Abstract
A single bout of exercise can result in inflammatory responses, increased oxidative stress and upregulation of enzymatic antioxidant mechanisms. Although low-volume high-intensity interval training (HIIT) has become popular, its acute responses on the above mechanisms have not been adequately studied. The present study evaluated the effects of HIIT on hematological profile and redox status compared with those following traditional continuous aerobic exercise (CET). Twelve healthy young men participated in a randomized crossover design under HIIT and CET. In HIIT session, participants performed four 30-sec sprints on a cycle-ergometer with 4 min of recovery against a resistance of 0.375 kg/kg of body mass. CET consisted of 30-min cycling on a cycle-ergometer at 70% of their VO2max. Blood was drawn at baseline, immediately post, 24h, 48h and 72h post-exercise and was analyzed for complete blood count and redox status (thiobarbituric acid reactive substances, [TBARS]; protein carbonyls, [PC]; total antioxidant capacity, [TAC]; catalase and uric acid). White blood cells (WBC) increased after both exercise protocols immediately post-exercise (HIIT: 50% and CET: 31%, respectively). HIIT increased (+22%) PC post-exercise compared to baseline and CET (p < 0.05). HIIT increased TAC immediately post-exercise (16%) and at 24h post-exercise (11%, p < 0.05), while CET increased TAC only post-exercise (12%, p < 0.05) compared to baseline, and TAC was higher following HIIT compared to CET (p < 0.05). Both HIIT and CET increased uric acid immediately post- (21% and 5%, respectively, p < 0.05) and 24h (27% and 5%, respectively, p < 0.05) post-exercise and the rise was greater following HIIT (p < 0.05). There were no significant changes (p > 0.05) for TBARS and catalase following either exercise protocol. Low-volume HIIT is associated with a greater acute phase leukocyte count and redox response than low-volume CET, and this should be considered when an exercise training program is developed and complete blood count is performed for health purposes.
Key points.
HIIT perturbates indices of redox status to a greater extent compared to continuous aerobic exercise
HIIT elevates white blood cells post exercise to a greater extent compared to continuous aerobic exercise
Participation in HIIT one or two days prior to a complete blood count might result in false positive results.
Key words: High-intensity interval exercise, aerobic exercise, oxidative stress, immune system, recovery
Introduction
Oxidative stress is defined as a shift in the balance between the production of Reactive Oxygen and Nitrogen Species (RONS, free radicals) and the antioxidant defenses, favoring the oxidants (Halliwell, 2001). RONS are produced at all times as part of normal cell metabolism, as well as the environment. During exercise the production of free radicals can increase up to 20-fold in comparison to resting states and even up to 100-fold in active muscle groups (Jackson, 2008). While exercising, the high energy demands of active muscles cause an increase of oxygen (O2) consumption. This influx of O2 in the mitochondrial electron transport chain makes for the large jumps in RONS production during exercise. If the amount of free radicals produced exceeds the amount that can be handled by the antioxidant defensive mechanisms, then we have what we call exercise-induced oxidative stress. It has been proposed that a single bout of exercise can result in an increase of oxidative stress and as a result in an upregulation of enzymatic antioxidant mechanisms (Michailidis et al. 2007). Redox changes are part of an exercise-induced inflammatory response that also includes the acute phase response, release of inflammatory mediators (cytokines), and immune cells mobilization and activation (Brenner et al., 1999; Nieman et al., 2012).
Regarding immune cells responses, an increase in leukocytes has been reported during and after short intense and longer submaximal exercise and resistance exercise (Brenner et al., 1999; Nieman et al., 2007; Natale et al., 2003), which may also trigger the increase in several cytokines (Nieman et al., 2006). Exercise-induced leukocytosis is characterized by a biphasic response in which the rise of leukocytes during and immediately after exercise is mainly due to an increase in both neutrophils and lymphocytes, while the delayed rise peaking several hours post-exercise is mainly due to circulating neutrophils (Hansen et al., 1991; McCarthy et al., 1991; Natale et al., 2003; Rowbottom and Green, 2000). Additionally, the magnitude of the response seems to be exercise intensity- and duration-related (Hansen et al., 1991; Rowbottom and Green, 2000).
Low volume high-intensity interval training (HIIT) has become popular since it has been shown to result in increased aerobic and anaerobic performance (Burgomaster et al. 2005; 2006). HIIT is characterized by a combination of intense anaerobic exercise with less intense recovery periods and is considered an excellent way to maximize a workout that is limited on time. Several studies have reported positive effects of HIIT on mitochondrial biogenesis (Little et al., 2010), glucose metabolism (Laursen and Jenkins, 2002), body composition (Zhang et al., 2017) and muscle and bone mass (Nybo et al., 2010). However, there are not many studies that compared redox status and inflammatory responses following acute HIIT and traditional continuous aerobic exercise (CET). A rise in lipid and protein oxidation indices as well as in antioxidant status markers have been reported following acute HIIT (Baker et al., 2004; Bloomer et al., 2006; Bogdanis et al., 2013). HIIT and weight training exercise have been reported to result in similar upregulation of redox status markers (Bloomer et al., 2006). On the other hand, a CrossFit workout (performed in an interval fashion and at a high intensity), resulted in lower protein carbonyl and total antioxidant capacity and higher lipid peroxides compared to a treadmill bout (Kliszczewicz et al., 2015). The duration of both exercise sessions was 20 minutes and the intensity of the treadmill exercise bout was 90% of maximal heart rate. It is evident that both of these exercise protocols were intense without differentiating the anaerobic from the aerobic component and therefore direct comparisons cannot be made. CET, usually characterized by low intensity, has been prescribed by exercise professionals as means to increase aerobic capacity and promote health and well-being. Increases in peak power outputs during exercise result in increased metabolic demands which in turn may compromise the integrity of skeletal muscle that could lead to early onset of fatigue and exhaustion and thus selection of exercise intensity should be done with caution in order to avoid undesirable outcomes.
