Abstract
The implications of impact-induced muscle damage (IIMD) that results from participation in contact-sport are not well understood. The purpose of the present study was to implement a novel method of generating IIMD and characterise the implications of this on perceptual, biochemical and exercise performance parameters. Eighteen male recreational contact-sport athletes completed a single-group time series with measures assessed at baseline (PRE) and immediately following (POST) an IIMD protocol, with repeat testing 24, 48, and 72 h following the IIMD protocol. Biochemical indices of muscle damage (myoglobin [Mb]) and inflammation (high-sensitivity C-reactive protein [hs-CRP]), 15 m sprint performance, squat jump peak power (SJ-PP), and perceived soreness were compared to PRE using a one-way (time) repeated measures ANOVA with post-hoc t tests. Speed over 5 and 15 m were impaired for 48 h (7.5 ± 4%, p < 0.01) and SJ-PP was impaired for 48 h following the IIMD protocol (9.5 ±3 %, p < 0.01). Subjective soreness was elevated from baseline for 72 h (P < 0.01) following the IIMD protocol. No change in [CRP] or [Mb] was observed (p > 0.01). IIMD resulted in impaired ability to produce power and speed, whilst negatively influencing perceived soreness. These changes were most pronounced in the 48 h following the IIMD protocol. No change in muscle damage or inflammation indices were observed, primarily due to the highly variable response. Thus, the experimental protocol used in the present study may be used as a model to further investigate other aspects of IIMD.
Key points.
Repeated collisions induced by a novel collision simulator can lead to muscle damage which has negative effects on exercise performance and soreness.
Examination of the timeline of recovery suggests that performance and soreness are affected for at least 48 h following IIMD.
Muscle damage and inflammatory markers were not significantly different from baseline, although this might reflect methodological consideration of the testing protocol.
This model could be used to study broader aspects of recovery following IIMD and examine strategies that may accelerate athletic recovery.
Key words: Muscle damage, rugby, collisions, performance, recovery
Introduction
Sports such as Rugby League (RL) and Union (RU) are characterised by intermittent periods of high-intensity movement such as sprinting, jumping, and tackling, separated by periods of relatively low-intensity activity such as standing, walking, and jogging (Waldron et al., 2011a). In elite RL match play, athletes are required to run at 90-100 m·min-1 and execute repeated high-intensity efforts, which have the potential to delay recovery of neuromuscular function by up to 48 h (McLellan et al., 2011). This can be partly attributed to a rise in exercise induced muscle damage (EIMD) (Cheung et al., 2003). EIMD occurs in response to movements that involve repetitive eccentric or high-intensity contractions such as wrestling (Kraemer et al., 2001), and repeated sprints (Howatson and Milak, 2009), which cause disruption to sarcomere integrity (Clarkson and Hubal, 2002), elevate blood markers of muscle damage and inflammation, and impair the ability to produce force/torque (Warren et al., 1999). In addition, EIMD often leads to an increase in subjective pain following a latency period which typically peaks 24-48 h following injury (Cheung et al., 2003), a phenomenon known as delayed onset muscle soreness (DOMS) (Clarkson and Hubal, 2002).
In addition to the aforementioned physical demands and muscle damaging exercise, rugby athletes frequently engage in collisions with opponents, team mates and the playing surface. These collisions have been found to lead to decrements in performance (McLellan and Lovell, 2012), increased energy expenditure (Costello et al., 2018), and a rise in markers of muscle damage following match play (Lindsay et al., 2015b; Smart et al., 2008; Takarada, 2003). Moreover, collisions have been linked to soreness experienced in the hours and days following match play, that may persist throughout a competitive season (Fletcher et al., 2016). Indeed, an emerging body of research from combat sports such as mixed martial arts has linked impacts and collisions to a rise in muscle damage and inflammatory markers (Lindsay et al., 2016; 2017). Mixed martial arts can include a severe collision and impact component, occurring within a relatively confined area, and depending on the individual athlete’s fighting style, may occur independently of high intensity exercise. These data suggest skeletal muscle ultrastructural damage results from blunt force trauma and may profoundly delay recovery. However, training and match play data provides insight into changes related to accumulated neuromuscular fatigue, and the aforementioned parameters are often used as criteria to evaluate individual changes in response to both EIMD and impact induced muscle damage (IIMD) (Lindsay et al., 2015b; Naughton et al., 2017). Therefore, the possibility of conflation of the effects of EIMD and IIMD exists. As such, the magnitude and time course of alterations in neuromuscular function and performance specifically resulting from IIMD remains to be elucidated. Identification of these potential changes may provide insight into the potential implications and subsequent management of IIMD (Naughton et al., 2017).
Research investigating the physiological effects and associated functional implications of IIMD in a controlled anner is limited. To date, studies have explored the effects of additional physical collisions in the context of a RL small-sided game (Johnston et al., 2014), intermittent-sprint protocol (Pointon and Duffield, 2012), and a team-sport conditioning circuit (Singh et al., 2011). These investigations have identified a significant attenuation in performance, and increases in indices of muscle damage, when compared to a non-contact condition. Collectively, these data suggest that the addition of physical collisions to exercise results in IIMD, an exacerbated decrement in muscle function, and consequently performance. However, the inclusion of high-intensity running and/or wrestling in these studies introduces eccentric muscle action and associated EIMD as a possible source of such changes (Howatson and Milak, 2009; Kraemer et al., 2001). Such exercise modalities have the potential to cause EIMD and may confound any conclusions specifically relating to the implications of IIMD. As such, whilst these studies have merit in examining impacts in somewhat of an ecologically valid manner, in the context of exploring IIMD considerable limitations exist. As we previously have highlighted (Naughton et al., 2017), to minimise conflation, research exploring IIMD should aim to do so in the absence of EIMD and possible muscle damaging exercise.
