Abstract
The effect of two soccer-training seasons on the growth, development and somatotype hormone concentrations of elite youth soccer players were evaluated. Eighteen elite soccer players and 18 age-matched non-athletic control subjects participated in the study. Anthropometric-measurements, aerobic and anaerobic performance tests and serum concentrations of insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), and growth hormone (GH) were assessed at 5 time points across two competitive seasons. Soccer players revealed higher GH, IGF-1 and IGFBP-3 than the control group across all-time points. Significant moderate correlations were observed only in soccer players between hormonal concentrations (IGF-1 and IGFBP-3) and the jumping tests (r = 0.45-0.48; p < 0.01). Somatotropic axis hormones, anthropometric and physical parameters increased to a greater degree with growth and soccer training combined compared to growth alone. Results from this investigation revealed that intense training did not impair growth or development in these young soccer players across 2-year period.
Key points.
The present study investigated the effect of two soccer-training seasons on the growth, development and somatotype hormone concentrations of elite youth soccer players.
Somatotropic axis hormones, anthropometric and physical parameters increased to a greater degree with growth and soccer training combined compared to growth alone.
The present investigation revealed that intense training did not impair growth or development in these young soccer players across 2-year period.
Key words: Football, Exercise, Training, Hormones, Growth hormone
Introduction
Physical activity plays an important role in the bone health (Mackelvie et al., 2002) and overall growth and development of children (Roemmich et al., 2001). The effectiveness of exercise-training on performance and physical adaptation depends on the training load and on the individual’s ability to adapt to training. Too little or too much training will have relative impact on the desired physical, mental and performance outcomes. Therefore, finding objective measures to quantify the balance between training load and athlete’s tolerance is essential. Intensive sports training and competition are often associated with endocrine deficiencies in adult men (Tanskanen et al., 2011). In young athletes, the stress of intense physical training, combined with calorie restriction, can alter cellular homeostasis and the normal pattern of pubertal development (Adiyaman et al., 2004; Roemmich et al., 2001). The long term benefit from intense training in adolescents and the mechanisms for how intense exercise training may affect tissue anabolism without taking into account the normal growth are not well known (Roemmich et al., 2001). The impact of strenuous exercise on the pubertal development of child and adolescent athletes in several sports is still not well understood.
Normal growth in children and adolescents is regulated to a great extent through the actions of the growth hormone/insulin-like growth factor-I (GH/IGF-I) axis (Adiyaman et al., 2004). Physical exercise plays an important role in the regulation of the GH/IGF-I axis by increasing GH secretion (Kanaley et al., 1997; Kraemer and Ratamess, 2005). Literature data concerning the effect of training on GH response to exercise training are mixed. In over-trained athletes, endogenous GH production is suppressed, exercise tolerance is reduced and performance is decreased (Schmikli et al., 2012). Therefore, the GH response to exercise is complex and appears to be affected by many variables (eg, exercise-type, intensity, duration, age...).
The effects of physical training on IGF-1 responses remain incompletely understood. Some investigations have found that endurance-training increased IGF-1 circulating levels (Maimoun et al., 2004), whereas other authors have reported a decrease (Eliakim et al., 1998). Moreover, acute exercise and exercise-training have been reported to increase IGFBP-3 (Di Luigi et al., 2001). Divergent results concerning GH, IGF-1 and IGFBP-3 may be explained by differences in exercise-type, intensity and duration as well as the subject’s training level, dietary status, body composition, and age.
Soccer is the most popular sport in the world, especially among children and adolescents. Optimizing the physical potential of young soccer players is one of the main objectives of youth soccer academies. Indeed, elite soccer players must be prepared to perform and sustain high-load training. The most important variables for measuring performance in soccer are physical fitness and technical and tactical performance (Rösch et al., 2000). The physical fitness of soccer players is usually measured in terms of endurance, speed, power, and strength (Hoff, 2005). It is relatively easy to test the physical fitness of young players, but it is a more challenging task to differentiate between the adaptations of soccer training and growth-mediated development (Vänttinen et al., 2011).
Some cross-sectional studies have demonstrated that soccer practice induces positive hormonal adaptations (Mejri et al., 2005; Vänttinen et al., 2011). However, effects of long-term intense soccer training on the adaptations of GH/IGF-I axis are very scarce. To the best of our knowledge, only one study examined the effects of intense soccer training over a competitive season on hormones related to growth. Mejri et al. (2005) observed, in young adult soccer players (19 years of age), that soccer training decreased exercise-stimulated GH levels throughout the competitive season, but did not have any effect on basal IGF-1 and IGFBP-3 levels. However, this study had subjects at the end of adolescent growth and they did not include a control group to differentiate exercise training from normal growth (Bouix et al., 1997).
To the best of our knowledge no longitudinal investigation (more than 1-yr.) has studied the impact of intense exercise training in elite adolescent soccer players on markers of growth and development. Therefore, the purpose of this study was to compare changes in growth related hormones (GH, IGF-1 and IGFBP-3) between elite youth soccer players belonging to youth national team and non-athletic controls over a 2-year period. We hypothesized that intense soccer-training would elicit greater physical, hormonal and physiological changes than just growth alone.
Methods
Participants
Thirty-six adolescent boys were recruited to participate in our study. Eighteen were residents at the youth academy of elite soccer players (age: 14.5 ± 0.4 years at the start of the study) and were members of the U-17 national team. These athletes were preparing for the U-17 Championship of African Nations which is a qualifier for the world cup. This group of elite players were selected from among 800 young male soccer players from six regional centres of football throughout Tunisia. The criteria used for selection was based on technical tests and physical fitness parameters. They had been playing soccer, in addition to their school physical education, for 11 months of the year, for at least 5 years, at a rate of 5 practice sessions and one competitive game per week. In general, soccer training sessions lasted ~1.5 hours, with about 15-20 min of warm up consisting of low-intensity games and stretching exercises, 15-25 min of technical soccer exercises (kicking, dribbling, jumping, and running with fast accelerations and decelerations), 20-30 min of match practice, and 10-15 min of active recovery.
Eighteen subjects (age: 14.3 ± 0.3 years at the beginning of the study) were assigned to the control group (non-athletic boys). They participated only in the compulsory physical education curriculum at school (two weekly sessions of 50 min). Individuals in the control group were randomly chosen from nearby schools, were healthy and were representative of the general population. Written informed consent was obtained from the parents of each subject before the study, and the study was approved by the Ethical Committee on Human Research of the University of Manouba, Tunisia.