As with redox status, not many studies have compared the effects of intense exercise and aerobic exercise on parameters related to immune cells changes such as white blood cell (WBC) count. In one of those, Mathes et al. (2017) found that a 4x30 sec all-out protocol resulted in significant perturbations of leukocytes, lymphocytes and neutrophils compared to high volume exercise (4x4 min at 90 – 95% VO2max). Furthermore, HIIT and moderate intensity training effectively depressed apoptosis and promoted autophagy in CD4 lymphocytes (Weng et al., 2013). However, the latter study was a training study and no firm conclusion can be drawn if the acute response of WBC-related variables is different between HIIT and CET. Nevertheless, such information is important when an exercise training program is developed and complete blood count is performed for health purposes. Therefore, the purpose of this investigation was to evaluate the effects of low-volume HIIT on WBC count and redox status and compare them with the effects of CET. The total time of involvement with exercise during HIIT was two times less compared to that during CET.
Methods
Participants
Twelve young males (22.4 + 0.5y) volunteered to participate in this two-trial, crossover investigation. In the present study, females were not included to avoid possible sex-dependent variability in the results, as it has been shown that sex accounts for different outcomes in exercise-induced inflammatory, oxidative stress, and cardiovascular parameters in both animals (Pósa et al., 2013), and humans (Pepe et al., 2009; Wiecek et al., 2017). Additionally, the participants were about the same age, to avoid any age-related variability in the results of the study, since there is evidence that the response of oxidative stress biomarkers and anti-oxidants to exercise varies within different age groups (Barranco-Ruiz et al., 2017; Deli et al., 2017). Selection criteria included: a) absence of musculoskeletal and/or other health problems (cardiovascular disease or any other related health condition that could affect energy metabolism), b) no use of nutritional supplements, and medications before (≥6 months) and during the study, c) participants were non-smokers. The anthropometric and physiological characteristics of participants are shown in Table 1. The procedures were conformed to the Helsinki declaration of 1975 and were approved by the Human Participants Committee of the local University (ref #:799/10-12-13).
Table 1.
Participants’ personal characteristics (n = 12).
| Variables | Mean (±SD) |
|---|---|
| Age (years) | 22.4 (.5) |
| Weight (kg) | 75.3 (8.9) |
| Height (m) | 1.80 (.1) |
| Body Fat (%) | 10.8 (4.1) |
| VO2max (ml/kg/min) | 45.3 (8.4) |
| HR max (bpm) | 185.2 (7.1) |
VO2max: Maximum oxygen consumption; HR: Heart rate.
Study design
The participants reported to the laboratory three times in total. All measurements took place in the morning (08.00-10.00 a.m). At the first visit, anthropometric characteristics and maximal oxygen consumption (VO2max) were measured. Body mass was measured to the nearest 0.5 kg with the participants lightly dressed and barefoot (Beam Balance 710, Seca, UK) and standing height was measured to the nearest 0.5 cm (Stadiometer 208, Seca, UK). Percent body fat was calculated from 7 skinfold measures (average of 2 measurements at each site), using a Harpenden calliper (John Bull, St. Albans, UK). Maximal oxygen consumption (VO2max) was determined using a cycle ergometer (Monark 834E) test to exhaustion. The results of the initial maximal test were used to determine the exercise intensity of CET (70% VO2max). During the second and third visit (a washout period of one week between the second and third visit was included), the participants performed in random order either a CET protocol, or a HIIT protocol.
Maximal oxygen consumption
Each subject started pedaling at 70 revolutions per minute (rpm) with no additional workload for 150 s. Work rate (300g) was then added incrementally every 120 s with the intent of reaching the subject’s VO2max within 6 to 12 min (Lundgren, et al. 2001). Criteria used to determine VO2max were: i) participants’ exhaustion, ii) a <2 mL·kg-1·min-1 increase in VO2 with an increase in work rate, iii) a respiratory exchange ratio greater than or equal to 1.10, iv) a heart rate within 10 bpm of the theoretical maximum heart rate (220 - age). Respiratory gas variables were measured using a metabolic cart (Vmax29, Sensormedics, USA), which was calibrated before each test using standard gases of known concentration. Exercise heart rate was monitored by telemetry (Polar Tester S610TM, Electro Oy, Finland).
Submaximal exercise
Submaximal CET bout was performed on the cycle ergometer for 30 min. Exercise intensity was set at 70% of the participant’s VO2max. The intensity of the exercise was checked through respiratory and heart rate measurements throughout the workout and adjustments were made so that participants were exercising at the prescribed intensity. In order to calculate the energy expenditure during the exercise, respiratory gas variables were measured for 30 min using a metabolic cart (Vmax29, Sensormedics, USA).
HIIT
The HIIT exercise bout involved the performance of four 30-sec sprints on a cycle ergometer (against a resistance of 0.375 kg/kg of body mass) interspersed with 4 min of recovery. In order to calculate the energy expenditure during the exercise, respiratory gas variables were measured for 30 min using a metabolic cart (Vmax29, Sensormedics, USA).
