Abstract
Acid–base abnormalities are frequently encountered in veterinary emergency and critical care, but information regarding the prognostic value of these findings is limited. Several systems for analysing acid–base disturbances have been reported, but the prognostic abilities of these systems have not been compared in dogs. The objectives of this retrospective study were to determine if the commonly used acid–base interpretation methods (Henderson–Hasselbalch, Stewart and semi‐quantitative) have prognostic value, and to compare the performance of the three methods. Electronic medical records were searched to create a database containing point‐of‐care blood‐gas, electrolyte and serum chemistry values for 1024 dogs assessed at a university teaching hospital. Dogs with contemporaneous blood‐gas analysis, blood lactate and serum biochemistry samples were eligible for study, and only the first recorded analyses for each patient visit were included. Components of the Henderson–Hasselbalch, Stewart and semi‐quantitative methods were calculated. To assess prognostic ability and to compare analysis system performance, receiver‐operating characteristic (ROC) curves for survival to hospital discharge were created. Of the 1024 dogs identified, case fatality rate was 23.8%. Area under the ROC curve did not exceed 0.63 for any calculated variable. Performance of all three analysis systems was similar. While some acid–base abnormalities identified were associated with mortality, no individual abnormality or system output yielded sensitive and specific cut‐off values for mortality prediction, and no interpretation method outperformed the others. This study suggests that initial acid–base abnormalities have limited prognostic utility and that various analysis systems can be used to assess acid–base disturbances in critically ill dogs.
Keywords: Acid–base equilibrium, dogs, mortality, emergencies, critical care
Introduction
Dogs with conditions that cause acid–base disturbances are frequently encountered in veterinary emergency and critical care practice (Hopper et al. 2014a,2014b). Acid–base analysis provides insights into the nature and extent of a patient's disease process and can help with identification of the underlying disorder (Boag et al. 2005; Durocher et al. 2008). In addition to treatment of the root cause of the disturbance, management of the perturbations of acid–base balance themselves may offer opportunities to improve outcomes (Benjamin et al. 1994; Chen et al. 2013). The severity of electrolyte disturbances has been associated with outcome in dogs (Goggs et al. 2017), but there is scant information about the prognostic value of parameters derived from acid–base analysis in the veterinary literature.
Three systems for analysing acid–base status are in common clinical usage. The traditional approach uses measurements of pH and PaCO2 to describe the respiratory component of acid–base status, and calculates the bicarbonate concentration using the Henderson–Hasselbalch equation to describe the metabolic component (Constable 2000). The traditional approach categorizes acid–base disturbances into four causal groups: respiratory acidosis or alkalosis and metabolic acidosis or alkalosis. Respiratory disorders are identified by abnormal values for PaCO2, while abnormalities of bicarbonate concentration are taken to indicate metabolic disorders. The anion gap (AG), which is the difference between the sum of the two primary measured cations and the two primary measured anions, can also be calculated to help in the detection of unmeasured anions such as lactate and ketones. These parameters are straightforward to measure and calculate and the approach is easily understood and applied. However, it has been argued that the traditional approach is overly simplistic (Torrente et al. 2014), fails to detect derangements in many cases of metabolic disorders (Hopper et al. 2014a) and misleadingly indicates that bicarbonate is an independent causal variable in the regulation of acid–base status (Siegling‐Vlitakis et al. 2007). This misconception relates to the equilibrium that exists between carbon dioxide and bicarbonate, such that marked changes in carbon dioxide partial pressures will affect the bicarbonate concentration irrespective of production of acid or consumption of base by metabolic processes (Constable 2000).