Therefore, the purpose of the present study was to capture and characterise the typical magnitude and duration of changes resulting from a standardised IIMD experimental protocol somewhat simulating collisions experienced during rugby match-play in athletes who are habitually exposed to blunt force trauma. The IIMD protocol was adapted to this population by exposing participants to a similar frequency, derived from published match-play research (Gabbett and Seibold, 2013), to which they typically experience.
Methods
Participants
Eighteen young, healthy men (Table 1) who regularly participate in contact sports such as RL or RU volunteered to participate in the present study. Participants had no recent history of lower-limb muscle, joint, bone or blood-related injury or health issue and provided written informed consent. The present study was approved by the University Human Research Ethics Committee. Participants were required to refrain from unaccustomed exercise or vigorous physical activity for the two weeks prior to testing and for the duration of participation. Similarly, participants were instructed not to use anti-inflammatory medication or engage in recovery strategies such as cold-water immersion or wear compression garments for the duration of the study. Finally, testing occurred during the off-season to ensure participants were not exposed to additional game or training related fatigue or muscle damage. The sample size (n=18) was derived from the mean and variance from two previous related studies (Johnston et al., 2014; Singh et al., 2011), and based on the effect size of 1, alpha level of 0.05 and power (1-β) of 0.80.
Table 1.
Descriptive characteristics including dual energy x-ray absorptiometry (DEXA) determined body composition data of the 18 participants (mean ± SD).
| Variables | Mean (± SD) |
|---|---|
| Age (years) | 24.8 ± 4.4 |
| Stretch stature (m) | 1.80 ± 0.08 |
| Body mass (kg) | 85.3 ± 10.4 |
| Fat mass (kg) | 16.4 ± 5.8 |
| Lean tissue mass (kg) | 65.3 ± 9.3 |
| Bone mineral content (kg) | 3.6 ± 0.4 |
Experimental protocol
Participants were familiarised with testing procedures at least two weeks prior to testing (Vaile et al., 2008). During familiarisation, participants were familiarised with the questionnaire and all testing equipment and procedures, including the completion of four maximal 15 m sprints and four submaximal squat jumps separated by a 3 min recovery period. Participants were visually familiarised with the impact simulating equipment; however, no impacts were implemented prior to testing to ensure no muscle damage was introduced that may have influenced the study outcomes.
In the week prior to testing participants undertook Duel Energy X-ray Absorptiometry (DXA) to assess body composition. At the commencement of each testing session, participants had a venous blood sample collected for analysis of biochemical parameters. Thereafter, 15 m sprint and squat jump performance was assessed, and finally a recovery questionnaire was administered to assess perceived soreness. Following completion of baseline measures, the IIMD protocol was implemented. The same biochemical, performance and subjective measures were replicated immediately after the IIMD protocol, and again at 24, 48, and 72 h thereafter (Figure 1).
Figure 1.
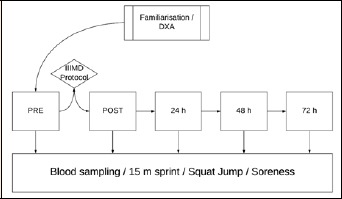
A schematic timeline of the flow of testing from familiarisation/dual x-ray absorptiometry (DXA) body composition testing, through to pre (PRE) and post (POST) impact-induced muscle damage (IIMD) protocol, with follow up testing at 24, 48, and 72 h following IIMD.
Dual energy X-ray absorptiometry (DXA)
Prior to undertaking the IIMD protocol, participants completed body composition assessment using DXA (Lunar iDXA, GE Healthcare, UK) fan-beam scan undertaken with current best-practice methodology (Nana et al., 2015). Participants were overnight fasted and free from exercise on the morning of assessment. Body mass was measured to the nearest 0.1 kg on electronic scales (SECA GmBH, Germany) and stretch stature to the nearest 0.01 m on a wall-mounted stadiometer (Harpenden, Holtain Limited, Crymych, United Kingdom), using previously described protocols (Scafoglieri et al., 2012).
Total body bone mineral density, fat mass and lean tissue mass were assessed. The DXA was calibrated using phantoms as per the manufacturer’s guidelines on each testing day prior to measurement. Participants wore minimal clothing and were positioned centrally on the scanning bed with foam hand and feet positioning aids. All tests were conducted by the same experienced and licenced operator. The scans were analysed automatically using GE enCORE v.13 software (GE Healthcare, UK) with the Geelong reference database and regions of interest subsequently confirmed by the operator. Whole body composition data was included for analysis.
Blood markers
Venous blood samples were collected at the beginning of each testing time-point. Each sample (5-8 mL) was collected from the superficial antecubital vein using standard venepuncture techniques. All samples were collected into serum separator tubes (SSI, Victoria, Australia) allowed to clot, and the serum separated at 1200 rcf (centrifugal force) for 10 min. Each serum sample was frozen and stored at -20 °C until analysis for concentrations of Myoglobin ([Mb]) and high-sensitivity C-reactive protein ([hs-CRP]). Concentrations of [Mb] were determined using Integra 800 (Roche Diagnostics, NSW, Australia) immunoturbidimetric assay method (Le Moigne et al., 2002). Sample [hs-CRP] were determined using an Abbott C16000 (Abbott Diagnostics, CA, USA) immunoturbidimetric technique with commercially available assay kits (Denham et al., 2007). All samples were analysed by Douglass Hanly Moir commercial pathology laboratory (Macquarie Park, NSW, Australia).