Dietary analysis
To establish that macronutrient and antioxidant intake levels were similar in both conditions, participants were asked to record their diet for three consecutive days prior to participation in the first exercise condition (HIIT or CET) and replicate their diet for three days before the participation in the second exercise condition (HIIT or CET). Participants were provided with a written set of guidelines for monitoring dietary consumption and a record sheet for recording food intake. Diet recalls were analyzed with ScienceFit Diet 200A (Science Technologies, Athens, Greece). The nutritional analysis is presented in Table 2.
Table 2.
Nutritional analysis of the 3-day diet records prior to aerobic and high intensity interval training exercise protocol.
| CET (n = 12) | HIIT (n = 12) | |
|---|---|---|
| Energy (kcal) | 1772 ± 425 | 1766 ± 406 |
| Protein (gr) | 74.3 ± 24.1 | 71.9 ± 25.7 |
| Carbohydrate (gr) | 190.4 ± 56.9 | 193.3 ± 40.4 |
| Fat (gr) | 81.3 ± 21.7 | 76.8 ± 18.1 |
| Vitamin A (IU) | 4246 ± 2193 | 4443 ± 2895 |
| Vitamin C (mg) | 89.3 ± 36.4 | 87.6 ± 29.0 |
| Vitamin D (mg) | 109.1 + 81.3 | 113.3 + 65.0 |
| Vitamin E (mg_RE) | 8.7 ± 9.5 | 9.1 ± 9.9 |
| Iron (mg) | 14.3 ± 3.2 | 15.7 ± 4.3 |
| Selenium (μg) | 121.4 ± 33.6 | 115.0 ± 40.5 |
CET, continuous aerobic exercise; HIIT, high intensity interval training.
Blood sampling and assays
Participants were asked not to engage in any intense physical activity and not to consume alcohol or caffeine products for at least 72h before reporting to the laboratory. Blood samples (20 mL) were drawn before the exercise, immediately post, 24, 48 and 72 hours post exercise from a forearm vein with participants in a seated position. A portion (1–2 mL) of the blood collected was used to determine the parameters of the complete blood count measured by an automatic hematology analyzer (Mythic 18 Orphee, Orphee Medical, Geneva, Switzerland). For plasma collection, a portion of blood was placed in separate tubes mixed with EDTA (20 uL/mL of blood) and centrifuged (1370 g, 10 min, 4°C) and the supernatant was transferred into Eppendorf tubes that were stored at -80°C for later measurement of protein carbonyls (PC), thiobarbituric acid reactive substances (TBARS) and total antioxidant capacity (TAC). Packed erythrocytes were diluted with distilled water (1:1 v/v), vortexed vigorously, and centrifuged (4000 g, 15 min, 4°C) for red blood cell lysate preparation and the resultant supernatant was transferred into Eppendorf tubes that were stored at -80°C for later analysis of catalase activity. Finally, another portion of blood was collected in plain tubes, left at room temperature for 20 min to clot, centrifuged (1370 g, 10 min, 4°C) for serum separation and the supernatant was transferred into Eppendorf tubes that were stored at -80°C for later determination of uric acid (UA). Capillary blood for lactate measurements was collected 3 minutes after the end of each exercise session.
TBARS were measured according to Keles et al. (2001). Protein carbonyls were analyzed according to procedures described by Patsoukis et al. (2004). Total antioxidant capacity (TAC) was measured as described by Janaszewska and Bartosz (2002). Catalase activity was measured as described by Aebi (1984). Hb was determined with a commercially available kit (Dutch Diagnostics BV, The Netherlands). UA was measured in a Clinical Chemistry Analyzer Z 1145 (Zafiropoulos Diagnostica, Athens, Greece) with commercially available kits (Zafiropoulos, Athens, Greece). Lactate was measured in a microphotometer (DR LANGE, Berlin) with commercially available kits (LCQ 140, DR LANGE, Berlin). Each variable was analyzed in duplicates on the same day. Inter- and intra-assay coefficients of variation for all assays ranged from 2.4% to 6.8% and from 2.8% to 7.1%, respectively. Spectrophotometric assays were performed on a Hitachi 2001 UV/VIS (Hitachi Instruments Inc., U.S.) in triplicates.
Statistical analysis
Data normality was examined using the Kolmogorov–Smirnov test and was not found to differ significantly from normal. A 2x5 (condition x time) repeated measures ANOVA and a Bonferroni post-hoc test were used to analyze the data. SPSS was used for all analyses (SPSS Inc., Chicago, Ill.) The level of statistical significance was set at p < 0.05. The G*Power program (G*Power 3.0.10) was utilized to perform power analysis. With our sample size of 12 we obtained a statistical power greater than 0.80 at an α error of 0.05.
Results
Analysis of the diet records did not reveal any significant differences in the macro- and micro-nutrients between exercise trials (Table 2). The intensity of CET was 70.0 ± 2.0% VO2max whereas mean power during HIIT was 412.5 ± 77.3 W. Mean energy expenditure during the 30 minutes of aerobic exercise was 348 ± 90 kcal whereas during HIIT was 200 ± 69 kcal. Post-exercise mean lactate values after the aerobic exercise and HIIT were 2.7 ± 0.4 mM and 15.3 ± 1.8 mM, respectively.