Experimental design
The experiment was conducted over five time-points for both soccer players and the control group (Figure 1). Testing was conducted over 2 days. On both testing days, all subjects performed the identical standardized 15 min warm-up consisting of low-intensity running, a series of dynamic stretching exercises (high knee lifts, butt kicks, straight line skipping, etc.) and short accelerations. On the first test day, subjects performed a squat jump, counter movement jump using arms, a 5 consecutive jump test and a 30m sprint. On the second testing day, the subjects performed a Yo-Yo Intermittent Recovery Test - Level 1(2).
Figure 1.
Study design: The experiment time-points for both soccer players and control groups. T0: first test; T1: second test; T2: third test; T3: fourth test; T4: fifth test
Puberty stage assessment
Puberty (Tanner) stage was determined and recorded by a pediatrician experienced in the assessment of secondary sex characteristics according to the method of Tanner (1975). It was determined that all subjects were at Tanner stage 2-3 at the beginning of the study and 2 years after they attained Tanner stage 4 and 5.
Anthropometric characteristics
Each participant came to the laboratory for a medical examination and anthropometric measurements performed by a pediatrician at each time period (T0, T1, T2, T3 and T4). Body height and body mass were measured with standard techniques to the nearest 0.1 cm and 0.1 kg, respectively for each subject. To estimate the adiposity, skin-fold thickness was measured at four sites (triceps, biceps, subscapular and suprailiac) (During and Webster., 1985) using a Harpenden skin-fold calliper (British Indicators Ltd., Luton) . All measurements were taken in the morning between 07:30 and 08:30 am by the same investigator for all time periods.
Physical fitness characteristics
Vertical Jump: Each subject performed three maximal jumps of the following type: 1) a squat-jump (SqJ), starting with knees bent at 90° and without previous counter movement; 2) a counter-movement-jump (CMJ), starting from a standing position allowing for counter movement with the intention of reaching knee bending angles of around 90° just before propulsion; and 3) a five-jump-test (5J), consisting of 5 consecutive long jumps. There was a 1-minute rest period between jumps and jump types. The ground reaction force generated during these vertical jumps was measured with an ergo jump (Opto Jump Microgate - ITALY) and converted to jump height (cm). The best (highest) jump of each type was used for analysis.
Running speed test: The participants performed three maximal 30-m sprints, measured with an infrared photoelectric cell (Cell Kit Speed Brower, USA). During the 3-minute recovery periods in-between sprints, the participants walked-back to the starting line and then waited for the next sprint. The participants commenced the sprint from a standing start, 0.5 m behind the first timing gate. Stance for the start was consistent for all participants. The best (fastest) 30-m sprint time was selected for analysis. The results show that these tests were highly repeatable: 30-m sprint (intra-class correlation) (ICC = 0.96), SJ (ICC = 0.93), CMJ (ICC = 0.90), 5-J (ICC = 0.89).
Endurance performance: The Yo-Yo Intermittent Recovery Test Level 1 (YYIRT1) was used to estimate maximal oxygen consumption (VO2max) (Bangsbo, 1994; Bangsbo et al. 2008). Briefly, the YYIRT1consisted of repeated 20m runs back and forth between the starting, turning, and finishing line at a progressively increased speed controlled by an audio metronome from a calibrated CD player. The subjects had a 10-s active rest period (decelerating and walking back to the starting line) between each running bout. When the subjects failed twice to reach the finishing line in time, or decided that they could no longer run at the imposed pace, the total distance covered was recorded. VO2max was estimated from the equation: VO2max (ml/kg/min) = YYIRT1 distance completed (m) × 0.0084 (ml/kg/min)/m + 36.4 ml/kg/min (Bangsbo et al. 2008). All tests were conducted by the same investigators, scheduled at the same time of day, carried out in the same order and using the same apparatus at each period as to retain maximum amount of validity and levels of reliability. All jumping tests were performed on a concrete surface with the players wearing running shoes, whereas the running speed test and the Yo-Yo IRT Level 1 tests were performed on a soccer pitch with the players wearing soccer cleats. Each player was instructed and verbally encouraged to provide maximal effort during all tests.
Blood analysis
Blood samples were drawn from all participants at each of the five testing periods, during the same week as the physical tests and anthropometric measurements. Blood samples were taken between 7:00 and 8:30 am following an overnight fast. The blood samples were centrifuged for 10 minutes at 4°C and 3000 rpm and the extracted serum was stored frozen at - 80°C until analysis. Growth hormone (GH) was measured by a sensitive chemiluminescent assay (Immulite, Diagnostic Products Corp., Los Angeles, CA, USA). Inter-assay coefficient of variation (CV) was 5.7-10% and the intra-assay CV was 4.9-8.3%. Assay sensitivity was 0.1 ng/ml. Serum concentrations of total insulin-like growth factor-1 (IGF-1) were measured using the Non-Extraction Insulin-Like Growth Factor-1 IRMA Kit (Diagnostic Systems Laboratories, Webster, TX, USA). The sensitivity, or minimum detection limit, was 2 ng/ml. The inter-assay coefficients of variability (CVs) were 7.4 and 4.2%, respectively, for concentrations 35.5 and 383.9 ng/ml. The inter-assay CVs were 7 and 3.9%, respectively, for the mean concentrations 34.0 and 373.9 ng/ml. Serum concentrations of total insulin-like growth factor binding protein-3 (IGFBP-3) were estimated using the Non-Extraction Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3) Immunoradiometric Assay Kit (Diagnostic Systems Laboratories, Webster, TX, USA). The minimum detection limit was approximately 0.5 ng/ml. The intra-assay CVs were 1.8 and 3.9%, respectively, for the mean concentrations 82.7 and 7.4 ng/ml. The inter-assay CVs were 1.9 and 0.6%, respectively, for the mean concentrations 76.9 and 8.0 ng/ml.