Performance Measures: 15 m sprint
Following venous blood collection, participants completed a standardised warm-up consisting of a 5 x 30 m jog, during which participants were instructed to maintain a low intensity (2/10) on Borg’s CR10 rating of perceived exertion (RPE) scale (Borg, 1998). Participants were then required to complete two 15 m sprints, separated by 2 min recovery, on a tartan running track. Duel-beam timing gates (SMARTSPEED, Fusion Sport, AUS) were placed at 0, 5, and 15 m to accurately record split 0-5 and 5-15 m times (s). The mean of two attempts was recorded for statistical analysis. Participants were instructed to begin within 1 m of the initial timing gate and sprint as explosively as possible. Verbal encouragement was given by the investigator throughout testing. Using a similar system the test-retest reliability of sprinting with rugby athletes has shown to be appropriate (<5% CV) (Waldron et al., 2011b).
Squat jump (peak power)
Following completion of the 15 m sprints, participants were required to complete two squat jumps (separated by 3 min) using a 20 kg standard Olympic bar, in accordance with prior guidance (Cormie et al., 2008). Participants were instructed to lower the bar to a 90° knee flexion position, pause for 2 s, and then jump upward as explosively as possible (Vaile et al., 2008). Squat jump peak power (SJ-PP; Watts (W)) was calculated using a GymAware linear position transducer (Kinetic Performance, AUS) interfaced with an iPod (Apple, CA, USA). Data derived from linear position transducers has been found to be both valid and reliable in the measurement of jump variables (Cronin et al., 2004). The mean of two attempts was recorded for analysis.
Soreness
Finally, Visual Analogue Scales (VAS) were used to monitor changes in subjective soreness and pain. Participants completed a VAS for pain and discomfort at passive rest (VAS-PAS), following stepping up and off a 40 cm bench with either leg three times (VAS-ACT), and after five seconds of palpation of the anterior impact sites (VAS-PALP).
IIMD Protocol
A ‘drop-mass’ model based on animal experimental research was adapted for use in the present study (Souza and Gottfried, 2013). A Smith Machine was modified to include a flat 35 x 10 cm metallic impact surface which was covered in ~6 cm of rubber to mimic the density of human skeletal muscle tissue (~1.1 g·cm3) (Ward and Lieber, 2005) (Figure 2).
Figure 2.

The rubber padding designed to mimic the density of skeletal muscle tissue affixed to a metallic impact surface used in the impact induced muscle damage (IIMD) collision simulator.
On arrival, participants superior border of each patella and the inguinal fold was landmarked. A landmark was then made at 1/3 (DIST), and 2/3 (PROX) the distance between the superior patella border and the inguinal fold. The DIST landmark was extended parallel to the axis of the ground from the initial anterior thigh position through the lateral and posterior thigh. Similarly, the PROX landmark was extended parallel to the axis of the ground and through the lateral and posterior thigh (Figure 3).
Figure 3.
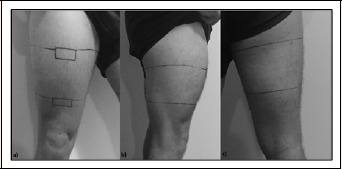
Representative reference markings for distal (DIST) and proximal (PROX) a) anterior, b) lateral, and c) posterior impact sites targeted during the impact induced muscle damage (IIMD) protocol.
The total system mass (bar and load) was ~60 kg, a load lower than reported body mass (kg) data from athletes of a similar recreational playing level (Gabbett, 2002). In order to replicate the frequency of impacts observed amongst semi-professional RL athletes (Gabbett and Seibold, 2013), the total frequency of impacts was set at 26 over 80 min (~0.33 impacts·min-1). Participants rotated through landmarks in a cyclic manner from anterior (DIST then PROX), to lateral (DIST then PROX) and finally posterior (DIST then PROX) (Figure 3), until the total of 26 impacts had been completed. There were no adverse injurious outcomes to the testing procedures during the study and participants did not report any adverse outcomes following testing.
At the completion of all baseline testing procedures, participants were seated below the Smith Machine impact surface, with knees at 90° flexion, underneath the DIST landmark (Figure 4a). The bar was situated transversely and was lowered to a position ~20 cm above the DIST landmark, the impact surface was then released to impact on this location. The bar was reset and participants were instructed they could move freely, if necessary. Participants were then seated below the PROX landmark and the identical protocol completed. For lateral impacts, participants were instructed to lie on their side, with padding provided to minimise knee collisions (Figure 4b). For posterior impacts, participants lay prone on a standard bench at each of the impact landmarks (Figure 4c). Following completion of 26 total impacts, the aforementioned measures were assessed (POST), with testing parameters replicated at 24, 48, and 72 h thereafter (Figure 1).
Figure 4.
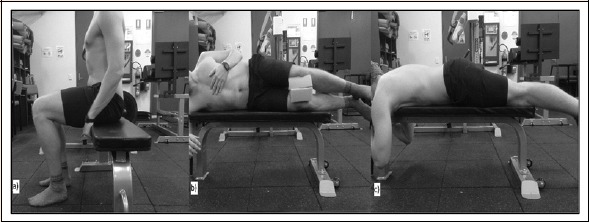
Representative positioning of the participants for the a) anterior impacts, b) lateral impacts, and c) posterior impacts during the impact induced muscle damage (IIMD) protocol.
Statistical analysis
Data are reported as mean ± standard deviation (SD). To assess changes in each dependent variable following the experimental protocol, repeated measures one-way analysis of variance (ANOVA) were utilized for parametric variables and Friedman’s ANOVA for nonparametric variables. Maulchy’s test was used to establish sphericity of the parametric dependent variables and on identification of a violation of sphericity, the Greenhouse-Geiser correction was applied. For the one-way repeated measures ANOVA, Mauchly’s test of sphericity indicated that [hs-CRP], [Mb], 5 and 15 m sprint split times output violated the assumption of non-sphericity and as such significant main effects for these variables were assessed using Greenhouse-Gessier corrections.