Redox status responses
PC increased significantly (p < 0.05) following HIIT immediately post-exercise compared to baseline and that increase was also significantly higher compared to CET (Figure 1). TBARS did not change significantly (p > 0.05) following neither type of exercise (Figure 2). TAC increased significantly (p < 0.05) following HIIT immediately post- and 24h post-exercise, while only immediately post-exercise following CET (Figure 3). TAC was significantly higher (p < 0.05) immediately post- and 24h post-exercise following HIIT compared to CET (Figure 3). Catalase did not change significantly (p > 0.05) following neither type of exercise (Figure 4). UA increased significantly (p < 0.05) following HIIT immediately post- and 24h post-exercise compared to baseline (Figure 5). UA responses 24h post-exercise following HIIT were significantly higher (p < 0.05) compared to CET.
Figure 1.

Protein carbonyls (PC) responses following high intensity interval training (HIIT) and continuous aerobic exercise (CET). *Sig. vs. pre; †Sig. vs. CET.
Figure 2.

Thiobarbituric acid reactive species (TBARS) responses following high intensity interval training (HIIT) and continuous aerobic exercise (CET).
Figure 3.

Total antioxidant capacity (TAC) responses following high intensity interval training (HIIT) and continuous aerobic exercise (CET). *Sig. vs. pre; †Sig. vs. CET.
Figure 4.
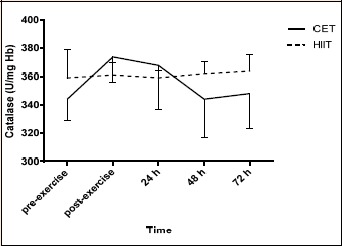
Catalase responses following high intensity interval training (HIIT) and continuous aerobic exercise (CET).
Figure 5.

Uric acid responses following high intensity interval training (HIIT) and continuous aerobic exercise (CET). *Sig. vs. pre; †Sig. vs. CET.
Hematological responses
No significant results for condition or condition x time were found for red blood cell (RBC) count, hemoglobin or hematocrit (Table 3). A significant increase was found for mean corpuscular volume (MCV) following HIIT and mean corpuscular hemoglobin concentration (MCHC) following HIIT and CET (Table 3). Platelets increased after CET (Table 3).
Table 3.
Hematological response following high intensity interval training (HIIT) and continuous aerobic exercise (CET).
| Variable | Pre-exercise | Post-exercise | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|
| RBC (X106/μl) | HIIT | 5.15 ± .67 | 5.26 ± .52 | 4.95 ± .46 | 4.93 ± .47 | 4.83 ± .47 |
| CET | 4.88 ± .50 | 5.3 ± .93 | 4.91 ± .56 | 4.94 ± .66 | 4.94 ± .66 | |
| Hemoglobin (g/dl) | HIIT | 14.46 ± 1.44 | 14.93 ± 1.20 | 14.14 ± 1.48 | 14.46 ± 2.06 | 13.59 ± 1.24 |
| CET | 13.89 ± 1.75 | 15.33 ± 2.80 | 14.06 ± 1.86 | 13.98 ± 1.80 | 13.85 ± 1.60 | |
| Hematocrit (%) | HIIT | 44.38 ± 4.43 | 45.8 ± 3.55 | 42.91 ± 4.16 | 42.84 ± 4.50 | 41.66 ± 3.54 |
| CET | 41.8 ± 4.71 | 46.04 ± 8.12 | 42.16 ± 5.35 | 42.33 ± 4.55 | 42.16 ± 4.42 | |
| MCV (fl) | HIIT | 86.68 ± 7.33 | 88.02 ± 7.48* | 86.95 ± 7.43 | 87.05 ± 7.44 | 86.64 ± 7.68 |
| CET | 85.87 ± 7.96 | 87.12 ± 7.09* | 86.00 ± 7.91 | 86.17 ± 8.20 | 85.89 ± 8.45 | |
| MCH (pg) | HIIT | 28.25 ± 2.61 | 29.81 ± 6.24 | 28.68 ± 2.93 | 28.55 ± 2.82 | 28.32 ± 3.28 |
| CET | 28.55 ± 3.17 | 29.03 ± 2.94 | 28.72 ± 3.17 | 28.47 ± 3.46 | 28.24 ± 3.42 | |
| MCHC (g/dl) | HIIT | 32.58 ± 1.00 | 34.02 ± 6.22* | 32.94 ± 1.11 | 32.78 ± 1.64 | 32.63 ± 1.31 |
| CET | 33.19 ± 1.24 | 33.31 ± 1.29* | 33.38 ± 1.73 | 33.03 ± 2.13 | 32.81 ± 1.22 | |
| Platelets (X103/µL) | HIIT | 213.8 ± 66.0 | 233.8 ± 38.8 | 223.7 ± 44.9 | 214.1 ± 45.5 | 216.9 ± 54.6 |
| CET | 215.2 ± 50.9 | 260.5 ± 83.4* | 225.7 ±53.3 | 224.1 ±47.0 | 216.0 ± 39.7 |
CET, continuous aerobic exercise; HIIT, high-intensity interval training.
*Sig. vs. pre-exercise.
WBC increased significantly (p < 0.05) following HIIT and CET immediately post-exercise compared to baseline with the increase in HIIT being significantly greater compared to the one of CET (Table 4). Lymphocytes (%) increased significantly (p < 0.05) following HIIT immediately post-exercise compared to baseline with the increase in HIIT being significantly greater compared to the one of CET (Table 4). Monocytes did not change significantly (p > 0.05) by either exercise protocol (Table 4). Granulocytes (%) decreased significantly (p < 0.05) following HIIT immediately post-exercise compared to baseline with HIIT inducing a decline of greater (p < 0.05) magnitude than CET (Table 4).