Statistical analysis
Results are expressed as means ± standard deviations (SD). An analysis of variance (ANOVA) with repeated measures was used to check significant differences between groups and across time points in anthropometric and physical parameters and hormonal concentrations (SPSS for Windows, version 16.0; SPSS Inc, Chicago). Significant differences were assumed when p < 0.05. All variables used in the study were checked for normality of distribution before the analyses (Kolmogorov-Smirnov tests). After confirming significant group differences over time, Newman-Keul’s test post hoc was performed. The reliability of each test was assessed by intra-class correlations (ICCs). The correlations between independent variables (GH, IGF-1, IGFBP-3) and the dependent variables (physical performances) were determined by simple regression. Effect size was calculated to document the size of the statistical effects observed and defined as small for r > 0.1, medium for r > 0.3, and large for r > 0.5. The magnitude of the effect for the correlations was determined using the modified scale as proposed by Hopkins: r < 0.1, trivial; 0.1–0.3, small; > 0.3–0.5, moderate; > 0.5–0.7, large; > 0.7–0.9, very large; > 0.9, nearly perfect; and 1 perfect (Hopkins, 2009).
Results
The anthropometric data of the soccer players and control group are summarized in Table 1. Significant differences (p < 0.01) are observed concerning weight, height and % of body fat measurements between soccer players and the controls.
Table 1.
Anthropometric characteristics of soccer players and control subjects determined during two-soccer seasons (mean±SD).
| Measurements | Soccer players (n = 18) | Controls (n = 18) | P Values | Δ | |
|---|---|---|---|---|---|
| Weight (kg) | T0 | 70.1 ± 5.3 | 53.5 ± 12.1 | 0.001 | 16.6** |
| T1 | 66.6 ± 5.5 | 53.1 ± 11.9 | 0.001 | 13.5** | |
| T2 | 67.9 ± 5.3 | 54.7 ± 11.7 | 0.001 | 13.2** | |
| T3 | 68.7 ± 5.4 | 55.9 ± 12.3 | 0.001 | 12.8** | |
| T4 | 70.3 ± 5.1 | 57.2 ± 11.3 | 0.001 | 13.1** | |
| Height (cm) | T0 | 175.1 ± 3.3 | 167.9 ± 9.1 | 0.01 | 7.2* |
| T1 | 176.2 ± 3.6 | 169.1 ± 7.9 | 0.01 | 7.1* | |
| T2 | 178.5 ± 3.6 | 171.6 ± 8.7 | 0.001 | 6.9* | |
| T3 | 181.7 ± 3.8 | 172.1 ± 8.5 | 0.001 | 9.6** | |
| T4 | 184.6 ± 3.7 | 173.5 ± 9.1 | 0.001 | 11.1** | |
| %Body fat | T0 | 12.3 ± 2.8 | 17.7 ± 2.6 | 0.01 | -5.4* |
| T1 | 11.8 ± 2.6 | 16.4 ± 2.8 | 0.01 | -4.6* | |
| T2 | 11.1 ± 2.1 | 16.8 ± 2.9 | 0.01 | -5.7* | |
| T3 | 10.5 ± 1.2 | 16.5 ± 2.7 | 0.001 | -6.0** | |
| T4 | 10.2 ± 1.8 | 16.9 ± 2.3 | 0.001 | -6.7** | |
| Body fat (kg) | T0 | 8.6 ± 1.8 | 9.5 ± 1.8 | 0.01 | -0.9* |
| T1 | 7.9 ± 1.9 | 8.7± 1.7 | 0.01 | -0.8* | |
| T2 | 7.5 ± 1.7 | 9.2 ± 1.8 | 0.01 | -1.7* | |
| T3 | 7.2 ± 1.9 | 9.2 ± 1.7 | 0.01 | -2.0* | |
| T4 | 7.2 ± 1.8 | 9.7 ± 1.8 | 0.01 | -2.5* | |
| Lean body mass (kg) | T0 | 61.5 ± 1.7 | 44.0 ± 1.8 | 0.001 | 17.5** |
| T1 | 58.7 ± 1.9 | 44.4 ± 1.4 | 0.001 | 14.3** | |
| T2 | 60.4 ± 1.4 | 45.5 ± 1.9 | 0.001 | 14.9* | |
| T3 | 61.5 ± 1.7 | 46.7 ± 2.1 | 0.001 | 14.8** | |
| T4 | 63.1 ± 1.8 | 47.5 ± 1.5 | 0.001 | 15.6** |
Δ: difference between groups; T0: First Test in October 2008; T1: Second Test in February 2009; T2: Third Test in May 2009; T3: Fourth Test in November 2009; T4: Fifth Test in May 2010; Significant differences between soccer players and control subjects.
* p < 0.01
** p < 0.001.
Results show changes in anthropometric parameters (weight, height and % body fat) for soccer players and the control group throughout the various time periods: T0-T1 (first follow-up period); T1-T2 (second follow-up period); T2-T3 (third follow-up period); T3-T4 (fourth follow-up period) and T0-T4 (Total follow-up period). For soccer players, the change values (Δ) are statistically significant for height and weight (p < 0.01). However, for % body fat, significant differences are only observed in total period T0-T4. For control subjects, significant differences of the Δare observed for most of the follow-up periods for height and weight, but there are no significant changes in % body fat for any time period.
The results for the physical fitness testing parameters (CMJ, SqJ, 5J, 30m Sprint, YYIRT (m) and estimated VO2max) in soccer players compared with the control group during each time period are presented in Table 2. The results demonstrate that most of the Δdifferences were significantly greater for young soccer players compared with controls. Over the Total period (T0-T4) significantly greater changes are observed in the soccer players compared to controls for all parameters (Table 2). At T4, regression analysis showed significant correlations between FFM and CMJ (r = 0.47, p < 0.05, moderate), SqJ (r = 0.57, p < 0.01, large) and 5J (r = 0.42, p < 0.05, moderate) for soccer players only.
Table 2.