For parametric variables, on identification of a significant F-ratio, paired sample t-tests were undertaken with Bonferroni adjustment for multiple comparisons to determine statistical significance difference compared to PRE values. For nonparametric variables, post-hoc Wilcoxon signed-rank t tests were undertaken following significant Friedman’s ANOVA identification. Significance was set at P ≤ 0.01 due to multiple comparisons. Standardised residuals were calculated from each observation and a value greater than 3.0 was an outlier and removed from analysis and these are noted on the table and figure captions. Statistical analysis was undertaken in SPSS version 17 (IBM, USA).
Results
Biochemical markers
No significant main effects for time were observed for either [hs-CRP] or [Mb] (p > 0.01) (Table 2).
Table 2.
Results (mean ± SD) of biochemical markers of inflammation (high-sensitivity C-reactive protein [hs-CRP]) and muscle damage (Myoglobin [Mb]) including probability (P). NB - n = 17 complete datasets included for [hs-CRP] analysis.
| Variable | Time | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | 24 h | 48 h | 72 h | F | P | |
| [hs-CRP] (mg/L) | 1.1 ± 1.0 | 2.6 ± 3.2 | 3.1 ± 2.1 | 2.3 ± 2.3 | 1.6 ± 3.0 | 2.421 | 0.108 |
| [Mb] (ng/mL) | 35.9 ± 15.4 | 46.5 ± 20.4 | 48.6 ± 31.9 | 49.4 ± 32.0 | 33.4 ± 16.8 | 2.353 | 0.098 |
Sprints
ANOVA revealed a significant main effect for time with speed over 5, and 15 m (5 m: F(4,68) = 21.382, p < 0.001; and 15 m: F(1.99,33.83) = 20.547, p < 0.001). When compared to PRE, the percentage change in 0-5 and 5-15 m split times were significantly increased immediately following (POST), and at 24 and 48 h, but not at 72 h following the IIMD protocol (Figure 5).
Figure 5.
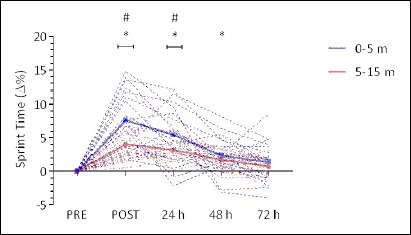
Percent change in 15 m sprint time before, immediately after and 24, 48, and 72 h following impact induced muscle damage (IIMD). Data represents individual 0-5 m (blue dotted trace), and 5-15 m (red dotted trace) split times expressed as a percentage of PRE values. Mean values are highlighted in solid lines with error bars omitted for clarity. *Significant (p < 0.01) difference from PRE. # Significant (p < 0.01) difference between 0-5 m and 5-15 m Δ% from baseline.
The percentage change at POST was significantly greater for sprint time for 0-5 m when compared to 5-15 m (p < 0.001) (Figure 5). Similarly, at 24 h, 0-5 m sprint time percentage change from PRE was significantly greater than 5-15 m (p = 0.006). There were no significant differences at any other time points (p ≥ 0.052).
Squat jump (peak power)
Squat jump ANOVA analysis revealed a significant main effect for time (F(4,68) = 41.621, p < 0.001). The percent-age change in peak power was significantly reduced immediately following the IIMD protocol (p < 0.001), at 24 (p < 0.001), and 48 h post-IIMD (p = 0.004), with no difference at 72 h post-IIMD (p = 0.800) (Figure 6).
Figure 6.
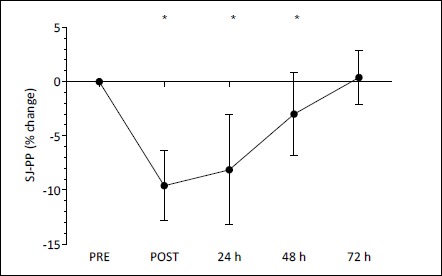
Squat jump peak power (SJ-PP) expressed as a percentage of PRE values (mean ± SD) before (PRE), immediately after (POST), 24, 48, and 72 h following the impact induced muscle damage (IIMD) protocol. *Significant (p < 0.01) difference from PRE.
Recovery questionnaire
Analysis of VAS-PASS, VAS-ACT, and VAS-PALP revealed significant main effects for time (VAS-PASS: χ2(4) = 37.432, p < 0.001; VAS-ACT: χ2(4) = 48.997, p < 0.001; VAS-PALP: χ2(4) = 50.112, p < 0.001). Compared to PRE, VAS-PASS was significantly elevated immediately following the IIMD protocol (Z = -3.517, p < 0.001) (Figure 7). Similarly, when compared to PRE, VAS-PASS was elevated 24 (Z = -3.433, p < 0.001), 48 (Z = -3.517, p < 0.001) and 72 h following the IIMD protocol (Z = -2.926, p = 0.003).
Figure 7.
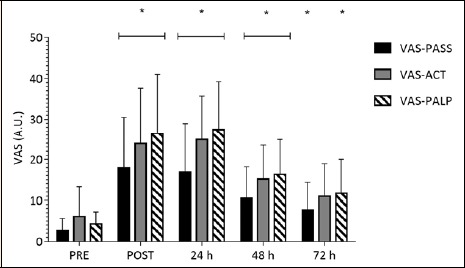
Change in Visual Analogue Scale (VAS) for subjective soreness (0-100 mm) PRE, POST, 24, 48 and 72 h following the impact induced muscle damage (IIMD) protocol. VAS-PASS – VAS following a period of passive standing, VAS-ACT – VAS following a period of active bench step-ups, VAS-PALP – VAS following palpation of the impact sites. Data are mean ± SD. *Significant (p <0.01) difference from PRE.
