Abstract
This study aimed to investigate and compare the effects of repeated-sprint (RSH) and sprint interval training in hypoxia (SIH) on sea level running and cycling performance, and to elucidate potential common or divergent adaptations of muscle perfusion and -oxygenation as well as mitochondrial respiration of blood cells. Eleven team-sport athletes performed either RSH (3x5x10s, 20s and 5min recovery between repetitions and sets) or SIH (4x30s, 5min recovery) cycling training for 3weeks (3 times/week) at a simulated altitude of 2,200m. Before and three days after the training period, a Wingate and a repeated cycling sprint test (5x6s, 20s recovery) were performed with a 30min resting period between the tests. Four to five days after the training, participants performed a repeated running sprint test (RSA, 6x17m back and forth, 20s recovery) and a Yo-Yo intermittent recovery test (YYIR2) with 1 hour active recovery between tests. The order of the tests as well as the duration of the resting periods remained the same before and after the training period. During the cycling tests near-infrared spectroscopy was performed on the vastus lateralis. In four participants, mitochondrial respiration of peripheral blood mononuclear cells (PBMC) and platelets was measured before and after training. YYIR2 running distance increased by +96.7 ± 145.6 m after RSH and by +100.0 ± 51.6 m after SIH (p = 0.034, eta² = 0.449). RSA mean running time improved by -0.138 ± 0.14s and -0.107 ± 0.08s after RSH and SIH respectively (p = 0.012, eta² = 0.564). RSH compared to SIH improved re-oxygenation during repeated sprinting. Improvements in repeated cycling were associated with improvements in re-oxygenation (r = 0.707, p <0.05). Mitochondrial electron transfer capacity normalized per PBMC count was decreased in RSH only. This study showed that cycling RSH and SIH training improves sea-level running performance. Our preliminary results suggest that RSH and SIH training results in different patterns of muscular oxygen extraction and PBMC mitochondrial respiration, without effect on platelets respiration.
Key points.
Cycling RSH and SIH improve sea level cycling and running performance to a similar extent.
RSH compared to SIH led to higher de- and re-oxygenation during repeated sprinting.
RSH conceivably modifies mitochondrial function in PBMC.
Key words: Repeated-sprint training, sprint interval training, altitude, adaptive mechanisms
Introduction
In recent years, new hypoxia training concepts for team sport athletes have emerged. These concepts, originally based on classical normoxic sprint training, include repeated-sprint training (RSH) and sprint interval training (SIH) performed in hypoxia. Repeated-sprint training is characterized by repeated maximal exercise bouts of short duration (≤ 10 s) interspersed with brief recovery periods (usually ≤60 s) (Girard et al., 2011a; 2017). Sprint interval training, in contrast, includes repeated bouts with a duration of approximately 30 s interspersed with 2–4 min of passive recovery (Buchheit and Laursen, 2013b). Similar to training in normoxia (Buchheit and Laursen, 2013a; Buchheit and Laursen, 2013b), slightly different anaerobic and aerobic energy contributions during RSH and SIH might be expected depending on bout and relief duration (Faiss et al., 2013a). Consequently, the physiological adaptations may differ to some extent as well. As comparative studies investigating different sprint training protocols in hypoxia are scarce, identification of possible divergent adaptations is difficult. For instance, RSH was found to enhance muscle perfusion (Faiss et al., 2015; Montero and Lundby, 2017) and changes in the proportion of type IIx muscle fibers have been described after SIH (De Smet et al., 2016). Yet, it is unknown if SIH is equally able to enhance perfusion and RSH will change fiber type. Additionally, an enhanced anaerobic metabolism (Faiss et al., 2013b; Puype et al., 2013) and increased muscle buffer capacity (Faiss et al., 2013a) have been reported for both, RSH and SIH, yet the magnitude of differences is hard to assess. Furthermore, Faiss et al. (2013b) reported decreases in factors involved in mitochondrial biogenesis after RSH, whereas no such data are available for SIH. Especially for this adaptation, the importance of comparing different sprinting protocols as well as hypoxia doses is evident as applying a slightly different RSH protocol and including hypoxia living led to an opposite finding (i.e., increased mRNA levels for PGC - 1α) (Brocherie et al., 2018).
In regard to performance, RSH compared to normoxia training was found to improve sea-level repeated sprinting abilities (Brocherie et al., 2017; Faiss et al., 2013b; Gatterer et al., 2014; Hamlin et al., 2017; Kasai et al., 2015) and to some extent intermittent endurance exercise performance (Galvin et al., 2013). SIH, in contrast, improved the anaerobic threshold to a greater extent (Puype et al., 2013). However, other reports suggest that adding hypoxia to both training modalities does not affect performance outcomes at all (De Smet et al., 2016; Montero and Lundby, 2015; 2017; Richardson and Gibson, 2015).
As outlined before, muscle perfusion and oxygenation changes might constitute adaptations related to performance improvements after RSH whereas such changes have not yet been reported for SIH. Additionally, to the best of our knowledge, no study investigated actual mitochondrial function after RSH and SIH mostly because invasive procedures are necessary. Recent research, however suggests that the mitochondrial function of peripheral blood mononuclear cells (PBMC) may provide a measure of physical ability similar to skeletal muscle mitochondrial function (Tyrrell et al., 2015a). Moreover, high-intensity interval training in normoxia enhanced oxidative phosphorylation of lymphocyte, which again indicates the usefulness of this analysis (Tsai et al., 2016). The effect of RSH and SIH on blood cells mitochondrial function is not established yet and its relationship to muscle oxygenation and performance has not been studied so far. Thus, the present study aimed at investigating and comparing the effects of RSH and SIH on sea level running and cycling performance, and to elucidate potential common or divergent adaptations of muscle perfusion and -oxygenation as well as mitochondrial respiration of blood cells, possibly related to performance improvements.
Methods
Participants
Twelve healthy subjects competing in intermittent sports (i.e. basketball, handball, soccer) at an amateur level (2-3 regular training sessions per week and one competition game) were recruited and provided their written informed consent to participate in the study. The final sample size consisted of eleven participants (age 24.0 ± 2.4 yr., height: 1.83 ± 0.05 m, weight: 84.0 ± 9.3 kg) since one subject dropped out during the study due to personal reasons. The study was approved by the Institutional Review Board of the Department of Sport Science of the University of Innsbruck.