Changes in physical fitness parameters of soccer players and control subjects determined during two-soccer seasons (mean ± SD).
| Variables | CMJ (cm) | SqJ (cm) | 5J (cm) | 30m (s) | YYIRT (m) | VO2max (ml/kg/min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | SP | C | SP | C | SP | C | SP | C | SP | C | SP | C |
| T0-T1 | 3.18 ** | 0.89£ | 1.89 ** | 0.31£ | 1.35 ** | 0.43*£ | -0.03 | -0.03 | 769 ** | 21£ | 5.37 ** | 0.11£ |
| T1-T2 | 2.27** | 1.51*£ | 1.38* | 0.93£ | 0.38 | 0.40 | -0.04 | -0.01 | 355* | 89£ | 2.38* | 0.04£ |
| T2-T3 | 5.35 ** | 0.91£ | 3.40 ** | 0.69£ | 0.59* | 0.65* | -0.16* | -0.05£ | 235** | 79£ | 1.17* | 0.59£ |
| T3-T4 | 2.17 ** | 1.15£ | 0.37* | 0.49*£ | 1.28** | 0.57£ | -0.05 | -0.06 | 271 ** | 11£ | 1.86 ** | 0.63£ |
| T0-T4 | 12.66** | 3.78**£ | 6.98 ** | 1.43 **£ | 2.77 ** | 1.17**£ | -0.29** | -0.17**£ | 1119 ** | 179**£ | 8.47 ** | 1.41 **£ |
CMJ, countermovement jump; SqJ, squat jump; 5J, highest of five consecutive jumps; 30m, 30-m sprint; YYIRT, distance covered in the Yo-Yo Intermittent Recovery Test - Level 1; VO2max, maximal oxygen consumption; SP, Soccer player; C, Controls; Significant differences in change between time points
* p < 0.01
** p < 0.001. Significant differences in change between soccer players and control subjects £: p < 0.01
Table 3 shows that the hormonal concentrations of GH, IGF-1 and IGFBP-3 of the soccer players are significantly higher than those of the control subjects at all the assessment periods (p < 0.001). Similarly, results showed that the ratio IGF-1/IGFBP3 is significantly (p < 0.001) higher in soccer players compared to the control group at all five evaluation sessions. Figure 2 (A and B) shows the change in GH in young soccer players (Figure 2A) and the control group (Figure 2B). Significant GH increases are observed in soccer players during all the time periods (p < 0.01). However, no significant changes are recorded for the control group (B). Figure 2 (C and D) illustrates the change in IGF-1 for soccer players (Figure 2C) and the control subjects (Figure 2D). Significant changes are observed from baseline for the soccer players during all time periods (T0-T1, T0-T2, T0-T3) (p < 0.01). Similarly, significant differences are observed across all periods for the soccer players (T1-T2; T2-T3; T3-T4 and T0-T4) (p < 0.01). For control subjects, significant differences are observed only in IGF-I at T0-T1 and for the total time period (T0-T4) (p < 0.05) (B). The changes in IGFBP-3 in young soccer players and controls are represented in Figure 2 (E and F). During the different time periods, significant changes are observed in young soccer players (Figure 2E) for T0-T1, T0-T2 and T0-T3 (p < 0.01) and T1-T2, T2-T3, T3-T4 and T0-T4 (A). For the control group (Figure 2F) significant differences are observed (p < 0.01) in IGFBP-3 only at T0-T1 and following the final phase of the longitudinal study (T0-T4) (B).
Table 3.
Hormonal Concentrations (GH, IGF-1 and IGFBP3) of soccer players and control subjects determined during two-soccer seasons (mean ± SD).
| Measurements | Soccer players (n = 18) | Controls (n = 18) | P Values | Δ | |
|---|---|---|---|---|---|
| GH (nmol/L) | T0 | 1.69 ± 0.44 | 1.19 ± 0.90 | 0.01 | 0.50* |
| T1 | 2.07 ± 0.48 | 1.15 ± 0.62 | 0.01 | 0.92* | |
| T2 | 2.62 ± 0.41 | 1.39 ± 0.67 | 0.001 | 1.23** | |
| T3 | 2.70 ± 0.43 | 1.25 ± 0.55 | 0.001 | 1.45** | |
| T4 | 2.02 ± 0.47 | 1.05 ± 0.58 | 0.01 | 0.97* | |
| IGF-1 (nmol/L) | T0 | 765.35 ± 62.71 | 455.95 ± 71.29 | 0.001 | 309.4** |
| T1 | 847.95 ± 38.02 | 515.70 ± 61.56 | 0.001 | 332.25** | |
| T2 | 914.20 ± 34.68 | 523.75 ± 58.87 | 0.001 | 390.45** | |
| T3 | 970.60 ± 34.45 | 552.90 ± 80.91 | 0.001 | 417.7** | |
| T4 | 851.65 ± 43.97 | 396.55 ± 95.78 | 0.001 | 455.10** | |
| IGFBP-3 (nmol/L) | T0 | 45.75 ± 5.36 | 38.11 ± 3.09 | 0.001 | 7.64** |
| T1 | 49.35 ± 4.52 | 41.70 ± 2.12 | 0.001 | 7.65** | |
| T2 | 51.30 ± 3.77 | 42.15 ± 3.24 | 0.001 | 9.15** | |
| T3 | 55.70 ± 5.84 | 43.45 ± 4.55 | 0.001 | 12.25** | |
| T4 | 57.55 ± 5.86 | 42.00 ± 5.54 | 0.001 | 15.55** | |
| IGF-1/IGFBP3 | T0 | 16.72 ± 3.29 | 11.96 ± 3.64 | 0.001 | 4.76** |
| T1 | 17.18 ± 3.64 | 12.36 ± 3.12 | 0.001 | 4.82** | |
| T2 | 17.82 ± 3.68 | 12.42 ± 2.96 | 0.001 | 5.4** | |
| T3 | 17.42 ± 3.22 | 12.72 ± 2.58 | 0.001 | 4.7** | |
| T4 | 14.79 ± 2.82 | 9.44 ± 2.75 | 0.001 | 5.35** |
GH, growth hormone; IGF-1, insulin-like growth factor-1, IGFBP3, insulin-like growth factor binding protein-3; T0: first test; T1: second test; T2: third test; T3: fourth test; T4: fifth test. Significant differences between soccer players and control subjects
* p < 0.01
** p < 0.001
Figure 2.