The strong ion approach was developed to address these shortcomings (Stewart 1983). This system of clinical acid–base analysis defines six potential disorders. The Stewart approach defines respiratory acidosis and alkalosis on the basis of PaCO2. The metabolic contributions are subdivided into disorders of strong ions and disorders of non‐volatile weak acids (Atot). The strong cations are sodium, potassium, calcium and magnesium, while the principal strong anion is chloride. The normal imbalance between the concentrations of strong cations and strong anions gives rise to the strong ion difference (SID) (Stewart 1983). The Stewart approach also helps to define the strong ion gap (SIG) as the difference between all unmeasured strong anion charges and all unmeasured strong cation charges (Russell et al. 1996; Constable et al. 1998; Corey 2003; Slawuta et al. 2010; Hopper et al. 2014a,2014b). Analysis of the SIG provides a specific method for detecting the presence of unmeasured strong ions (such as lactate) and may be more specific than the anion gap which is also affected by non‐volatile buffer ions such as albumin, globulins and phosphate. The Stewart approach is perhaps the most comprehensive method for acid–base assessment and is based on the laws of electroneutrality, dissociation equilibria for incompletely dissociated solutes and conservation of mass (Stewart 1983). However, the resulting polynomial equations are too complex to be solved by hand and the approach is therefore too cumbersome for use in clinical practice (Fencl & Leith 1993; Corey 2003; Slawuta et al. 2010).
The third commonly used method is the semi‐quantitative approach (Fencl et al. 2000; Hopper et al. 2014a,2014b). This system also uses PaCO2 to define the respiratory component. The semi‐quantitative approach incorporates the strong ion and weak acid concepts from Stewart's work but uses them to determine their effect on the base excess in order to confirm that all metabolic factors have been accounted for, or to identify the presence of unmeasured anion (XA−) or cation species (XA+). Specifically, the semi‐quantitative approach uses sodium concentration as an indicator of free water changes, and assesses the contributions of corrected chloride, albumin, phosphate and lactate to determine if XA− or XA+ must be present to explain the known base excess value (Fencl et al. 2000). Although the computational burden of this semi‐quantitative approach is less than for the Stewart method, it still requires multiple calculation elements and the measurement of some variables not typically measured at the point‐of‐care.
To inform clinical practice, acid–base analysis should be rapid to perform. Given the varying time demands of the three available analysis systems, it is rational to enquire whether the additional measurements and computational effort required by the Stewart or semi‐quantitative approaches are worthwhile. It has been demonstrated that in dogs presented to an emergency room, the semi‐quantitative and Stewart methods were more likely to detect acid–base abnormalities than the traditional method, with the semi‐quantitative method detecting some type of acid–base derangements in all dogs evaluated (Siegling‐Vlitakis et al. 2007; Slawuta et al. 2010; Slawuta & Glinska‐Suchocka 2012; Hopper et al. 2014a,2014b). To date, however, no study has analysed the association between patient outcome and acid–base disturbances using strong ion analyses approaches. Several studies in the veterinary literature have assessed the relationship between acid–base balance and outcome (de Papp et al. 1999; Hume et al. 2006). Those studies evaluated small numbers of dogs, were focused on specific disease processes, and assessed individual acid–base parameters such as lactate. It is unknown if a strong ion approach to acid–base analysis offers prognostic information that might be of value to clinicians assessing and managing patients with a heterogeneous group of complex disorders. This is the knowledge gap that the present study sought to fill.
The aims of this study were to evaluate the associations between acid–base disturbances identified using three different analysis systems (traditional, Stewart and semi‐quantitative) and mortality for a heterogeneous group of dogs evaluated in an emergency and critical care setting, and to compare the prognostic abilities of the three systems with each other. It was hypothesized that the derived parameters of SID, SIG and Atot and the presence of unmeasured anions (XA−) are associated with increased mortality, and that these derived parameters are better predictors of mortality than pH, bicarbonate or base excess.
Materials and methods
Electrolyte and metabolite analyses
Venous blood‐gas and electrolyte analyses were conducted with point‐of‐care analysers (RapidPoint 405; Siemens, Malvern, PA, USA) equipped with ion‐selective electrodes using blood samples heparinized with dry balanced lithium/zinc heparin (Arterial Blood Gas Sampler, Westmed Inc., Tucson, AZ, USA). Serum chemistry analyses were conducted using an automated chemistry analyser (Cobas ModP, Roche‐Hitachi, Indianapolis, IN, USA). Blood lactate concentrations were measured with a handheld point‐of‐care lactate meter (Lactate Pro, Arkray, Minneapolis, MN, USA) using heparinized whole blood samples.
