppress NF-kappaB activation through modulation of I kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human gastric carcinoma cells. Biomed Pharmacother. 2008;62:659–666. doi: 10.1016/j.biopha.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Huch K, Stove J, Puhl W, Gunther KP. Review and comparison of culture-techniques for articular chondrocytes. Z Orthop Ihre Grenzgeb. 2002;140:145–152. doi: 10.1055/s-2002-31532. (In German) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.