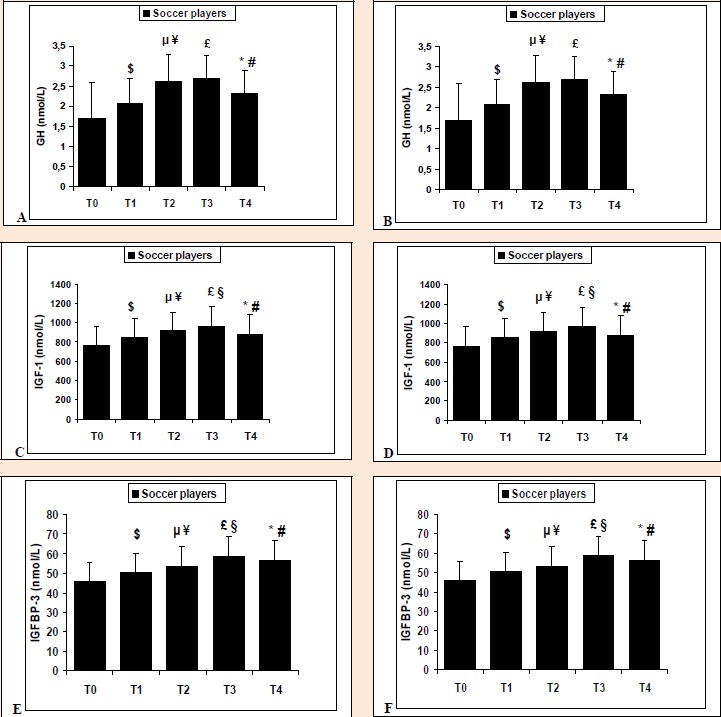
Change in GH, IGF-1 and IGFBP3 between each time period across 2 soccer seasons in young soccer players (A, C, E) and control subjects (B, D, F). GH, growth hormone; IGF-1, insulin-like growth factor-1, IGFBP3, insulin-like growth factor binding protein-3; T0: first test; T1: second test; T2: third test; T3: fourth test; T4: fifth test. $, (p < 0.001): Hormonal change from T0-T1; µ, (p < 0.001): Hormonal change from T0-T2; £, (p < 0.001): Hormonal change from T0-T3; ¥, (p < 0.001): Hormonal change from T1-T2; §, (p < 0.001): Hormonal change from T2-T3; #, (p < 0.001): Hormonal change from T3-T4; *, (p < 0.001): Hormonal change from T0-T4.
Results show that IGF-1 is significantly correlated with the FFM (r = 0.46, p < 0.05, moderate), CMJ (r = 0.49, p < 0.05, moderate), SqJ (r = 0.58, p < 0.01, large) and 5J (r = 0.52, p < 0.01, large) for soccer players at T0. In addition, at T4 the same significant correlations are observed between IGF-1 and the same physical parameters (CMJ, SqJ and 5J). Regression analysis showed significant correlations between IGFBP-3 and CMJ (r = 0.45, p < 0.05, moderate), SqJ (r = 0.58, p < 0.01, large) and 5J (r = 0.47, p < 0.01, moderate) for soccer players at T0. Similar correlations are recorded between IGFBP-3 and the same physical parameters (CMJ, SqJ and 5J) for young soccer players compared to control subjects at T4. No significant relationship is observed between IGFBP-3 and any physical parameters for the control group at T0 and T4. In addition, no significant correlations were found between GH and physical performances for either group at any time point. However, results show that IGF-1/IGFBP-3 ratio is significantly correlated with the CMJ (T0: r = 0.47, p < 0.05 and T4: r = 0.49, p < 0.05), SqJ (T0: r = 0.59, p < 0.01, large and T4: r = 0.57, p < 0.01, large) and 5J (T0: r = 0.47, p < 0.01, moderate and T4: r = 0.49, p < 0.01, moderate) for soccer players only (Table 4).
Table 4.
Relationship between physical fitness parameters and hormonal concentrations (GH, IGF-1, IGFBP-3 and IGF1/IGFBP3 ratio) for the soccer players and the controls.
| Variables (r) |
GH (ng/ml) | IGF-1 (ng/ml) | IGFBP-3 (ng/ml) | IGF1/IGFBP-3 (ng/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T 0 | T 4 | T 0 | T 4 | T 0 | T 4 | T 0 | T 4 | |||||||||
| Groups | S P | C | S P | C | S P | C | S P | C | S P | C | S P | C | S P | C | S P | C |
| CMJ (cm) | 0.07 | -0.12 | 0.24 | -0.11 | 0.49 * | 0.01 | 0.45 * | 0.05 | 0.45* | 0.15 | 0.47* | 0.05 | 0.47* | 0.15 | 0.49* | 0.03 |
| SqJ (cm) | 0.04 | -0.15 | 0.13 | -0.04 | 0.58 ** | 0.13 | 0.57 ** | 0.24 | 0.58** | 0.04 | 0.57** | 0.03 | 0.59** | 0.04 | 0.57** | 0.04 |
| 5J (cm) | 0.25 | -0.18 | 0.27 | -0.16 | 0.52 ** | 0.04 | 0.47 * | 0.05 | 0.47* | 0.27 | 0.52** | 0.11 | 0.47* | 0.27 | 0.49** | 0.10 |
| 30m (s) | -0.02 | -0.11 | -0.11 | -0.16 | 0.02 | 0.11 | 0.14 | 0.19 | 0.17 | 0.09 | 0.21 | 0.14 | 0.19 | 0.08 | 0.28 | 0.16 |
| YYIRT (m) | -0.36 | -0.10 | -0.20 | -0.16 | 0. 22 | 0.04 | 0.19 | 0.14 | 0.36 | 0.16 | 0.13 | 0.12 | 0.47 | 0.16 | 0.15 | 0.13 |
| VO2 max | -0.05 | -0.02 | -0.19 | -0.02 | -0.23 | 0.03 | 0.13 | 0.18 | 0.35 | 0.14 | 0.12 | 0.12 | 0.35 | 0.16 | 0.14 | 0.13 |
GH, growth hormone; IGF-1, insulin-like growth factor-1, IGFBP3, insulin-like growth factor binding protein-3; CMJ, countermovement jump; SqJ, squat jump; 5J, highest of five consecutive jumps; 30m, 30-m sprint; YYIRT, distance covered in the Yo-Yo Intermittent Recovery Test - Level 1; VO2max, maximal oxygen consumption (ml/min/kg) ; SP, Soccer player; C, Controls; *: Significant correlations
*: p< 0.05
**: p< 0.01.
Discussion
The main findings of the study showed significant alterations in the hormonal concentrations (GH, IGF1 and IGFBP3) and physical fitness parameters (jumping tests and 30m sprint) in the young soccer players compared to the control subjects matched for age during the two-season follow-up. Specifically these changes were mainly seen between T0 and T4. These differences can mainly be explained by an adaptation to the physical exercise occurring with soccer training.