Compared to PRE, VAS-ACT was significantly elevated immediately post-IIMD (Z = -3.115, p = 0.002). Furthermore, when compared to PRE, VAS-ACT was significantly elevated at 24 (Z = -3.485, p < 0.001), and 48 h (Z = -3.181, p = 0.001). There was no significant difference in VAS-ACT at 72 h (Z = -2.122, p = 0.034). A similar response was observed for VAS-PALP compared to VAS-PASS throughout the testing period (Z = -3.725 to -3.080, p ≤ 0.002).
Discussion
The present study is the first to develop and implement a systematic method of producing IIMD in contact-sport populations, in the absence of EIMD. The implementation of this protocol resulted in IIMD and associated impairment in performance variables such as sprint speed and squat jump peak power, persisting for 48 h following the IIMD protocol. The IIMD intervention produced changes in subjective soreness that persisted for 72 h and which were related to change in performance at 48 and 72 h. Conversely, no significant differences in [hs-CRP] or [Mb] were observed when compared to baseline, primarily due to the variance in response. Collectively, these data confirm that in the absence of EIMD, IIMD has independent adverse effects on several parameters including performance and soreness that can persist for upwards of 72 h.
The findings provide confirmation of the strong relationships previously observed between game-related collisions and performance impairment (McLellan and Lovell, 2012; Twist et al., 2012). The change in squat jump peak power observed in the present study are substantially lower than those reported for countermovement jump peak power immediately (9.6 vs. 31.2%), and 24 h (8.1 vs. 23.3%) following elite RL match-play (McLellan et al., 2011). This is expected given that neuromuscular performance following match-play is likely to be negatively influenced by a number of factors including EIMD (Clarkson and Hubal, 2002), as well as the physiological and cognitive/psychological aspects of fatigue. Previous studies have reported altered neuromuscular function extending beyond 48 h of recovery following match-play in elite contact-sport populations (West et al., 2014). This is consistent with the present findings, suggesting this delayed athletic recovery from match-play may be, at least in part, due to the deleterious effects of IIMD. When exploring the limited research investigating IIMD in a controlled manner, decrements in jumping performance reported in the present study were slightly higher than those previously reported using similar methods immediately (~6%) and 24 h (~5%) following an IIMD protocol (Johnston et al., 2014). A potential explanation is the difference in methods, with Johnston et al. using primarily upper body collisions and wrestling in the context of a small sided conditioning game (Johnston et al., 2014). Collisions resulted in a greater upper body performance decrement than lower body performance change immediately (~15% vs. ~6%) and 24 h post-exercise (~9% vs. ~5%). These results suggest that the lower body would have experienced primarily EIMD through the running activity whilst the upper body would have been exposed to IIMD and EIMD through collisions and wrestling respectively. Compared to the upper body performance changes, peak power in the present study is lower immediately, and at 24 h following IIMD.
The lack of an observed significant rise in Mb, a marker of muscle damage, in the present study is somewhat surprising, given that previous research has highlighted a strong relationship between [Mb] and game-related collisions (Takarada, 2003). This lack of change might suggest that IIMD was not present in the participants, and the concomitant performance decrements were due to another aspect of muscle damage/fatigue. However, given the lack of plausible alternative fatigue inducing stimuli in the study, and the lack of comparative research investigating IIMD in athletes, this assertion would be speculative and require further research to investigate. Furthermore, as others have suggested when investigating EIMD (presently the closest well-researched analogue to IIMD (Naughton et al., 2017)), muscle damage markers can track relatively poorly with histological evidence of damage, and as such performance variables should be considered with a focus on blood variables “de-emphasized” (Warren et al., 1999). The peak systemic concentrations reported immediately following the present IIMD protocol (46.5 ± 20.4 ng·mL-1) are far below those reported at 45 min following RU match-play (~500 ng·mL-1) (Takarada, 2003), and 15 min following American Football match-play (~250 ng·mL-1) (Hoffman et al., 2002). A potential explanation for the lower concentrations observed in the present study include that the IIMD protocol was undertaken in the absence of EIMD, which would have likely contributed to amplified muscle damage and [Mb] in the aforementioned studies. Furthermore, [Mb] is influenced by intracellular release and circulatory uptake kinetics (Hoffman et al., 2002), with peak concentrations observed within an hour following muscle damaging exercise (Lindsay et al., 2015a). Therefore, the true peak concentrations may have occurred between the POST and 24 h testing time points and have been missed by the pattern of testing. The use of creatine kinase, an alternative biomarker which peaks 36-72 hours following muscle damaging exercise, may have offered utility based on the testing time points. Overall, the lack of significant findings with respect to [hs-CRP] and [Mb] may highlight the variability that is inherent in the primary and secondary muscle damage response, as is often observed following EIMD (Warren et al., 1999).
Perceived soreness was elevated following the IIMD protocol and did not subside by the completion of the 72 h data collection period. Additionally, subjective soreness was lower following IIMD than data previously reported at the completion of elite RL match-play (Twist et al., 2012), and following a RL simulation protocol, involving no collisions (Twist and Sykes, 2011). The pattern of the soreness response following IIMD is in contrast with the DOMS response which is often observed following EIMD in which soreness peaks 24-48 h post-injury, before subsiding by 96 h (Connolly et al., 2003). However, in the present study, soreness was observed immediately following the IIMD protocol, peaking 24 h later, and remaining elevated for at least 72 h. This finding is reflected in a recent study that observed soreness in the upper-, and lower-body were significantly related to total collisions in the initial days following RL match-play (Fletcher et al., 2016), with observed upper-, and lower-body soreness similar in recovery. This further highlights the divergence in the magnitude and progression of damage and associated symptomology between IIMD and EIMD (Warren et al., 2002).