Table 4.
White blood cells response following high intensity interval training (HIIT) and continuous aerobic exercise (CET).
| Variable | Pre-exercise | Post-exercise | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|
| WBC (X103/µL) | HIIT | 6.5 ± .8 | 9.8 ± 2.1*† | 6.1 ± .7 | 6.1 ± .7 | 5.9 ± 1.1 |
| CET | 5.8 ± .7 | 7.6 ± 1.5 * | 5.9 ± .8 | 6.0 ± .9 | 6.0 ± 1.1 | |
| Lymphocytes (%) | HIIT | 37.4 ± 8.0 | 42.2 ± 9.6 *† | 35.3 ± 6.9 | 35.5 ± 7.9 | 34.8 ± 5.3 |
| CET | 35.6 ± 7.7 | 36.7 ± 6.5 | 34.8 ± 8.1 | 34.5 ± 6.7 | 33.4 ± 5.5 | |
| Monocytes (%) | HIIT | 8.5 ± 1.4 | 8.4 ± 1.9 | 8.7 ± 2.3 | 8.6 ± 1.7 | 8.4 ± 2.3 |
| CET | 9.9 ± 3.5 | 9.2 ± 2.3 | 9.8 ± 3.7 | 9.8 ± 4.7 | 9.7 ± 4.1 | |
| Granulocytes (%) | HIIT | 54.1 ± 8.1 | 49.4 ± 10.2 *† | 56.0 ± 7.5 | 56.1 ± 8.3 | 56.8 ± 5.3 |
| CET | 54.5 ± 6.0 | 54.1 ± 6.7 | 55.4 ± 6.5 | 55.7 ± 5.7 | 56.9 ± 5.0 |
HIIT, high intensity interval training; CET, continuous aerobic exercise
*Sig. vs. pre-exercise
†Sig. vs. CET.
Discussion
To our knowledge this is the first study that compared the acute effects of HIIT and CET on hematological profile and redox status. Results revealed that low-volume HIIT results in greater perturbations in WBC count and redox status than CET. It has to be noted, however, that the absolute duration of the HIIT exercise bout was 15 times less than that of CET, and that energy expenditure was higher in CET compared to HIIT. Our intention was to compare HIIT and CET protocols usually employed for fitness development.
Redox status responses
A single bout of HIIT exercise that comprised of four 30-sec intense sprints induced a large rise in PC immediately post-exercise and that increase was significantly greater compared to CET. The results obtained by this study are different compared with those reported previously and revealed either no differences in lipid peroxidation and protein carbonylation following an intense HIIT bout (Bloomer et al., 2006) or lower protein and lipid oxidation and total antioxidant capacity in response to a CrossFit workout compared to an acute CET protocol (Kliszczewicz et al., 2015). However, our results coincide with those reported by Bogdanis et al. suggesting a rise in protein oxidation following a HIIT-type protocol (Bogdanis et al., 2013). The increase in PC in response to HIIT could be explained by several mechanisms. It is well known that intense exercise results in increased levels of catecholamines to fuel the muscles with energy from nutrient breakdown among other things (Wahrenberg et al., 1987). An increased autoxidation of catecholamines could be one mechanism of the elevated levels of protein carbonyls following HIIT (Zouhal et al., 1998). Another possible reason for the increased levels of PC could be the increased catabolism of purines that results in elevated levels of hypoxanthine and UA (Starling et al., 1996). The latter explanation can be supported by the uric acid responses in this study. UA was significantly higher immediately-post and 24 hours post-exercise following the HIIT protocol compared to CET. As mentioned previously, high intensity exercise increases the activation of the purine nucleotide system resulting in the formation of adenosine monophosphate which in turn, due to hypoxanthine activation, UA is formed (Starling et al., 1996). This may explain the greater rise of UA following the HIIT protocol compared to CET which relies more on mitochondrial metabolism. Nevertheless, the response of UA in this study differs with the one of Baker et al. study where decreases in UA concentration were seen following a 30-sec high intensity exercise (Baker et al., 2004). Our protocol included four 30-sec cycling sprints and differences in the intensity and duration of the exercise bout could explain discrepancies between these two studies.
TAC responses were higher following the HIIT protocol. Previous results reported elevated levels of TAC (Bogdanis et al., 2013) whereas others found decrements in TAC a few hours after the end of an intense workout (Kliszczewicz et al., 2015). The elevated TAC levels in the present study could be explained by the significant increase of UA since it is known that this molecule represents almost 50% of the plasma antioxidant capacity. Catalase, an antioxidant enzyme found primarily in the RBCs, responses were not different due to time or protocol implying that the RBCs were not stressed due to exercise. That is further supported by the non-significant differences of the RBC count following either exercise protocol.