The study results revealed significant increases in height of all the adolescent subjects across the 2-year period and the elite soccer players remained taller compared to the control group across time points monitored. From the initiation of this particular investigation, the soccer players started a taller and heavier baseline than the control subjects; however, they were closely matched for age and Tanner stage of pubertal development. Results from the study support the idea that sport, even intense soccer training, has beneficial effects on developmental growth (Mackelvie et al., 2002) and concur to other studies reporting how youth elite soccer players are taller (Gil et al., 2007) and skeletally more mature (Malina et al., 2000) when compared to the non-athletic subjects.
Additionally, throughout this investigation substantial differences were observed in weight between the young soccer players and control subjects over the two soccer seasons across all five time-points. Soccer players assessed were heavier at all testing points compared to the control group, which is in line with findings from Gil et al. (2007) who suggested in general that elite soccer players are heavier than their non-elite-counterparts. The data generated showed how weight within the elite soccer players decreased significantly from T0 to T3, and increased slightly at T4, whereas for the control; a significant increase in weight was observed from T0 to T4.
Furthermore, significant differences in percentage of body fat were observed between the two testing groups. Soccer players were leaner than their control counterparts throughout the entire study period and similar results were observed by other authors comparing elite soccer players to non-elite players (Gil et al., 2007). For youth soccer players, the body fat decreased significantly over the study period (T0-T4) (p < 0.01), but for control subjects no significant changes were observed.
Physical exercise is known to play an important role in the regulation of the GH-IGF-I axis (Kanaley et al., 1997). The GH response to exercise is dependent on the duration and intensity of the exercise, the fitness of the exercising subject, and other environmental factors such as the ambient temperature (Wheldon et al., 2006). During teenage years, GH also plays an essential role in the regulation of anthropometric characteristics and has a key role in regulating body composition. In addition, GH can modify physiological responses to exercise training, especially somatotype hormone responses (Duclos, 2001). In adolescents, separating out the GH effects of exercise training from the effects of maturation can be difficult. Our results showed significant changes in plasma GH concentrations during the different time periods and after two-soccer seasons in young soccer players but no change was seen in the age matched, sedentary control group. Similar results have been found with longer periods of training being associated with stable or with increases in circulating GH and IGF-1 levels (Eliakim et al., 2010).
However, our results are not consistent with those reported by the only study, to our knowledge, which monitored plasma GH concentrations during one soccer season (Mejri et al., 2005). In fact, in this previous study, the authors demonstrated that GH levels at rest and in response to exercise were considerably greater at the beginning of the soccer season (S1) than in its middle (S2) or at its end (S3) (Mejri et al., 2005). These differences in findings could be explained by different factors known to affect GH responses, such as the intensity and duration of training sessions and chronological age (Kraemer and Ratamess, 2005). In fact, soccer players involved in the study conducted by Mejri et al. (2005) were young adults (19 ± 1 yr.) and their relative training load was lower than that of our soccer players. It was suggested that a threshold training intensity was required to stimulate a changes in GH with a long period of training (Manetta et al., 2002). Also, the study of Mejri et al. (2005) had no control group, so it is difficult to separate changes in normal growth versus changes due to training.
Serum IGF-1 levels increase steadily around pre-puberty, with a peak occurring in late puberty, and declining quickly thereafter (Juul et al., 1995). Contradictory results have been reported regarding the effect of physical exercise on serum IGF-1 (Eliakim et al., 1998). Data from the present study illustrates a change in total serum IGF-I for both soccer players and the control subjects, but the change was greater for the soccer players. As well, there were significant differences across all periods in soccer players compared to control subjects after two soccer seasons (p < 0.01). Similar increases have been seen in resting IGF-1 during long-term endurance training in young men and women (Kraemer and Ratamess, 2005; Kanaley et al., 1997). Poelman et al., (1994) found that endurance training also increased the fasting level of IGF-1 in older adults. These results suggested that the age-associated decline in somatotropic hormones may be attenuated by endurance training.
As with our GH results, our findings of increased IGF-I basal values with exercise training are not in accordance with those reported by Mejri et al. (2005). In young people, high-intensity exercise is associated with increased activity of the IGF system favoring an anabolic state (Eliakim et al., 1998). The IGFBP3 is the major binding protein for IGF-1 in human circulation. This hormone is a glycoprotein, which is synthesized in many tissues. IGFBP3 is not only a transport protein, but also has other complex actions such as modulating both the endocrine and paracrine actions of IGF-1, influencing IGF-1 bioavailability and may also exert IGF-independent effects on target cells. IGFBP3 could be a way to prolong the effects of GH (which changes rapidly with physical exercise) (Bouix et al., 1997; Juul et al., 1995). IGFBP3 levels increased in both groups throughout our study, but to a much greater extent in the soccer players. The significant increase of serum IGFBP-3 is in agreement with the other data examining IGFBP-3 in children and adolescents (Juul et al., 1995). Thus, the average concentrations of IGFBP-3 increase progressively during puberty to reach the maximal values about the age of 14-15 (Bouix et al., 1997). Moreover, exercise and training have been reported to increase IGFBP-3 to a greater extent than with growth alone (Di Luigi et al., 2001) and there is general agreement about this finding.
Previous findings demonstrated that levels of physical fitness in adults or young men are known to be correlated with concentration levels of IGF-1. Serum IGF-1 and serum IGFBP3 levels are identified to positively correlate with normal growth rate in children and adolescents and also with aerobic performance in children (Brun et al., 1996; Manetta et al., 2002). Significant correlations between IGF-1 and fitness parameters (CMJ, SqJ and 5J) were found in our young soccer players in the beginning of the study and after two soccer seasons. The correlation existing between fitness parameters and serum IGF-I, confirm that in healthy adolescents, high-intensity exercise is associated with increased activity of the IGF system favoring an anabolic state (Eliakim et al., 1998). Maimoun et al. (2004) observed that the bioavailability IGF-1 index (IGF-1/IGFBP-3) increased, while IGFBP3 concentrations were unchanged after a triathlon season.