The present study had several limitations which may have influenced the findings. Firstly, the force of impacts was not directly quantified. However, during pilot testing, the simulator was tested using a method for quantifying displacement from video (Dartfish Analyser, Dartfish, Switzerland) using a known reference in image (Hendricks et al., 2012), and subsequently velocity (velocity (m/s-1) = Δ displacement ÷ Δ time), acceleration (acceleration (m/s-2) = Δ velocity ÷ Δ time), and force (force (N) = mass x acceleration) data were derived using standard equations. The force involved per collision was substantially lower than that derived using published velocity and acceleration into contact data (~900 – 1100 N) from a similar recreational contact-sport athletic population (Hendricks et al., 2012). Similarly, the inclusion of padding between the participants legs to minimise intralimb collisions for the lateral impacts may have further reduced the impact forces. Given that this was a novel method of generating IIMD, safety of participants was paramount and as such increasing the load to produce greater impact forces equivalent to match-play wasn’t possible. Secondly, there was a lack of a control testing phase. However, to minimise the effects of initial testing on a subsequent testing bout (the repeated bout effect), a substantial wash-out period of six to eight months was potentially necessary (Nosaka et al., 2001; Vaile et al., 2008). This was not practical due to the availability of the athletes and limitations of the season structure. Finally, inclusion of a control condition featuring participants who were naïve to physical collisions would allow for the exploration of potential chronic habituation effects to IIMD, although this would need to be balanced with participant safety considerations.
Future research may look to implement IIMD in contact-sport populations using the current method and explore existing protective equipment which may attenuate the magnitude and timeframe of damage responses including protective equipment, such as strapping or padding (Hrysomallis, 2013, Tatar et al., 2014). The current model of IIMD can be used by researchers and practitioners to explore recovery strategies which have already been examined in the context of promoting recovery from EIMD including hydrotherapy (Bieuzen et al., 2013), cryotherapy (Bleakley et al., 2014), compression garments (Hill et al., 2013), and dietary interventions (Souza and Gottfried, 2013), amongst others. Given the current results and the potential differences between EIMD and IIMD (Warren et al., 2002), it is likely that certain recovery strategies could preferentially affect IIMD and exploration of novel methods should be an area of future research. Identification of effective strategies, both existing and novel, would be of interest to athletes and practitioners to promote the effective prescription of strategies to individuals who may be habitually exposed to a greater collision load. Other potential areas of research include exploring the existence of a dose-response effect between IIMD and dependent variables, and ‘contact adaptation’ (Fletcher et al., 2016; Kraemer et al., 2009), a phenomenon analogous to the repeated-bout effect in EIMD.
Conclusion
In summary, the present investigation has demonstrated performance impairment that was sustained for at least 48 h following a novel IIMD protocol. Subjective soreness was negatively affected and followed a similar time course. Finally, biochemical markers of muscle damage and inflammation were not significantly changed throughout testing. Compared to EIMD, where soreness develops following a latency period, the time course of changes in subjective soreness displayed a divergent pattern with soreness being evident immediately following the IIMD protocol. Conversely, functional capacity was impaired immediately following IIMD, which is consistent with the time course of changes following EIMD. These data demonstrate that the IIMD protocol described here was safe for the participants, athletes who are habitually exposed to blunt force trauma, with no adverse injurious outcomes for the participants. The creation of a repeatable, systematic method of generating IIMD in contact-sport populations such as the method described herein, allows for the exploration of IIMD and its effects, plus potential interventions to minimise the implications of this.
Acknowledgements
The authors have no conflicts of interest to declare. All experiments comply with the current laws of the country.
Biographies

Mitchell NAUGHTON
Employment
University of the Sunshine Coast
Degree
MSc
Research interests
Recovery strategies for team sport athletes in collision sports; environmental physiology as it relates to heat, cooling, and altitude as physiological stressors.
E-mail: mrn004@student.usc.edu.au

Joanna MILLER
Employment
Senior Recovery Physiologist at the Australian Institute of Sport
Degree
PhD
Research interests
To evaluate strategies to enhance recovery in high performance athletes.
E-mail: Jo.miller@ausport.gov.au

Gary J. SLATER
Employment
Assoc. Prof. in Nutrition and Dietetics at University of the Sunshine Coast
Degree
PhD
Research interests
Enhancing the reliability and validity of physique assessment techniques, enhancing the reliability and validity of metabolic assessment techniques, field based estimates of energy expenditure, nutrient timing and its impact on body composition and performance, health and performance implications of adjustments in protein intake, and dietary strategies to enhance performance of rugby union athletes.