Hematological responses
It is well known that physical exercise results in a series of immune response changes (Keast et al., 1988; Fatouros and Jamurtas, 2016) whereas exercise of varying intensity and duration results in increased WBC (Nieman et al., 2007). Acute exercise can result in physiological changes in the quality and quantity of WBC affecting therefore the capacity to resist against common infections. Exercise-induced leukocytosis is accompanied by cytokines production after prolonged exercise (Nieman et al., 2012), and blood leukocytes may produce IL-8, IL-10, IL-1ra (Nieman et al., 2006). In the present study, although cytokines were not measured, the observed leukocytosis is in accordance with previous results that show increases in total leukocytes and lymphocytes following aerobic exercise (Benoni et al., 1995; Nieman et al., 2012). In agreement with our results, all-out bouts of rowing exercise increased counts of leukocytes and lymphocytes but not monocytes (Nielsen et al., 1996) suggesting that intense exercise provokes significant short-term immune system perturbations immediately post-exercise. Perhaps this response is attributed to the increased catecholamine levels. Previous work with beta-adrenergic blockade has shown that after propranolol (a beta adrenergic blocker) administration during exercise the increase in neutrophils, leukocytes and lymphocytes was abolished (Ahlborg and Ahlborg, 1970). The exercise-induced leukocytosis response is transient since white blood cells returned to the resting values within 24 hours post-exercise. Platelet count appears to be elevated following moderate intensity CET (Warlow and Ogston, 1974) as well as prolonged (Lippi et al., 2014) and intense exercise (Belviranli et al., 2017). This response appears to be transient and platelets return to baseline within 24 hours post-exercise. These results are in accordance with those of previous studies regarding the aerobic and anaerobic types of exercise. One possible explanation for the increased levels of platelets could be the release of fresh platelets from the spleen, bone marrow or other stored places (Freedman et al., 1977; Schmidt and Rasmussen, 1984).
Conclusions
In conclusion, redox status and CBC responses following HIIT appear to be exacerbated compared to the ones following CET. Some of these variables are still elevated 24 hours post-exercise indicating a prolonged perturbation in metabolism following this type of exercise. These responses should be taken into consideration when an exercise training program is developed and complete blood count is performed for health purposes.
Acknowledgements
The authors thank all participants for their contributions. The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare.
Biographies
Athanasios Z. JAMURTAS
Employment
Professor, University of Thessally, School of Physical Education and Sport Science.
Degree
PhD
Research interest
Exercise induced inflammation and oxidative stress
E-mail: ajamurt@pe.uth.gr
Ioannis G. FATOUROS
Assoc. Prof., University of Thessally, School of Physical Education and Sport Science.
Degree
PhD
Research interest
Exercise induced inflammation and oxidative stress
E-mail: fatouros@otenet.gr
Chariklia K. DELI
Employment
Post-Doctoral Researcher, Assistant Lecturer, University of Thessally, School of Physical Education and Sport Science
Degree
PhD
Research interest
Exercise and inflammation in adults and pediatric population, Exercise-related health adaptations, Sports nutrition
E-mail: delixar@pe.uth.gr
Kalliopi GEORGAKOULI
Employment
Assistant Lecturer at the Technological Educational Institute of Thessaly (Greece) and Research Associate at the SmArt Lab (University of Thessaly, Greece)
Degree
PhD
Research interest
The effects of functional foods, food supplements and exercise on various health aspects in both healthy and clinical populations
E-mail: kgeorgakouli@gmail.com
Athanasios POULIOS
Employment
Ph.D. candidate, University of Thessally, School of Physical Education and Sport Science.
Degree
MSc
Research interest
Metabolic responses following soccer games, protein supplementation
E-mail: athanpoul@gmail.com.
Dimitrios DRAGANIDIS
Employment
Ph.D. candidate, University of Thessally, School of Physical Education and Sport Science.
Degree
MSc
Research interest
Inflamaging, protein supplementation
E-mail: dimidraganidis@gmail.com.
Konstantinos PAPANIKOLAOU
Employment
Ph.D. candidate, University of Thessally, School of Physical Education and Sport Science.
Degree
MSc
Research interest
Satellite cell response following exercise, exercise induced inflammation
E-mail: guspapa93@gmail.com
Panagiotis TSIMEAS
Employment
Associate Lecturer, University of Thessally, School of Physical Education and Sport Science.
Degree
Ph.D.
Research interest
Strength and Conditioning, Basketball training,
E-mail: ptsimeas@pe.uth.gr
Athanasios CHATZINIKOLAOU
Employment
Ass. Professor, Democritus University of Thrace, School of Physical Education and Sport Science.
Degree
Ph.D.
Research interest
Strength and Conditioning, Basketball training, exercise induced inflammation
E-mail: achatzin@phyed.duth.gr.
Alexandra AVLONITI
Employment
Ass. Professor, Democritus University of Thrace, School of Physical Education and Sport Science.
Degree
Ph.D.
Research interest
Pediatric exercise physiolgy
E-mail: alavloni@phyed.duth.gr
Athanasios TSIOKANOS
Employment
Professor, University of Thessally, School of Physical Education and Sport Science.
Degree
Ph.D
Research interest
Biomechanical responses to exercise in health and disease
E-mail: atsiokan@pe.uth.gr
Yiannis KOUTEDAKIS
Employment
Professor, University of Thessally, School of Physical Education and Sport Science.
Degree
Ph.D.