Similar to serum IGF-1 levels, the levels of serum IGFBP-3 and were also positively correlated with fitness parameters (CMJ, SqJ and 5J) in young players following two soccer seasons, but not in control subjects. Moreover, in the current study significant correlations were observed between the ratio IGF1/IGFBP3 and CMJ, SqJ and 5J. Similarly, significant correlation between serum IGFBP3 and physical parameter (VO2max) has been described in young male subjects (Brun et al., 1996). Thus, IGFBP3 and especially the ratio IGF1/IGFBP3 may be considered as an endocrine marker of physical fitness (Manetta et al., 2002).
Data in the literature concerning the effect of training on GH levels are controversial. However, in our study, no association was observed between GH and physical performances in young soccer players and control subjects in all time points throughout two soccer seasons. In contrast, Eliakim et al. (2010) found a positive correlation between fitness and overnight GH levels in adolescent females. Training increased not only the resting GH levels but also its response to exercise (Manetta et al., 2002). Thus, the GH response to training may be different depending on the age of the subjects, on the level intensity of training and the physical activity practiced (Kraemer and Ratamess, 2005).
Despite the novelty and applicability of the current investigation, some limitations concerning our experimental design should be discussed. First, the blood samples were taken only at rest, which does not allow us to report adaptations to exercise or to soccer match play. It would be interesting to take blood samples during the match or at least at half time and finally match to specify the hormonal responses to this kind of test. Second, in the current study, psychological monitoring was not integrated. This may increase our knowledge concerning the connection between physiological and psychological adaptations during a soccer season in young elite soccer players.
Conclusions
Due to the lack of literature within this area, this study is the first of its type to monitor somatotype hormones and the longitudinal effect of a soccer training load stimulus (two season follow-up) in elite adolescent soccer players. The investigation provides results concerning anthropometric characteristics, physical fitness performances and hormonal concentrations for highly trained young soccer players in comparison to a controlled group. As a result based on the data obtained, it can be suggested that soccer participation of young elite players may lead to increased GH concentrations compared to control subjects, and this may represent an adaptation to exercise with soccer training. Furthermore, total IGF-1 levels and IGFBP3 remained strongly correlated with fitness performances (CMJ, SqJ, 5J) and serum IGF-1 and serum IGFBP3 levels may be considered as endocrine markers of physical fitness in young soccer players.
Acknowledgements
The authors thank all the subjects for their voluntary participation in this investigation. The authors have no conflicts of interest that are directly relevant to the contents of this manuscript. The study complied with the laws of the country of the authors’ affiliation.
Biographies

Mohamed Ali HAMMAMI
Employement
Movement, Sport and Health Laboratory (M2S), UFR-STAPS, University of Rennes 2, ENS Cachan, Rennes, France.
Degree
PhD
Research interests
Sport physiology, Physical training.
E-mail: medaliest@yahoo.fr

Abderraouf BEN ABDERRAHMAN
Employment
High Institute of Sport and Physical Education, Ksar- Saïd, Manouba University, Tunisia.
Degree
Professor
Research interests
Human physiology, Physical training, Physical activity quantification.
E-mail:
benabderrahmanabderraouf@yahoo.fr

Fatma RHIBI
Employment
Laboratory of Biomonitoring of the Environment, Faculty of Science of Bizerte, University of Carthage, Tunisia.
Degree
PhD. Student.
Research interests
Human physiology, Physical training.
E-mail: rhibi.fatma@yahoo.fr

Ammar NEBIGH
Employment
Laboratory of Physiology and Functional Explorations, Ibn Eljazzar Faculty of Medicine, University of Sousse, Tunisia.
Degree
PhD
Research interests
Sport Sciences, Physical training.
E-mail: ammarnebigh@yahoo.fr

Sullivan COPPALLE
Employment
Movement, Sport and Health Laboratory (M2S), UFR-STAPS, University of Rennes 2, ENS Cachan, Rennes, France.
Degree
PhD
Research interests
Sport Sciences, Soccer training, Physical training.
E-mail: sullivan.coppalle@hotmail.fr

Guillaume RAVÉ
Employment
Stade Lavallois Mayenne Footbal Club, Laval, France.
Degree
PhD
Research interests
Sport Sciences, Soccer training, Physical training.
E-mail:
guillaume.rave@stade-lavallois.com

Zouhair TABKA
Employment
Laboratory of Physiology and Functional of explorations. Faculty of Medicine “IBN EL JAZZAR» Sousse, Avenue Mohamed Karoui. 4002 Sousse, University of Sousse
Degree
MD, PhD
Research interests
Physiology and physiopathology of exercise.
E-mail: tabkazouhair@yahoo.fr

Hassane ZOUHAL
Employement
Movement, Sport and Health Laboratory (M2S), UFR-STAPS, University of Rennes 2, ENS Cachan, Rennes, France.
Degree
PhD
Research interests
Sport Sciences, Exercise Physiology, Physical training.