E-mail: Gslater@usc.edu.au
References
- Bieuzen F., Bleakley C.M., Costello J.T. (2013) Contrast water therapy and exercise induced muscle damage: a systematic review and meta-analysis. PLoS One 8, e62356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakley C.M., Bieuzen F., Davison G.W., Costello J.T. (2014) Whole-body cryotherapy: empirical evidence and theoretical perspectives. Open Access Journal of Sports Medicine 5, 25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. 1998. Borg’s Perceived Exertion and Pain Scales, Champaign, IL: Human Kinetics. [Google Scholar]
- Chueng K., Hume P. A., Maxwell L. (2003). Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Medicine 33, 145-164. [DOI] [PubMed] [Google Scholar]
- Clarkson P.M., Hubal M.J. (2002) Exercise-induced muscle damage in humans. American Journal of Physical Medicine and Rehabilitation 81, 52-S69. [DOI] [PubMed] [Google Scholar]
- Connoly D.A.J., Sayers S.P., McHugh M.P. (2003) Treatment and prevention of delayed onset muscle soreness. Journal of Strength and Conditioning Research 17, 197-208. [DOI] [PubMed] [Google Scholar]
- Cormie P., McBride J.M., McCauley G.O. (2008) Power-time, force-time, and velocity-time curve analysis during the jump squat: impact of load. Journal Applied Biomechanics 24, 112-20. [DOI] [PubMed] [Google Scholar]
- Costello N., Deighton K., Preston T., Matu J., Rowe J., Sawczuk T., Halkier M., Read D.B., Weaving D., Jones B. (2018) Collision activity during training increases total energy expenditure measured via doubly labelled water. European Journal of Applied Physiology 118, 1169-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J.B., Hing R.D., McNair P.J. (2004) Reliability and validity of a linear position transducer for measuring jump performance. Journal of Strength and Conditioning Research, 18, 590-3. [DOI] [PubMed] [Google Scholar]
- Denham E., Mohn B., Tucker L., Lun A., Cleave P., Boswell D. R. (2007) Evaluation of immunoturbidimetric specific protein methods using the Architect ci8200: comparison with immunonephelometry. Annals of Clinical Biochemistry 44, 529-36. [DOI] [PubMed] [Google Scholar]
- Fletcher B.D., Twist C., Haigh J.D., Brewer C., Morton J.P., Close G.L. (2016) Season-long increases in perceived muscle soreness in professional rugby league players: role of player position, match characteristics and playing surface. Journal of Sports Sciences 34(11), 1067-1072. [DOI] [PubMed] [Google Scholar]
- Gabbett T.J. (2002) Influence of physiological characteristics on selection in a semi-professional first grade rugby league team: a case study. Journal of Sports Sciences 20, 399-405. [DOI] [PubMed] [Google Scholar]
- Gabbett T.J., Seibold A.J. (2013) Relationship between tests of physical qualities, team selection, and physical match performance in semiprofessional rugby league players. Journal of Strength and Conditioning Research 27, 3259-3265. [DOI] [PubMed] [Google Scholar]
- Hendricks S., Karpul D., Nicholls F., Lambert M. (2012) Velocity and acceleration before contact in the tackle during rugby union matches. Journal of Sports Sciences 30, 1215-24. [DOI] [PubMed] [Google Scholar]
- Hill J., Howatson G., Van Someran K., Leeder J., Pedlar C. (2013) Compression garments and recovery from exercise-induced muscle damage: A meta-analysis. British Journal of Sports Medicine 48, 1340-1346. [DOI] [PubMed] [Google Scholar]
- Hoffman J. R., Maresh C. M., Newton R. U., Rubin M. R., French D. N., Volek J. S., Sutherland J., Robertson M., Gomez A. L., Ratamess N. A., Kang J., Kraemer W. J. (2002) Performance, biochemical, and endocrine changes during a competitive football game. Medicine and Science in Sports Exercise 34, 1845-1853. [DOI] [PubMed] [Google Scholar]
- Howatson G., Milak A. (2009) Exercise-induced muscle damage following a bout of sport specific repeated sprints. Journal of Strength and Conditioning Research 23, 2419-2424. [DOI] [PubMed] [Google Scholar]
- Hrysomallis C. (2013) Injury incidence, risk factors and prevention in Australian rules football. Sports Medicine, 43, 339-54. [DOI] [PubMed] [Google Scholar]
- Johnston R.D., Gabbett T.J., Seibold A.J., Jenkins D.G. (2014) Influence of physical contact on neuromuscular fatigue and markers of muscle damage following small-sided games. Journal of Science and Medicine in Sport 17, 535-540. [DOI] [PubMed] [Google Scholar]
- Kraemer W.J., Fry A.C., Rubin M.R., Triplett-McBride T., Gordon S.E., Perry Koziris, L., Lynch J.M., Volek J.S., Meuffels D.E., Newton R.U., Fleck S.J. (2001) Physiological and performance responses to tournament wrestling. Medicine and Science in Sports Exercise, 33, 1367-1378. [DOI] [PubMed] [Google Scholar]
- Kraemer W.J., Spiering B.A., Volek J.S., Martin G.J., Howard R.L., Ratamess N.A., Hatfield D.L., Vingren J.L., Ho J.Y., Fragala M.S., Thomas G.A., French D.N., Anderson J.M., Hakkinen K., Maresh C.M. (2009) Recovery from a National Collegiate Athletic Association Division I Football Game: Muscle Damage and Hormonal Status. Journal of Strength and Condititoning Research 23, 2-10. [DOI] [PubMed] [Google Scholar]
- Le Moigne F., Beauvieux M.C., Derache P., Darmon Y.M. (2002) Determination of myoglobin: comparative evaluation of the new automated VIDAS assay with two other immunoassays. Clinical Biochemistry 35, 255-262. [DOI] [PubMed] [Google Scholar]
- Lindsay A., Carr S., Cross S., Petersen C., Lewis J. G., Gieseg S.P. (2017) The physiological response to cold-water immersion following a mixed martial arts training session. Applied Physiology, Nutrition, and Metabolism 42, 529-536. [DOI] [PubMed] [Google Scholar]