Research interest
E-mail: y.koutedakis@uth.gr
References
- Aebi H. (1984) Catalase in vitro. Methods in Enzymology 105, 121-126. [DOI] [PubMed] [Google Scholar]
- Ahlborg B., Ahlborg G. (1970) Exercise leukocytosis with and without beta-adrenergic blockade. Acta Medica Scandinavica 187(4), 241-246. [DOI] [PubMed] [Google Scholar]
- Baker J.S., Damian Z., Bailey M., Hullin Z.D., Young I., Davies B. (2004) Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. European Journal of Applied Physiology 92, 321-327. [DOI] [PubMed] [Google Scholar]
- Barranco-Ruiz Y., Martínez-Amat A., Casals C., Aragón-Vela J., Rosillo S., Gomes S.N., Rivas-García A., Guisado R., Huertas J.R. (2017) A lifelong competitive training practice attenuates age-related lipid peroxidation. J Physiol Biochem 73(1), 37-48. [DOI] [PubMed] [Google Scholar]
- Belviranli M., Okudan N., Kabak B. (2017) The Effects of Acute High-Intensity Interval Training on Hematological Parameters in Sedentary Subjects. Medical Sciences (Basel, Switzerland) 5(3), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoni G., Bellavite P., Adami A., Chirumbolo S., Lippi G., Brocco G., Cuzzolin L. (1995) Effect of acute exercise on some haematological parameters and neutrophil functions in active and inactive subjects. European Journal of Applied Physiology 70, 187-191. [DOI] [PubMed] [Google Scholar]
- Bloomer R.J., Falvo M.J., Fry A.C., Schilling B.K., Smith W.A., Moore C.A. (2006) Oxidative stress response in trained men following repeated squats or sprints. Medicine and Science in Sports and Exercise 38(8), 1436-1442. [DOI] [PubMed] [Google Scholar]
- Bogdanis G.C., Stavrinou P., Fatouros I.G., Philippou A., Chatzinikolaou A., Draganidis D., Ermidis G., Maridaki M. (2013) Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food and Chemical Toxicology 61, 171-177. [DOI] [PubMed] [Google Scholar]
- Brenner I.K.M., Natale V.M., Vasiliou P., Moldoveanu A.I., Shek P.N., Shephard R.J. (1999) Impact of three different types of exercise on components of the inflammatory response. European Journal of Applied Physiolog 80, 452-460. [DOI] [PubMed] [Google Scholar]
- Burgomaster K.A., Heigenhauser G.J., Gibala M.J. (2006) Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. Journal of Applied Physiology (1985) 100(6), 2041-2047. [DOI] [PubMed] [Google Scholar]
- Burgomaster K.A., Hughes S.C., Heigenhauser G.J., Bradwell S.N., Gibala M.J. (2005) Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. Journal of Applied Physiology (1985) 98(6), 1985-1990. [DOI] [PubMed] [Google Scholar]
- Deli C.K., Fatouros I.G., Paschalis V., Georgakouli K., Zalavras Avloniti A., Koutedakis Y., Jamurtas A.Z. (2017) A Comparison of Exercise-Induced Muscle Damage Following Maximal Eccentric Contractions in Men and Boys. Pediatric Exercise Science 29(3), 316-325. [DOI] [PubMed] [Google Scholar]
- Fatouros I.G., Jamurtas A.Z. (2016) Insights into the molecular etiology of exercise-induced inflammation: opportunities for optimizing performance. Journal of Inflammation Research 9, 175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Altszuler N., Karpatkin S. (1977) Presence of a nonsplenic platelet pool. Blood 50(3), 419-425. [PubMed] [Google Scholar]
- Halliwell B. (2001) Free radicals and other reactive species in disease. In: Encyclopedia of life sciences. Ed: J. Wiley & Sons; London: Nature Publishing Group; 1-7. [Google Scholar]
- Hansen J.B., Wilsgard L., Osterud B., (1991) Biphasic changes in leukocytes induced by strenuous exercise. European Journal of Applied Physiolog 62, 157-161. [DOI] [PubMed] [Google Scholar]
- Jackson M.J. (2008) Free radicals generated by contracting muscle: Byproducts of metabolism or key regulators of muscle function? Free Radical Biology and Medicine 44, 132-141. [DOI] [PubMed] [Google Scholar]
- Janaszewska A., Bartosz G. (2002) Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scandinavian Journal of Clinical and Laboratory Investigation 62(3), 231-236. [DOI] [PubMed] [Google Scholar]
- Keast D., Cameron K., Morton A.R. (1988) Exercise and the immune response. Sports Medicine 5(4), 248-267. [DOI] [PubMed] [Google Scholar]
- Keles M.S., Taysi S., Sen N., Aksoy H., Akçay F. (2001) Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Canadian Journal of Neurological Sciences 28(2), 141-143. [DOI] [PubMed] [Google Scholar]
- Kliszczewicz B., Quindry C.J., Blessing L.D., Oliver D.G., Esco R.M., Taylor J.K. (2015) Acute Exercise and Oxidative Stress: CrossFit(™) vs. Treadmill Bout. Journal of Human Kinetics 47, 81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen P.B., Jenkins D.G. (2002) The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. Sports Medine 32(1), 53-73. [DOI] [PubMed] [Google Scholar]
- Lippi G., Salvagno G.L., Danese E., Skafidas S., Tarperi C., Guidi G.C., Schena F. (2014) Mean platelet volume (MPV) predicts middle distance running performance. PLoS One 9(11), e112892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J.P., Safdar A., Wilkin G.P., Tarnopolsky M.A., Gibala M.J. (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. Journal of Physiology 588, 1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren R.A., Maier L.A., Rose C.S., Balkissoon R.C., Newman L.S. (2001) Indirect and direct gas exchange at maximum exercise in beryllium sensitization and disease. Chest 120(5), 1702-1708. [DOI] [PubMed] [Google Scholar]