Sample population
An electronic database of venous blood‐gas and electrolyte analyses conducted in the emergency room or intensive care unit at the institution hospital between 31/05/07 and 03/01/15 was searched for analyses of dogs. The database was visually inspected and manually curated to remove analyses of samples from other species, and analyses of arterial blood or of sample types other than blood (e.g. peritoneal fluid). Analyses with missing, erroneous or untraceable case numbers were also eliminated. Medical records from dogs with very high measured chloride concentrations were manually checked to identify dogs receiving potassium bromide as an anticonvulsant (Rossmeisl et al. 2006) to reduce the impact of a known interfering substance (Dimeski et al. 2010). In dogs for which multiple blood‐gas analyses were identified, only the first measurement was used.
Institution electronic medical record systems were then searched for data on patient signalment, presenting complaint, final diagnosis, outcome, hospitalization dates and for data from serum biochemistry analyses. Four separate databases were thereby created containing the electrolyte data, point‐of‐care analyses, biochemistry analyses and case demographics. A custom script (Visual Basic, Microsoft Visual 328 Studio for Windows; Microsoft, Redmond, WA, USA) was written to search each database via the unique patient identifier and create a final composite database containing the relevant data from each of the separate databases corresponding to the time and date stamp from the blood‐gas and electrolyte analyses. The final database was then manually checked for accuracy by cross‐referencing the database entries with the parent data sources for a randomized selection of cases. For calculation of acid–base variables, only cases where all necessary variables (electrolytes, biochemistry panel and lactate) were contemporaneously measured were selected. Survival was defined as discharge from the hospital. Dogs that were euthanized and those that died were classified as non‐survivors.
Acid–base status calculations
Calculated acid–base variables were derived from measured acid–base, electrolyte and metabolite values using a series of previously established equations (Table 1). Some calculations of components of the Stewart and semi‐quantitative acid base analysis used formulae developed for use in dogs. In other instances, where no canine specific equations were available, formulae developed for use in humans were used. For the purposes of acid–base calculations, phosphate and albumin concentrations were measured in mg/dL, while electrolyte and lactate concentrations were measured in mmol/L. Local reference intervals for the blood‐gas analyser were previously generated using heparinized blood samples collected from 20 healthy dogs that were not part of the study population. These animals were considered healthy on the basis of history, physical examination and the results of complete blood count and serum chemistry profiles. Where local reference intervals for calculated acid–base variables were not available, comparisons were made with published values (Vanova‐Uhrikova et al. 2017).
Table 1.
Formulae used for calculations of acid–base variables used in the present study
| Parameter | Equation | Reference |
|---|---|---|
| Cl− corr | (Na+ normal/Na+ patient) × Cl− patient [Where Na+ normal is the reference interval mid‐point 148 mmol/L] | Meltesen & Bohn (2012) |
| HCO3 − act | 0.0307 × pCO2 × 10(pH – 6.105) | NCCLS (1994) |
| BEecf | HCO3 − act – 24.8 + (16.2 × (pH – 7.40)) | Shapiro et al. (1989) |
| AG | (Na+ + K+) – (Cl− + HCO3 −) | Dibartola (1992) |
| AGcorrected | AG + (0.42 x (3.8 – Alb (g/dL)) | Dibartola (1992) |
| Atot | (phosphate (mg/dL) × 0.58) + (albumin (g/dL) × 2.8) | Corey (2003) and Stewart (1983) |
| SIDapparent | [(Na+ + K+) – (Cl− + Lactate)] | Constable (2000) |
| SIDeffective | [HCO3 − + (albumin (g/dL) × 2.8) + (phosphate (mg/dL) × 0.58)] | Hopper et al. (2014a) |
| SIG | [(Na+ + K+) – (Cl− + Lactate)] ‐ [HCO3 − + (albumin (g/dL) × 2.8) + (phosphate (mg/dL) × 0.58)] | Constable & Stampfli (2005) and Hopper et al. (2014a) |
| SIGsimplified | Anion gap – (albumin × 4.9) | de Morais & Leisewitz (2012) |
| Water BE effect | (0.25 x (Na+ patient – Na+ normal)) | Dibartola (1992) |
| Chloride BE effect | Cl− normal – Cl− corrected | Dibartola (1992) |
| Phosphate BE effect | (0.58 × (phosphatenormal – phosphatepatient (mg/dL))) | Hopper et al. (2014a) |
| Albumin BE effect | (3.7 × (albuminnormal – albuminpatient (g/dL))) | Dibartola (1992) |
| Lactate BE effect | (–1) x lactatepatient | Hopper et al. (2014a) |
| XA‐ | BE – Sum of water, chloride, phosphorous, albumin and lactate effects | Hopper et al. (2014a) |
AG, anion gap; Atot, total plasma weak acids; BEecf, base excess (extracellular fluid); HCO3 − act, actual bicarbonate; SID, strong ion difference; SIG, strong ion gap; XA−, unmeasured anions.