Design
After performing the pretests as outlined in detail below, the participants were randomly assigned to a short (RSH, n = 6, age 24.8 ± 2.5 yr., height: 1.84 ± 0.04 m, weight: 83.7 ± 10.7 kg) or long (SIH, n = 5, 23.0 ± 2.1 yr., height: 1.82 ± 0.06 m, weight: 84.5 ± 8.5 kg) repeated-sprint training regime, stratified for the pretest outcomes of the repeated-sprint ability (RSA, mean sprint time) and the Yo-Yo intermittent recovery test level 2 (YYIR2, running distance). The subsequent training intervention lasted for 3 weeks and included 3 training sessions per week (training procedures are detailed below). 3-5 days after the last training session, the posttests were performed using the same procedures described for the pretests. In a subgroup of 4 participants, venous blood samples were taken before and after the training intervention to analyze mitochondrial respiration of PBMCs.
Measurements
Before and 3 days after the training intervention, participants performed a 30 s anaerobic Wingate test followed by short repeated bursts of maximal cycling (5x6 s with 20 s recovery). Tests were separated by 30 min of rest and were performed on a cycle ergometer (Cyclus II, RBM elektronik-automation GmbH, Germany). Since residual fatigue from the Wingate test was probable, the testing procedure was identical for all subjects and at all training phases, allowing for comparison of changes due to the training. The cycle ergometer was set at a fixed torque corresponding to 0.85 x body weight during the Wingate test and 0.95 x body weight during the repeated sprints. The outcomes of the Wingate and the repeated sprinting tests included the peak and the mean power output. For the Wingate test, the fatigue index was calculated as the performance loss per second. Performance decrement of the repeated cycling sprint was calculated according to Girard et al. (2011b) (i.e., [1 – (SUM of mean power output accumulated / (best mean power output × 6)] × 100 for cycling)).
On a separate day (day 4 and 5 after the last training session), participants performed the RSA and YYIR2 test in a gym. The testing session started with performing the RSA test first, followed by the YYIR2 test. Tests were separated by 1 hour where participants freely moved in the gym. Similar to the cycling tests, residual fatigue could have influenced YYIR2 test outcome, but as testing procedures before and after the training period were the same, comparison of changes due to the training should be valid. The test procedures are outline elsewhere (Gatterer et al., 2014; 2015). Briefly, the RSA test consisted of 6 x 34 m sprints (17 m back and forth) with 20 s of passive recovery between sprints. Participants started 0.5 m ahead of a photocell system (Brower-Timing-System, Utah, USA), sprinted linear 17 m, touched a cone with one hand and sprinted back through the timing system as fast as possible. Best and mean RSA times were recorded. Furthermore, the performance decrement was calculated according to Girard et al. (2011b) (i.e., [total sprint time accumulated / (fastest sprint time × 6) – 1] × 100).
The YYIR2 consisted of repeated 2 x 20 m runs back and forth between the starting, turning, and finishing line at a progressively increasing speed until exhaustion. The speed was controlled by audio bleeps. Between each shuttle, the participants had a 10 s active rest period, consisting of 2 x 2.5 m walking. The end of the test was considered when participants failed twice to reach the front line in time (objective evaluation) or if they felt unable to complete another shuttle (subjective evaluation) (Krustrup et al., 2006). The completed shuttle and the resulting distance covered were registered for analysis.
During the Wingate tests and the repeated cycling sprints muscle oxygenation was measured continuously by NIRS (Niro 200, Hamamatsu Photonics K.K., Hamamatsu City, Japan). The NIRS optical sensor was placed longitudinally over the distal part of the belly of the vastus lateralis. NIRS provides the tissue oxygenation index (TOI) and the normalised total haemoglobin index (nTHI). TOI is the ratio of oxygenated to total tissue haemoglobin and reflects changes in tissue O2 saturation relative to rest, provides information on oxygen availability and rate of oxygen utilization and, therefore, indicates crude alterations in tissue oxygen extraction (Boushel et al., 2001; Gatterer et al., 2013; Ihsan et al., 2013). The nTHI on the other hand represents a measure of total haemoglobin and is suggested to reflect blood flow (Highton et al., 2013; Montero and Lundby, 2017). During the Wingate test oxygen extraction, i.e. deoxygenation, was calculated by subtracting the lowest TOI value during the test from the 1 min average before the start of the test. “Re-oxygenation” was calculated by subtracting a 1 min average TOI value after the end of the test from the lowest value during the test. During the repeated sprints the average of the lowest values (representing oxygen extraction during each sprint) and the average of the highest values (representing re-oxygenation after each sprint) were calculated. Overall deoxygenation during the repeated-sprint test was calculated as the average of the lowest values minus the 1 min average before the start of the test. Overall “re-oxygenation” was calculated by subtracting a 1 min TOI average after the end of the test from the average of the lowest values during the test. Moreover, re-oxygenation during the sprints was calculated by subtracting the average of the highest TOI values during the sprints from the average of the lowest values. nTHI changes during the Wingate test were calculated as the value at the end of the test minus a 1 min average before the test. Similar, nTHI changes during the repeated cycling sprints were calculated as the mean of the highest nTHI values minus the 1 min average before the first sprint.
Two to three minutes after both the Wingate test and the repeated sprints, capillary blood samples were obtained from the hyperaemized earlobe to measure lactate concentration ([La], Biosen C line, EKF Diagnostics, Germany). Additionally, participants were asked to rate their perceived exertion (RPE) according to the 6-20 BORG scale (Borg, 1974).