- Mathes S., Mester J., Bloch W., Wahl P. (2017) Impact of high-intensity and high-volume exercise on short-term perturbations in the circulating fraction of different cell types. The Journal of Sports Medicine and Physical Fitness 57(1-2), 130-137. [DOI] [PubMed] [Google Scholar]
- McCarthy D.A., Grant M., Marbut M., Watling M., Wade A.J., Macdonald I., Nicholson S., Melsom R.D., Perry J.D. (1991) Brief exercise induces an immediate and a delayed leukocytosis. British Journal of Sports Medicine 25(4), 191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidis Y., Jamurtas A.Z., Nikolaidis M.G., Fatouros I.G., Koutedakis Y., Papassotiriou I., Kouretas D. (2007) Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Medicine and Science in Sports and Exercise 39(7), 1107-1113. [DOI] [PubMed] [Google Scholar]
- Natale V.M., Brenner I.K., Moldoveanu A.I., Vasiliou P., Shek P., Shephard R.J. (2003) Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Medical Journal 121(1), 9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H.B., Secher N.H., Christensen N.J., Pedersen B.K. (1996) Lymphocytes and NK cell activity during repeated bouts of maximal exercise. American Journal of Physiology 271(1 Pt 2), R222-R227. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Davis J.M., Dumke C.L., Utter A.C., Murphy E.A., Pearce S., Gojanovich G., McAnulty S.R., McAnulty L.S. (2006) Blood Leukocyte mRNA Expression for IL-10, IL-1Ra, and IL-8, but Not IL-6, Increases After Exercise. J Interferon Cytokine Res 26(9), 668-674. [DOI] [PubMed] [Google Scholar]
- Nieman D., Henson D., Gojanovich G., Davis J.M., Dumke C., Utter A., Murphy A., Pearce S., McAnulty S., McAnulty L. (2007) Immune changes: 2 h of continuous vs. intermittent cycling. International Journal of Sports Medicine 28(7), 625-630. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Konrad M., Shanely R.A., Henson D.A., Kennerly K., Wallner-Liebmann S.J. (2012) Variance in the Acute Inflammatory Response to Prolonged Cycling Is Linked to Exercise Intensity. Journal of Interferon & Cytokine Research 32(1), 12-17. [DOI] [PubMed] [Google Scholar]
- Nybo L., Sundstrup E., Jakobsen M.D., Mohr M., Hornstrup T., Simonsen L., Bülow J., Randers M.B., Nielsen J.J., Aagaard P., Krustrup P. (2010) High-intensity training versus traditional exercise interventions for promoting health. Medicine and Science in Sports and Exercise 42(10), 1951-1958. [DOI] [PubMed] [Google Scholar]
- Patsoukis N., Zervoudakis G., Panagopoulos N.T., Georgiou C.D., Angelatou F., Matsokis N.A. (2004) Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neuroscience Letters 357(2), 83-86. [DOI] [PubMed] [Google Scholar]
- Pepe H., Balci S.S., Revan S., Akalin P.P., Kurtoğlu F., (2009) Comparison of oxidative stress and antioxidant capacity before and after running exercises in both sexes. Gender Medicine 6(4), 587-95. [DOI] [PubMed] [Google Scholar]
- Pósa A., Kupai K., Ménesi R., Szalai Z., Szabó R., Pintér Z., Pálfi G., Gyöngyösi M., Berkó A., Pávó I., Varga C., (2013) Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxidative Medicine Cellular Longevity 2013:521563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbottom D.G., Green K.J. (2000) Acute exercise effects on the immune system. Medicine & Science in Sports & Exercise 32(7), S396-S405. [DOI] [PubMed] [Google Scholar]
- Schmidt K.G., Rasmussen J.W. (1984) Are young platelets released in excess from the spleen in response to short-term physical exercise? Scandinavian Journal of Haematology 32(2), 207-214. [DOI] [PubMed] [Google Scholar]
- Starling R.D., Trappe T.A., Short K.R., Sheffield-Moore M., Jozsi A.C., Fink W.J., Costill D.L. (1996) Effect of inosine supplementation on aerobic and anaerobic cycling performance. Medicine and Science in Sports and Exercise 28, 1193-1198. [DOI] [PubMed] [Google Scholar]
- Wahrenberg H, Engfeldt P., Bolinder J., Arner P. (1987) Acute adaptation in adrenergic control of lipolysis during physical exercise in humans. American Journal of Physiology 253(4 Pt 1), E383-E390. [DOI] [PubMed] [Google Scholar]
- Warlow C.P., Ogston D. (1974) Effect of exercise on platelet count, adhesion, and aggregation. Acta Haematologica 52(1), 47-52. [DOI] [PubMed] [Google Scholar]
- Weng T.P., Huang S.C., Chuang Y.F., Wang J.S. (2013) Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One 8(11), e80248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecek M., Maciejczyk M., Szymura J., Szygula Z. (2017) Sex differences in oxidative stress after eccentric and concentric exercise, Redox Report 22(6), 478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tong T.K., Qiu W., Zhang X., Zhou S., Liu Y., He Y. (2017) Comparable Effects of High-Intensity Interval Training and Prolonged Continuous Exercise Training on Abdominal Visceral Fat Reduction in Obese Young Women. Journal of Diabetes Research 2017, 5071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhal H., Rannou F., Gratas-Delamarche A., Monnier M., Bentue-Ferrer D., Delamarche P. (1998) Adrenal medulla responsiveness to the sympathetic nervous activity in sprinters and untrained subjects during a supramaximal exercise. International Journal of Sport Medicine 19, 172-176. [DOI] [PubMed] [Google Scholar]