E-mail: hassane.zouhal@univ-rennes2.fr
References
- Adiyaman P., Ocal G., Berberoğlu M., Evliyaoğlu O., Aycan Z., Cetinkaya E., Bulca Y., Ersöz G., Akar N. (2004) Alterations in serum growth hormone (GH)/GH dependent ternary complex components (IGF-I, IGFBP-3, ALS, IGF-I/IGFBP-3 molar ratio) and the influence of these alterations on growth pattern in female rhythmic gymnasts. Journal of Pediatric Endocrinology and Metabolism 17(6), 895-903. [DOI] [PubMed] [Google Scholar]
- Bangsbo J. (1994) The physiology of soccer with special reference to intense intermittent exercise. Acta Physiologica Scandinavica, 619 (Suppl), 1-155. [PubMed] [Google Scholar]
- Bangsbo J., Iaia F. M., Krustrup P. (2008) The Yo-Yo intermittent recovery test: A useful tool for evaluation of physical performance in intermittent sports. Sports Medicine 38, 37-51. [DOI] [PubMed] [Google Scholar]
- Bouix O., Brun J. F., Fédou C., Micallef J.P., Charpiat A., Rama D., Orsetti A. (1997) Which medical survey for growth and puberty in adolscent gymnast? Sciences and Sport 1, 51-65. [Google Scholar]
- Brun J. F., Blachon C., Micallef J. P. (1996) Somatomedin carrier proteins and isometric strength of prehension in a group of adolescent gymnasts submitted at an intensive training. Science and Sports 11, 157-165. [Google Scholar]
- Di Luigi L., Guidetti L., Nordio M., Baldari C., Romanelli F. (2001) Acute effect of physical exercise on serum insulin-like Growth Factor-Binding protein 2 and 3 in healthy men: role of exercise linked Growth Hormone Secretion. International Journal of Sports Medicine 22(2), 103-110. [DOI] [PubMed] [Google Scholar]
- Duclos M. (2001) Effects of physical training on endocrine functions. Annales d’Endocrinologie 62, 19-32. [PubMed] [Google Scholar]
- During J. V., Webster C. I. (1985) A new method of assessing fatness and desirable weight for use in the Armed Service Army department. Technical Report: Ministry of Defence; USA. [Google Scholar]
- Eliakim A., Brassel J. A., Mohan S., Wong W.L.T., Cooper D.M. (1998) Increased physical activity and the growth hormone-IGF-1 axis in adolescent males. American Journal of Physiology 275, 308-314. [DOI] [PubMed] [Google Scholar]
- Eliakim A., Nemet D., Jürimäe J., Hills A. P., Jürimäe T. (2010) Cytokines, Growth Mediators and Physical Activity in Children during Puberty. Medicine and sport science 55, 128-140.20956865 [Google Scholar]
- Gil S., Ruiz F., Irazusta A., Gil J., Irazusta J. (2007) Selection of young soccer players in terms of anthropometric and physiological factors. Journal of Sports Medicine and Physical Fitness 47, 25-32. [PubMed] [Google Scholar]
- Hoff J. (2005) Training and testing physical capacities for elite soccer players. Journal of Sports Sciences 23, 573-582. [DOI] [PubMed] [Google Scholar]
- Hopkins WG. (2009) A scale of magnitudes for effect statistics. Available from URL: http://www.sportsci.org/resource/stats/index.html [Google Scholar]
- Juul A., Dalgaard P., Werner F. (1995) Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. Journal of Clinical Endocrinology and Metabolism 80, 2534-2542. [DOI] [PubMed] [Google Scholar]
- Kanaley J. A., Weltman J. Y., Veldhuis J. D., Rogol A. D., Hartman M. L., Weltman A. (1997) Human growth hormone response to repeated bouts of aerobic exercise. Journal of Applied Physiology 83, 1756-1761. [DOI] [PubMed] [Google Scholar]
- Kraemer W. J., Ratamess N. A. (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Medicine 35, 339-361. [DOI] [PubMed] [Google Scholar]
- Mackelvie K. J., Khan K. M., Mckay H. A. (2002) Is there a critical period for bone response to weight-bearing exercise in children and adolescents? A systematic review. British Journal of Sports Medicine 36, 250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimoun L., Galy O., Manetta J. (2004) Competitive season of triathlon does not alter bone metabolism and bone mineral status in male triathletes. International Journal of Sports Medicine 25(3), 230-234. [DOI] [PubMed] [Google Scholar]
- Malina R.M., Peña Reyes, M.E., Eisenmann J. C., Horta L., Rodrigues J., Miller R. (2000) Height, mass and skeletal maturity of elite Portuguese soccer players aged 11-16 years. Journal of Sports Sciences 8, 685-693. [DOI] [PubMed] [Google Scholar]
- Manetta J., Brun J. F., Maimoun L., Callis A., Prefaut C., Mercier J. (2002) Effect of training on the GH/IGF-1 axis during exercise in middle-aged men: relationship to glucose homeostasis. American Journal of Physiology-Endocrinology and Metabolism 283(5), 929-936. [DOI] [PubMed] [Google Scholar]
- Mejri S., Bechir F., Ben Rayana M. C., Ben Hamida J., Ben Slama C. (2005) Effect of training on GH and IGF-1 responses to a submaximal exercise in football players. European Journal of Applied Physiology 95, 496-503. [DOI] [PubMed] [Google Scholar]
- Poelman E. T., Rosen C. J., Copeland K. C. (1994) Influence of endurance training on insulin-like growth factor-1 in older individuals. Metabolism 43, 1401-1405. [DOI] [PubMed] [Google Scholar]
- Roemmich J. N., Richmond R. J., Rogol. A. D. (2001) Consequences of sport training during puberty. Journal of Endocrinological Investigation 24(9), 708-715. [DOI] [PubMed] [Google Scholar]
- Rösch D., Hodgson R., Peterson L., Graf-Baumann T., Junge A., Chomiak J., Dvorak J. (2000) Assessment and evaluation of football performance. American Journal of Sports Medicine 28, 29-39. [DOI] [PubMed] [Google Scholar]
- Schmikli S. L., de Vries W. R, Brink M. S., Backx F. J. (2012) Monitoring performance, pituitary-adrenal hormones and mood profiles: how to diagnose non-functional over-reaching in male elite junior soccer players. British Journal of Sports Medicine 46(14), 1019-1023. [DOI] [PubMed] [Google Scholar]
- Tanner J.M. (1975) Growth endocrinology of the adolescence. Endocrine Genetic Diseases of childhood and Adolescence. Ed: Gardner L. Philadelphia: WB Saunders; 14-64. [Google Scholar]
- Tanskanen M. M., Kyröläinen H., Uusitalo A. L., Huovinen J., Nissilä J., Kinnunen H., Atalay M., Häkkinen K. (2011) Serum sex hormone-binding globulin and cortisol concentrations are associated with overreaching during strenuous military training. Journal of Strength and Conditioning Research 25(3), 787-797. [DOI] [PubMed] [Google Scholar]
- Vänttinen T., Blomqvist M., Nyman K., Häkkinen K. (2011) Changes in body composition, hormonal status, and physical fitness in 11-, 13-, and 15-year-old Finnish regional youth soccer players during a two-year follow-up. Journal of Strength and Conditioning Research 25, 3342-3351. [DOI] [PubMed] [Google Scholar]
- Weltman A., Weltman J. Y., Womack C. J., Davis S. E., Blumer J. L., Gaesser G. A., Hartman M. L. (1997) Exercise training decreases the growth hormone (GH) response to acute constant-load exercise. Medicine and Science in Sports and Exercise 29(5), 669-676. [DOI] [PubMed] [Google Scholar]
- Wheldon A., Savine R. L., Sonksen P. H., Holt R. I. (2006) Exercising in the cold inhibits growth hormone secretion by reducing the rise in core body temperature. Growth Hormone and IGF Research 16, 125-131. [DOI] [PubMed] [Google Scholar]