For measurement of mitochondrial respiration, venous blood samples were taken from 4 participants (2 in each group) before and after the training intervention. Two 9 ml samples of whole blood were collected in VACUETTE® K3EDTA (tri-potassium ethylenediaminetetraacetic acid) in the morning after an overnight fasting and avoiding strenuous exercise for 48 h. The blood was transported within 1 hour after sampling to the diagnostic lab in thermo-insulating containers with hot-cold-gel packs at room temperature and protected from light. Whole blood was counted on the Sysmex XN-350 automated blood cell counter (Sysmex Corporation). Blood cells were isolated in 50 ml Leucosep® tubes (Greiner Bio-One) with 15 ml of Ficoll-Paque™ (Sumbalova et al., 2016). 18 ml of blood diluted 1:1 with DPBS was gently poured on the top of the polyethylene barrier and centrifuged at 1,000 g (10 min, acceleration 6, no brakes). The layer was collected containing peripheral blood mononuclear cells (PBMC) and platelets (PLT). PBMC were washed twice with 25 ml DPBS (120 g; 10 min, acceleration 6, brakes 2), resuspended in 0.5 ml DPBS and counted (1:10 dilution) with the Sysmex XN-350. For isolation of PLT, 5 ml of diluted plasma obtained in the first centrifugation of diluted blood were combined with supernatant obtained in the first washing step of PBMC, and EGTA was added at 10 mM final concentration. After centrifugation at 1,000 g (10 min, acceleration 6, brakes 2) the sediment was washed with 5 ml DPBS containing 10 mM EGTA (1,000 g; 5 min, acceleration 6, brakes 2), resuspended with 0.5 ml DPBS containing 10 mM EGTA and counted (1:10 dilution) with the Sysmex XN-350. Isolated blood cells were used for determination of respiratory characteristics of intact cells. In a typical measurement, 4 million PBMCs or 200 million PLT were added into the 2 ml chamber of the O2k High-Resolution FluoRespirometer (Oroboros Instruments, Innsbruck, Austria). A simple titration protocol was used, including (1) ROUTINE respiration of intact cells (R), (2) noncoupled respiration induced by optimum concentrations of the uncoupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP) corresponding to electron transfer capacity (E), and (3) antimycin A-inhibitied respiration corresponding to residual oxygen consumption (Rox). (Pesta and Gnaiger, 2012) All measurements were performed at 37 °C in respiration medium MiR05 (Gnaiger et al., 2000) with addition of 10 mM pyruvate. In all samples of isolated blood cells, citrate synthase (CS) activity was determined and used as a marker for mitochondrial content. Oxygen fluxes of the PBMC fraction were corrected for the contribution by contaminating PLTs, and normalized per cell count and CS activity. The advantage of this method in an exercise training setting is that only blood samples are needed compared to muscle biopsies, which is a more invasive approach and disliked by many athletes. An obvious disadvantage is that so far, only limited research on this method exists and conclusive comparisons to the gold standard (i.e., analyzing mitochondrial function from muscle biopsies) are still lacking.
Training
The training intervention lasted for 3 weeks and the participants trained 3 times/week. After a standardized warm up, including 10 min cycling at 100-150 watts, participants performed either a long (SIH, 4x 30s; 5min rest) or short (RSH, 3 sets of 5x10 s with 20 s rest between the intervals and 5 min rest between the series) normobaric hypoxia sprint training program as previously described in detail (Faiss et al., 2013b; Puype et al., 2013). The training was performed on a cycle ergometer (Cyclus 2) in a hypoxia chamber (LowOxygen, Germany) at a simulated altitude level of approximately 2,200 m (FiO2: 17.1%, Innsbruck). The Cycle ergometer was set at a fixed torque corresponding to 0.75 x body weight during the long intervals and 0.85 x body weight during the short repeated bouts. The torque was differently set to enable participants to deliver maximal power output in both training scenarios and not to limit power development due to either biomechanical insufficiencies related to excessive pedaling frequency or load. During each session, power output, oxygen saturation (SpO2), HR (Polar RS800CX, Finland), [La], and effort perception (RPE) were determined. A training altitude of 2,200 m was chosen because this altitude level corresponds to the elevation of the natural altitude training facilities in the county and results might thus be indicative for training outcomes at these training centers. The selection is furthermore justified by the recommendation of Goods et al. (2014) to use moderate simulated altitude of 2,000 to 3,000 m when implementing hypoxic repeat sprint training for team-sport athletes. Nonetheless, the altitude level of 2,200 m was lower than mostly applied during repeated-sprint training regimes (i.e., 2,800 – 3,000 m) (Brocherie et al., 2017).
Statistical analyses
Data analysis was performed using the SPSS statistical software package (PASW Statistic 21). Normal distribution of data was confirmed by the Kolmogorov Smirnov test. ANOVA with repeated measurement design (post hoc t-tests with Bonferroni correction, applied as adequate) was applied to investigate training effects and changes in physiological variables between RSH and SIH. Effect sizes (ES, partial η²) were reported for the performance and NIRS outcomes. Pearson correlation analyses were performed for the combined groups only to investigate parameters related to performance changes. Due to technical problems (NIRS and Cyclus II device) and drop outs during single testing the number of participants varies as shown in Tables 1 and 2. The level of significance was set at p<0.05. The number of participants for evaluation of blood cell respiration was limited to 2 participants per training mode, thus a descriptive statistical approach was applied for this data set.
Table 1.