- Lindsay A., Carr S., Draper N., Gieseg S. P. (2015a) Urinary myoglobin quantification by high-performance liquid chromatography: An alternative measurement for exercise-induced muscle damage. Analytical Biochemistry 491, 37-42. [DOI] [PubMed] [Google Scholar]
- Lindsay A., Healy J., Mills W., Lewis J., Gill N., Draper N., Gieseg S.P. (2015b) Impact-induced muscle damage and urinary pterins in professional rugby: 7,8-dihydroneopterin oxidation by myoglobin. Scandinavian Journal of Medicine and Science in Sports 26, 329-337. [DOI] [PubMed] [Google Scholar]
- Lindsay A., Othman M.I., Prebble H., Davies S., Gieseg S.P. (2016) Repetitive cryotherapy attenuates the in vitro and in vivo mononuclear cell activation response. Experimental Physiology 101, 851-865. [DOI] [PubMed] [Google Scholar]
- McLellan C.P., Lovell D.I. (2012) Neuromuscular responses to impact and collision during elite rugby league match play. Journal of Strength and Conditioning Research 26, 1431-1440. [DOI] [PubMed] [Google Scholar]
- McLellan C.P., Lovell D.I., Gass G.C. (2011) Markers of postmatch fatigue in professional rugby league players. Journal of Strength and Conditioning Research 25, 1030-1039. [DOI] [PubMed] [Google Scholar]
- Nana A., Slater G.J., Stewart A.D., Burke L.M. (2015) Methodology review: using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. International Journal of Sport Nutrition and Exercise Metabolism 25, 198-215. [DOI] [PubMed] [Google Scholar]
- Naughton M., Miller J., Slater G.J. (2017) Impact-induced muscle damage and contact-sport: aetiology, effects on neuromuscular function and recovery, and the modulating effects of adaptation and recovery strategies. International Journal of Sports Physiology and Performance Nov 28, 1-24. [DOI] [PubMed] [Google Scholar]
- Nosaka K., Sakamoto K., Newton M., Sacco P. (2001) How long does the protective effect on eccentric exercise-induced muscle damage last? Medicine and Science in Sports Exercise 33, 1490-1495. [DOI] [PubMed] [Google Scholar]
- Pointon M., Duffield R. (2012) Cold water immersion recovery after simulated collision sport exercise. Medicine and Science in Sports Exercise 44, 206-216. [DOI] [PubMed] [Google Scholar]
- Scafoglieri A., Tresignie J., Provyn S., Marfell-Jones M., Reilly T., Bautmans I., Clarys J.P. (2012) Prediction of segmental lean mass using anthropometric variables in young adults. Journal of Sports Sciences 30, 777-785. [DOI] [PubMed] [Google Scholar]
- Singh T. K., Guelfi K. J., Landers G., Dawson B., Bishop D. (2011) A comparison of muscle damage, soreness and performance following a simulated contact and non-contact team sport activity circuit. Journal of Medicine and Science in Sport 14, 441-446. [DOI] [PubMed] [Google Scholar]
- Smart D.J., Gill N.D., Beaven C.M., Cook C.J., Blazevich A.J. (2008) The relationship between changes in interstitial creatine kinase and game-related impacts in rugby union. British Journal of Sports Medicine, 42, 198-201. [DOI] [PubMed] [Google Scholar]
- Souza J.D., Gottfied C. (2013) Muscle injury: Review of experimental models. Journal of Electromyography and Kinesiology 23, 1253-1260. [DOI] [PubMed] [Google Scholar]
- Takarada Y. (2003) Evaluation of muscle damage after a rugby match with special reference to tackle plays. British Journal of Sports Medicine 37, 416-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar Y., Ramazonoglu N., Camliguney A. F., Saygi E. K., Cotuk H. B. (2014) The effectiveness of shin guards used by football players. Journal of Sports Science and Medicine 13, 120-127. [PMC free article] [PubMed] [Google Scholar]
- Twist C., Sykes D. (2011) Evidence of exercise-induced muscle damage following a simulated rugby league match. European Journal of Sports Science 11, 401-409. [Google Scholar]
- Twist C., Waldron M., Highton J., Burt D., Daniels M. (2012) Neuromuscular, biochemical and perceptual post-match fatigue in professional rugby league forwards and backs. Journal of Sports Sciences 30, 359-367. [DOI] [PubMed] [Google Scholar]
- Vaile J., Halson S., Gill N., Dawson B. (2008) Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. European Journal of Applied Physiology 102, 447-455. [DOI] [PubMed] [Google Scholar]
- Waldron M., Twist C., Highton J., Worsfold P., Daniels M. (2011a) Movement and physiological match demands of elite rugby league using portable global positioning systems. Journal of Sports Science 29, 1223-1230. [DOI] [PubMed] [Google Scholar]
- Waldron M., Worsfold P., Twist C., Lamb K. (2011b) Concurrent validity and test-retest reliability of a global positioning system (GPS) and timing gates to assess sprint performance variables. Journal of Sports Science 29, 1613-1619. [DOI] [PubMed] [Google Scholar]
- Ward S.R., Lieber R.L. (2005) Density and hydration of fresh and fixed human skeletal muscle. Journal of Biomechanics 38, 2317-2320. [DOI] [PubMed] [Google Scholar]
- Warren G.L., Ingalls C.P., Lowe D.A., Armstrong R.B. (2002) What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? Journal of Orthopaedic and Sports Physical Therapy 32, 58-64. [DOI] [PubMed] [Google Scholar]
- Warren G.L., Lowe D.A., Armstrong R.B. (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Medicine 27, 43-59. [DOI] [PubMed] [Google Scholar]
- West D.J., Finn C.V., Cunningham D.J., Shearer D.A., Jones M.R., Harrington B.J., Crewther B.T., Cook C.J., Kilduff L.P. (2014) Neuromuscular function, hormonal, and mood responses to a professional rugby union match. Journal of Strength and Conditioning Research, 28, 194-200. [DOI] [PubMed] [Google Scholar]