Running and cycling performance outcomes before and after SIH and RSH training
| SIH | RSH | ANOVA, p-value (partial η²) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | pre | post | Δ | n | pre | post | Δ | time effect training | interaction effect group x training | |
| Running | ||||||||||
| YYIR2, m | 4 | 430.0±50.3 | 530.0±50.3 | +100.0±51.6 | 6 | 486.7±134.9 | 583.3±215.6 | +96.7±145.6 | 0.034 (0.449) | 0.967 (0.000) |
| RSA mean, s | 4 | 6.60±0.26 | 6.50±0.26 | -0.11±0.08 | 6 | 6.59±0.32 | 6.45±0.36 | -0.14±0.14 | 0.012 (0.564) | 0.700 (0.020) |
| RSA best, s | 4 | 6.29±0.28 | 6.19±0.29 | -0.10±0.06 | 6 | 6.35±0.41 | 6.20±0.43 | -0.15±0.18 | 0.031 (0.460) | 0.637 (0.029) |
| RSA %decrement | 4 | 5.0±0.6 | 5.1±1.0 | +0.2±0.5 | 6 | 3.8±1.7 | 4.1±1.9 | +0.3±1.7 | 0.729 (0.016) | 0.760 (0.012) |
| Cycling | ||||||||||
| Wingate mean,W | 5 | 789±90 | 821±112 | +31.8±47.4 | 5 | 739±76 | 811±73 | +71.6±34.5 | 0.004 (0.661) | 0.168 (0.223) |
| Wingate peak, W | 5 | 1011±105 | 1132±129 | +120.7±45.4 | 6 | 969±112 | 1099±120 | +129.6±93.9 | <.001 (.766) | 0.852 (0.004) |
| Wingate FI, W/s | 5 | 19.8±2.7 | 21.7±3.4 | +1.9±2.6 | 5 | 19.1±2.5 | 21.1±3.0 | +2.0±4.5 | 0.124 (0.270) | 0.967 (0.000) |
| Wingate [La] | 5 | 10.6±2.9 | 8.8±2.9 | -1.8±2.2 | 6 | 10.3±2.5 | 10.7±2.3 | +0.4±2.5 | 0.339 (0.102) | 0.160 (0.206) |
| Wingate RPE | 5 | 17.0±1.4 | 16.2±1.3 | -0.8±1.6 | 6 | 17.0±2.3 | 17.8±1.0 | +0.8±1.7 | 0.975 (0.000) | 0.144 (0.221) |
| RS mean, W | 5 | 1000±118 | 1056±122 | +55.5±44.9 | 6 | 951±94 | 1043±89 | +91.8±58.1 | 0.001 (0.703) | 0.283 (0.126) |
| RS peak, W | 5 | 1141±109 | 1170±113 | +28.5±37.2 | 6 | 1051±128 | 1155±134 | +104.2±83.3 | 0.010 (0.544) | 0.095 (0.279) |
| RS % decrement | 5 | 8.9±2.0 | 7.6±3.7 | -1.4±3.0 | 6 | 8.5±4.2 | 8.8±2.9 | +0.3±3.7 | 0.633 (0.026) | 0.430 (0.071) |
| RS [La] | 5 | 10.8±1.9 | 10.2±3.5 | -0.6±2.3 | 5 | 9.9±3.1 | 11.3±2.6 | +1.4±2.1 | 0.602 (0.036) | 0.183 (0.210) |
| RS RPE | 5 | 16.2±2.3 | 16.4±2.7 | +0.2±1.3 | 4 | 16.3±1.0 | 17.5±0.6 | +1.4±1.1 | 0.136 (0.288) | 0.262 (0.175) |
YYIR2, Yo-Yo intermittent recovery test level 2; RSA, repeated-sprint ability; FI, fatigue Index; [La], lactate concentration (mmol/L); RPE, rating of perceived exertion; RS, repeated cycling sprint
Table 2.
Near infrared spectroscopy outcomes before and after SIH and RSH training
| SIH | RSH | ANOVA, p-value (partial η²) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | pre | post | Δ | n | pre | post | Δ | time effect training | interaction effect group x training | |
|
NIRS (cycling) Tissue oxygenation index [%] | ||||||||||
| Wingate (deoxygenation) | 5 | -18.8±9.7 | -20.3±11.3 | -1.5±5.9 | 4 | -20.9±10.7 | -22.2±13.6 | -1.3±3.4 | .429 (.092) | .943 (.001) |
| Wingate (re-oxygenation) | 5 | 16.0±6.9 | 18.5±8.7 | 2.5±4.9 | 4 | 18.0±11.1 | 19.7±12.8 | 1.7±2.5 | .174 (.246) | .770 (.013) |
| RS (deoxygenation) | 5 | -17.2±4.4 | -18.0±4.8 | -.7±2.3 | 5 | -15.4±6.4 | -25.3±11.1 | -9.9±5.8 | .005 (.642) | .012 (.570) |
| RS (re-oxygenation) | 5 | 17.4±5.0 | 20.0±3.6 | 2.6±3.4 | 4 | 17.8±6.0 | 29.5±7.6 | 11.7±1.8 | <.001 (.889) | .002 (.764) |
| RS (re-oxygenation during sprints) | 5 | 15.9±3.4 | 16.2±3.3 | .3±2.3 | 5 | 12.9±8.0 | 19.7±7.8 | 6.8±5.9 | .037 (.438) | .053 (.391) |
| Normalised total haemoglobin index (au) | ||||||||||
| Wingate (increase) | 5 | .037±.065 | .051±.087 | .014±.08 | 5 | .049±.117 | .059±.111 | .011±.067 | .634 (.034) | .950 (.001) |
| RS (increase) | 5 | .047±.024 | .086±.130 | .039±.134 | 5 | .034±.077 | .112±.073 | .079±.074 | .126 (.268) | .579 (.040) |
NIRS, near infrared spectroscopy; RS, repeated cycling sprint
Results
Table 1 summarizes the outcomes of the performance tests before and after the training period. Both RSH and SIH improved YYIR2 running distance and RSA mean and best time (time effect: training, p <0.05) with no interaction effects (group x training, p <0.05). Additionally, the training improved Wingate as well as repeated cycling sprints mean and peak power output (time effect: training, p > 0.05) with a trend toward an interaction effect in repeated cycling sprints peak power output (group x training, p = 0.095). Improvements in YYIR2 running distance was negatively associated with changes in Wingate and repeated cycling mean power (r = -0.743 and r = -0.756, respectively, p <0.05, Figure 1).
Figure 1.
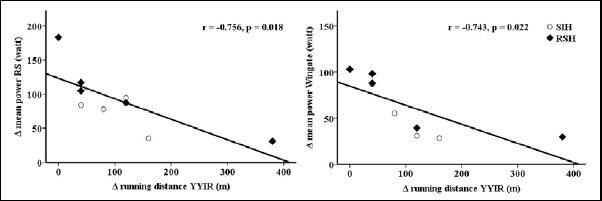
Relationship between changes in cycling performance and YYIR2 running performance.
During repeated cycling sprints de- and re-oxygenation was changed over time (time effect: training, p <0.05) with greater deoxygenation and re-oxygenation in the RSH group (interaction: group x training, p <0.05) (Table 2). Improvements in repeated cycling and Wingate mean power were associated with re-oxygenation during the sprints after training (r = 0.707 and r = 0.713, respectively, p <0.05, Figure 2). Table 3 shows individual changes of the PBMC respiration before and after the training.
Figure 2.
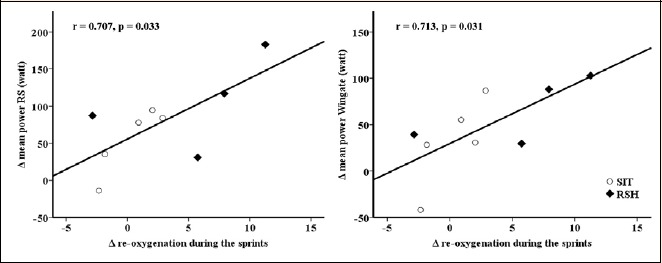
Relationship between changes in cycling performance and re-oxygenation during the sprints.
Table 3.
PBMC respiration (high-resolution respirometry) and muscle de- and re-oxygenation (NIRS) before and after training.
| O2 flow corrected for Rox | Overall sprinting | O2 flux corrected for Rox | Repeated sprinting | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IO2 [amol·s-1·cell-1] | Δ De-oxygenation | Δ Re-oxygenation | JO2/CS [pmol·s-1·IU-1] | Δ Re-oxygenation | ||||||
| before | after | Δ | TOI [%] | TOI [%] | before | after | Δ | [TOI%] | ||
| RSH 1 | R | 5.1 | 4.0 | 1.17 | 1.31 | |||||
| E | 23.4 | 13.7 | -9.7 | -9.7 | 12.82 | 5.39 | 4.43 | -0.96 | -2.9 | |
| RSH 2 | R | 3.6 | 3.0 | 0.93 | 0.94 | |||||
| E | 21.4 | 8.0 | -13.4 | -19.8 | 12.4 | 5.54 | 2.48 | -3.06 | 5.8 | |
| SIH 1 | R | 2.9 | 4.9 | 1.08 | 1.37 | |||||
| E | 16.8 | 16.0 | -0.7 | -2.3 | 7.7 | 6.34 | 4.49 | -1.86 | 2.9 | |
| SIH 2 | R | 3.3 | 5.5 | 1.13 | 1.26 | |||||
| E | 18.4 | 23.5 | 5.1 | 1.24 | -0.44 | 6.24 | 5.36 | -0.88 | -1.84 | |
RSH1, RSH2 – participants with RSH training, SIH1, SIH2 – participants with SIH training. ROUTINE respiration (R) and electron transfer capacity (E) were determined as described in the methods section. IO2 – oxygen flow per cell count [amol·s-1·cell-1 = pmol·s-1·10-6 cells], JO2 – oxygen flux per mitochondrial marker citrate synthase activity [pmol·s-1·IU-1] 37. Respiration was corrected for the contribution from PLTs contaminating the PBMC fraction. Rox – residual oxygen consumption.
The maximum electron transfer (E) capacity normalized per PBMC count was decreased in RSH after the training, whereas in SIH it was affected to a lesser extent. The E capacity normalized for CS activity showed a decrease after the training in all participants. A similar pattern was observed for changes in TOI and PBMC respiration (differentiated for O2 flow per cell count and O2 flux per citrate synthase activity; Table 3). Respiration of PLTs was not affected by any training modality (data not shown).
Discussion
The present investigation shows that hypoxia cycling training including either long (SIH) or short (RSH) duration repeated sprints and recovery intervals improved sea level cycling and running performance to a similar extent. However, athletes who improved most during the cycling task showed less improvement during running (Figure 1). Additionally, outcomes indicate an influence of the type of training on TOI during repeated cycling. RSH compared to SIH led to higher de- and re-oxygenation during repeated sprinting. Increases in re-oxygenation during sprinting were associated with improvements in Wingate and repeated cycling mean power output. Additionally, this study suggests for the first time that RSH modifies mitochondrial function in PBMC.
In the present study, the magnitude of the performance gains were similar between RSH and SIH and comparable to the results of studies investigating these training regimes individually (Faiss et al., 2013b; Gatterer et al., 2014; Galvin et al., 2013; Hamlin et al., 2017; Kasai et al., 2015; Puype et al., 2013). Data suggest that the two training regimes can interchangeable be used to improve athletes performance. Additionally, similar changes in [La] and RPE after the Wingate and repeated cycling sprint test were recorded indicating that anaerobic metabolism and perception of effort were similarly affected by both training regimes. Nonetheless, when considering the medium to large effect size (Table 1), cycling power output and [La] data indicate that RSH compared to SIH might be favorable for performance improvements (Δ repeated cycling sprint mean power: 55.5 ± 44.9 vs. 91.8 ± 58.1 W and Δ Wingate mean power:= 31.9 ± 47.4 vs. 71.6 ± 34.5 W, for the 30 s and 10 s group, respectively) and increases in anaerobic contribution (Δ Wingate [La]: -1.8 ± 2.2 vs. 0.4 ± 2.5 mmol/L; Δ repeated cycling sprint [La]: -0.6 ± 2.3 vs. 1.4 ± 2.1 mmol/l, for the 30 s and 10 s group, respectively). The somewhat higher overall training volume during RSH compared to SIH (overall sprinting time: 1350 s vs. 1080 s for RSH and SIH, respectively) might have led to these differences, which however have to be confirmed by further studies with a larger sample size. NIRS data on the other hand suggest a clear beneficial effect of RSH compared to SIH. De- and re-oxygenation during repeated sprinting were improved after RSH compared to SIH, indicating that both oxygen extraction and restoration of oxygen levels were enhanced. Accordingly, in studies investigating RSH, changes in the concentration of desoxyhemoglobin as well as total hemoglobin/myoglobin during repeated sprinting and in the recovery phase have been reported (Faiss et al., 2013b; 2015; Montero and Lundby, 2017). The major benefit of a higher re-oxygenation during short rest phases is a faster re-synthesis of phosphocreatine (McMahon and Jenkins, 2002; Tomlin and Wenger, 2001). Thus, higher re-oxygenation might be one mechanism responsible for performance improvements, which is supported by the positive correlation between changes in performance and re-oxygenation during the sprints (Figure 2).
A remarkable finding is that RSH and SIH seem to differently affect mitochondrial respiration of PBMCs, whereas platelet respiration remained unchanged. Few data are available on the effects of physical training on blood cell mitochondrial function (de Lucas et al., 2014; Tsai et al., 2016), whereas PBMC and PLT respiration is becoming progressively established as a biomarker of mitochondrial dysfunction in degenerative diseases (Li et al., 2015; Sjövall et al., 2010). The number of tested participants is not adequate to draw firm conclusions from the present preliminary study; nonetheless, it seems that O2 flow per cell count was reduced in RSH and unaffected by SIH, whereas O2 flux per mitochondrial marker showed comparable reductions after both training types (Table 3). The exact meaning of these changes in relation to mitochondrial dysfunction in stress and disease states needs to be explored in further studies. It could be speculated that in the amateur participants the unusual high training intensity and volume in combination with hypoxia, especially during RSH might have an impact on mitochondrial function due to excess reactive oxygen species (ROS) production (Pialoux et al., 2006; Trentadue et al., 2012), as was shown during overtraining (Kadaja et al., 2010). In accordance, Faiss et al. (2013b) found reductions in mRNA expression of genes implicated in mitochondrial biogenesis after RSH. Such changes might explain why most studies investigating RSH did not find improvements in aerobic capacity (Brocherie et al., 2017; Hamlin et al., 2017). Additionally, interleukin-6 (IL-6) produced during exercise could have played a role in PBMC respiration. IL-6 acts as an anti-inflammatory factor in muscles and as a pro-inflammatory factor in the blood stream (Pedersen et al., 2001), with different effects on PBMC and PLT. In fact, Tyrell et al. reported a negative correlation between plasma IL-6 and PBMC respiration (Tyrrell et al., 2015b). In contrast, O2 flow per cell count seems to be less affected by SIH training (Table 3). One might speculate that the more anaerobic characteristic of the training (i.e., the 5 min rest between the 30 s sprints likely allowed the aerobic system to return to nearly resting levels permitting a O2 deficit to occur at sprint onset (Buchheit and Laursen, 2013a)) might have led to lower ROS production of the mitochondria (Fisher-Wellman and Bloomer, 2009) and thus might have preserved mitochondrial function. Accordingly, this type of training, next to improve anaerobic capacity, was shown to enhance performance at the anaerobic threshold, which indicates up-regulation of muscular oxidative capacity (Puype et al., 2013). A further interesting finding was that TOI changes gathered from muscle show some comparable patterns to the mitochondrial PBMC respiration (Table 3) indicating some connection. Further studies are needed to explore the meaning of this finding.
A secondary outcome of this study was that cycling sprint training is able to improve running performance as was found by others (Hamlin et al., 2017). Thus, similar to the conclusion of Hamlin et al. (2017) both types of training (RSH and SIH) might be recommended as an opportunity to relieve on-feet training stress without reducing overall performance in intermittent sports involving large amounts of running exercise. However, it should be considered that athletes who improved most during the cycling task might improve less during running (Figure 1), highlighting individual responses.
Some limitations of the present study have to be acknowledged. The number of participants was low and due to drop outs and malfunction of measurement devices some data was lost. Nonetheless, the present study gives some insight into the specific performance changes and mechanisms of adaptations of RSH and SIH. A further issue that needs consideration is the somewhat different training volume of the two groups outlined before. The sprinting protocols have been selected as they represent typical protocols described in the literature; as such, outcomes represent characteristic training outcomes of the two training regimes. Clearly, a possible effect of the different training volume on the results cannot be excluded. Moreover, this study lacks normoxia control groups, thus, it cannot be stated whether hypoxia training leads to different adaptations compared to normoxia training. However, this study was not designed to investigate the effects of hypoxia compared to normoxia training but to study if the two types of training i.e. RSH and SIH differ in regard to performance outcomes and mechanisms of adaptation.
Conclusions
This study showed that RSH and SIH training performed on a cycle ergometer improves normoxia running performance even though the specificity of adaptation has to be recognized. Additionally, RSH and SIH training seem to lead to different adaptations in regard to muscular oxygen extraction and mitochondrial respiration of PBMC.
Acknowledgements
Erich Gnaiger is founder and CEO of Oroboros Instruments. There are no patents, products in development or marketed products to declare. This investigation was part of the K-Regio project MitoFit, which is funded by the Land Tirol within the program K-Regio of Standortagentur Tirol. We thank Verena Laner for administrative assistance. Contribution to COST Action CA15203 MitoEAGLE. We acknowledge the help of Julia Nirrnheim and Sina Straub during training and testing. The study complied with the laws of the country of the authors’ affiliation.
Biographies
Hannes GATTERER
Employment
High altitude medicine group leader at the Institute of Mountain Emergency Medicine, EURAC Research, Bolzano, Italy
Degree
PhD
Research interests
High altitude physiology/medicine, exercise physiology, intermittent hypoxia, body composition
E-mail: hannes.gatterer@eurac.edu
Verena MENZ
Employment
Researcher at the Department of Sport Science, University of Innsbruck, Austria
Degree
PhD
Research interests
High-intensity interval training, high altitude physiology, exercise physiology
E-mail: verena.menz@uibk.ac.at
Eduardo SALAZAR-MARTÍNEZ
Employment
Assistant Professor in Human Physiology at the University Studies Center, CEU Andalucía
Degree
PhD
Research interests
Sport performance and training adaptations with special interest on exercise in hypoxia
E-mail: eduardosm1989@gmail.com
Zuzana SUMBALOVA
Employment
Researcher at the Daniel Svarowski Research Laboratory, Department of Visceral, Transplant and Thoracic Surgery of the Medical University of Innsbruck
Degree
PhD
Research interests
Mitochondrial respiration, respirometry of human blood cells
E-mail:zuzana.sumbalova@fmed.uniba.sk
Luiz Felipe GARCIA-SOUZA
Employment
Rresearch assistant at the Department of Sport Science of the University of Innsbruck and is a PhD student at the Medical University of Innsbruck
Degree
MSc
Research interests
Blood cells bioenergetics and mitochondrial physiology
E-mail: luiz.garcia@student.i-med.ac.at
Beáta VELIKÁ
Employment
Research specialist at the Department of Medical and Clinical Biochemistry, Pavol Jozef Šafárik University in Košice, Slovakia
Degree
PhD
Research interests
Spectroscopic methods (UV/Vis, fluorescence spectroscopy), electrophoretic partitioning of lipoprotein fractions (lipoprint), cellular respiration
E-mail: bvelika@gmail.com
Martin BURTSCHER
Employment
Professor (retired) at the Department of Sport Science, University of Innsbruck, Innsbruck, Austria
Degree
PhD, MD
Research interests
Exercise and high-altitude (hypoxia) physiology and pathophysiology. Life-style interventions in health and disease. Medical aspects of harsh environments.
E-mail: martin.burtscher@uibk.ac.at
References
- Borg G.A. (1974) Perceived exertion. Exercise and Sport Sciences Reviews 2, 131-153. [PubMed] [Google Scholar]
- Boushel R., Langberg H., Olesen J., Gonzales-Alonzo J., Bülow J., Kjaer M. (2001) Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scandinavian Journal of Medicine and Science in Sports 11, 213-222. [DOI] [PubMed] [Google Scholar]
- Brocherie F., Girard O., Faiss R., Millet G.P. (2017) Effects of Repeated-Sprint Training in Hypoxia on Sea-Level Performance: A Meta-Analysis. Sports Medicine 47, 1651-1660. [DOI] [PubMed] [Google Scholar]
- Brocherie F., Millet G.P., D’Hulst G., Van Thienen R., Deldicque L., Girard O. (2018) Repeated maximal-intensity hypoxic exercise superimposed to hypoxic residence boosts skeletal muscle transcriptional responses in elite team-sport athletes. Acta Physiologica (Oxf) 222, 1. [DOI] [PubMed] [Google Scholar]
- Buchheit M., Laursen P.B. (2013a) High-intensity interval training, solutions to the programming puzzle. Part II: anaerobic energy, neuromuscular load and practical applications. Sports Medicine 43, 927-954. [DOI] [PubMed] [Google Scholar]
- Buchheit M., Laursen P.B. (2013b) High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sports Medicine 43, 313-338. [DOI] [PubMed] [Google Scholar]
- de Lucas R.D., Caputo F., Mendes de Souza K., Sigwalt A.R., Ghisoni K., Lock Silveira P.C., Remor A.P., da Luz Scheffer D., Guglielmo L.G., Latini A. (2014) Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. Journal of Sports Sciences 32, 22-30. [DOI] [PubMed] [Google Scholar]
- De Smet S., Van Thienen R., Deldicque L., James R., Sale C., Bishop D.J., Hespel P. (2016) Nitrate Intake Promotes Shift in Muscle Fiber Type Composition during Sprint Interval Training in Hypoxia. Frontiers in Physiology 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss R., Girard O., Millet G.P., (2013a). Advancing hypoxic training in team sports: from intermittent hypoxic training to repeated sprint training in hypoxia. British Journal of Sports Medicine 47, i45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss R., Leger B., Vesin J.-M., Fournier P.-E., Eggel Y., Deriaz O., Millet G.P. (2013b) Significant Molecular and Systemic Adaptations after Repeated Sprint Training in Hypoxia. Plos One 8, e56522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss R., Willis S., Born D.P., Sperlich B., Vesin J.M., Holmberg H.C., Millet G.P. (2015) Repeated double-poling sprint training in hypoxia by competitive cross-country skiers. Medicine and Science in Sports and Exercise 47, 809-817. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman K., Bloomer R.J. (2009) Acute exercise and oxidative stress: a 30 year history. Dynamic Medicine 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin H.M., Cooke K., Sumners D.P., Mileva K.N., Bowtell J.L. (2013) Repeated sprint training in normobaric hypoxia. British Jouranl of Sports Medicine 47 Suppl 1, i74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatterer H., Greilberger J., Philippe M., Faulhaber M., Djukic R., Burtscher M. (2013) Short-Term Supplementation with Alpha-Ketoglutaric Acid and 5-Hydroxymethylfurfural Does not Prevent the Hypoxia Induced Decrease of Exercise Performance Despite Attenuation of Oxidative Stress. International Journal of Sports Medicine 34, 1-7. [DOI] [PubMed] [Google Scholar]
- Gatterer H., Klarod K., Heinrich D., Schlemmer P., Dilitz S., Burtscher M. (2015) Effects of a 12-day maximal shuttle-run shock microcycle in hypoxia on soccer specific performance and oxidative stress. Applied Physiology Nutrition and Metabolism 40, 842-845. [DOI] [PubMed] [Google Scholar]
- Gatterer H., Philippe M., Menz V., Mosbach F., Faulhaber M., Burtscher M. (2014) Shuttle-run sprint training in hypoxia for youth elite soccer players: a pilot study. Jouranl of Sports Science and Medicine 13, 731-735. [PMC free article] [PubMed] [Google Scholar]
- Girard O., Brocherie F., Millet G.P., (2017) Effects of Altitude/Hypoxia on Single- and Multiple-Sprint Performance: A Comprehensive Review. Sports Medicine 47, 1931-1949. [DOI] [PubMed] [Google Scholar]
- Girard O., Mendez-Villanueva A., Bishop D. (2011a) Repeated-sprint ability - part I: factors contributing to fatigue. Sports Medicine 41, 673-694. [DOI] [PubMed] [Google Scholar]
- Girard O., Racinais S., Kelly L., Millet G.P., Brocherie F. (2011b) Repeated sprinting on natural grass impairs vertical stiffness but does not alter plantar loading in soccer players. European Journal of Applied Physiology 111, 2547-2555. [DOI] [PubMed] [Google Scholar]
- Gnaiger E., Kuznetsov A.V, Schneeberger S., Seiler R., Brandacher G., Steurer W., Margreiter R. (2000) Mitochondria in the cold. In: Life in the Cold. Eds: Heldmaier G., Klingenspor M. Heidelberg, Berlin, New York: Springer; 431-442. [Google Scholar]
- Goods P.S., Dawson B.T., Landers G.J., Gore C.J., Peeling P. (2014) Effect of different simulated altitudes on repeat-sprint performance in team-sport athletes. International Journal of Sports Physiology and Performance 9, 857-862. [DOI] [PubMed] [Google Scholar]
- Hamlin M.J., Olsen P.D., Marshall H.C., Lizamore C.A., Elliot C.A. (2017) Hypoxic Repeat Sprint Training Improves Rugby Player’s Repeated Sprint but Not Endurance Performance. Frontiers in Physiology 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton D., Panovska-Griffiths J., Ghosh A., Tachtsidis I., Banaji M., Elwell C., Smith M. (2013) Modelling cerebrovascular reactivity: a novel near-infrared biomarker of cerebral autoregulation? Advances in Experimental Medicine and Biology 765, 87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihsan M., Abbiss C.R., Lipski M., Buchheit M., Watson G. (2013) Muscle oxygenation and blood volume reliability during continuous and intermittent running. International Jouranl of Sports Medicine 34, 637-645. [DOI] [PubMed] [Google Scholar]
- Kadaja L., Eimre M., Paju K., Roosimaa M., Põdramägi T., Kaasik P., Pehme A., Orlova E., Mudist M., Peet N., Piirsoo A., Seene T., Gellerich F.N., Seppet E.K. (2010) Impaired oxidative phosphorylation in overtrained rat myocardium. Experimental and Clinical Cardiology 15, e116-127. [PMC free article] [PubMed] [Google Scholar]
- Kasai N., Mizuno S., Ishimoto S., Sakamoto E., Maruta M., Goto K. (2015) Effect of training in hypoxia on repeated sprint performance in female athletes. Springerplus 4, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P., Mohr M., Nybo L., Jensen J.M., Nielsen J.J., Bangsbo J. (2006) The Yo-Yo IR2 test: physiological response, reliability, and application to elite soccer. Medicine and Science in Sports and Exercice 38, 1666-1673. [DOI] [PubMed] [Google Scholar]
- Li P., Wang B., Sun F., Li Y., Li Q., Lang H., Zhao Z., Gao P., Zhao Y., Shang Q., Liu D., Zhu Z. (2015) Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Scientific Reports 5, 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S., Jenkins D. (2002) Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Medicine 32, 761-784. [DOI] [PubMed] [Google Scholar]
- Montero D., Lundby C. (2015) Enhanced Performance after Repeated Sprint Training in Hypoxia: False or Reality? Medicine and Science in Sports and Exercise 47, 2483. [DOI] [PubMed] [Google Scholar]
- Montero D., Lundby C. (2017) No Improved Performance With Repeated-Sprint Training in Hypoxia Versus Normoxia: A Double-Blind and Crossover Study. International Journal of Sports Physiology and Performance 12, 161-167. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Steensberg A., Schjerling P. (2001) Muscle-derived interleukin-6: possible biological effects. Jouranl of Physiology 536, 329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesta D., Gnaiger E. (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods in Molecular Biology 810, 25-58. [DOI] [PubMed] [Google Scholar]
- Pialoux V., Mounier R., Ponsot E., Rock E., Mazur A., Dufour S., Richard R., Richalet J.P., Coudert J., Fellmann N. (2006) Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. European Journal of Clinical Nutrition 60, 1345-1354. [DOI] [PubMed] [Google Scholar]
- Puype J., Van Proeyen K., Raymackers J.M., Deldicque L., Hespel P. (2013) Sprint interval training in hypoxia stimulates glycolytic enzyme activity. Medicine and Science in Sports and Exercise 45, 2166-2174. [DOI] [PubMed] [Google Scholar]
- Richardson A.J., Gibson O.R. (2015) Simulated hypoxia does not further improve aerobic capacity during sprint interval training. The Journal of Sports Medicine and Physical Fitness 55, 1099-106. [PubMed] [Google Scholar]
- Sjövall F., Morota S., Hansson M.J., Friberg H., Gnaiger E., Elmér E. (2010) Temporal increase of platelet mitochondrial respiration is negatively associated with clinical outcome in patients with sepsis. Critical Care 14, R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbalova Z., Hiller E., Chang S., Garcia L., Droescher S., Calabria E., Volani C., Krumschnabel G., Gnaiger E. (2016) Isolation of blood cells for HRR. Mitochondrial Physiology Network 2117, 1-15. [Google Scholar]
- Tomlin D.L., Wenger H.A. (2001) The relationship between aerobic fitness and recovery from high intensity intermittent exercise. Sports Medicine 31, 1-11. [DOI] [PubMed] [Google Scholar]
- Trentadue R., Raffaella T., Fiore F., Massaro F., Fabrizia M., Papa F., Francesco P., Iuso A., Arcangela I., Scacco S., Salvatore S., Santacroce L., Luigi S., Brienza N., Nicola B. (2012) Induction of mitochondrial dysfunction and oxidative stress in human fibroblast cultures exposed to serum from septic patients. Life Sciences 91, 237-243. [DOI] [PubMed] [Google Scholar]
- Tsai H.H., Chang S.C., Chou C.H., Weng T.P., Hsu C.C., Wang J.S. (2016) Exercise Training Alleviates Hypoxia-induced Mitochondrial Dysfunction in the Lymphocytes of Sedentary Males. Scientific Reports 6, 35170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.J., Bharadwaj M.S., Van Horn C.G., Kritchevsky S.B., Nicklas B.J., Molina A.J. (2015a) Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. The Journals of Gerontology Series A Biological Sciences and Medical Sciences 70, 1394-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.J., Bharadwaj M.S., Van Horn C.G., Marsh A.P., Nicklas B.J., Molina A.J. (2015b) Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Experimental Gerontology 70, 84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]