Statistical methods
Prior to test selection, variables were tested for normality using the D'Agostino Pearson test, and descriptive statistics calculated. Parametric variables are presented as mean ± SD, while non‐parametric variables are presented as median (interquartile range). Non‐parametric continuous variables were compared using the Mann–Whitney U test and with box and whisker plots. A Bonferroni correction was applied to account for multiple comparisons. For categorical variables, 2 × 2 contingency tables were constructed to compare frequencies between survivors and non‐survivors, and analysed by chi‐squared test with Yates’ continuity correction, and calculation of odds ratios. For categorical variables, sensitivity, specificity, positive predictive value and negative predictive value were calculated. Receiver‐operator characteristic (ROC) curves were drawn to predict survival to discharge. The area under the ROC curve (AUROC) was calculated by the trapezoidal rule. The optimal cut‐off values for sensitivity and specificity were identified from ROC curves by maximizing the Youden index (J) where J = (Sensitivity + Specificity)‐1 (Youden 1950). Statistical analyses were conducted using commercial software (Prism 7 for Mac OS X; GraphPad Software, La Jolla, CA, USA).
Results
A total of 1024 dogs were identified that satisfied inclusion criteria. Of the 1024 dogs, 780 (76.2%) survived to discharge, 215 (21.0%) were euthanized and 29 (2.8%) died. The median duration of hospitalization in survivors was 3 days (2–5) and in non‐survivors 2 days (1–3). The median age was 7 years (4–10), n = 1009. Breed and sex information were available for 1022 dogs. There were 424 castrated males, 95 intact males, 408 spayed females, and 97 intact females. Most dogs in the population were mixed breeds (n = 214, 20.8%). There were 106 different pure breeds of dog represented. The 10 most common purebred dogs were Labrador retriever (n = 101, 9.9%), golden retriever (n = 50, 4.9%), German shepherd (n = 34, 3.3%), Dachshund (n = 25, 2.5%), Rottweiler (n = 25, 2.5%), Great Dane (n = 23, 2.3%), boxer (n = 23, 2.3%), shih tzu (n = 19, 1.9%), Newfoundland (n = 18, 1.8%) and Australian shepherd (n = 17, 1.7%). One or more diagnoses were coded for all patients (total 1676 from 1024 cases). From the records available, it was not possible to determine which of these was contemporaneously associated with the blood‐gas and electrolyte analyses. More than 180 separate diagnoses were listed (Table S1). After grouping together common types of disease, the 10 most common diagnoses were cancer (n = 191), infectious disease (n = 165), trauma (n = 174), gastrointestinal disturbances excl. GDV (n = 142), endocrine disease (n = 109), acute or chronic kidney injury (n = 65), immune‐mediated disease (n = 52), pancreatitis (n = 43), GDV syndrome (n = 37) and toxicity (n = 36). An open diagnosis was recorded in 48 cases.
Summary acid–base, electrolyte and biochemistry data for the whole population are presented in Tables 2 and 3. Summary data for survivors and non‐survivors are summarized in Table 4. Receiver‐operating characteristic curve analysis suggested that the best performing acid–base parameters for prediction of survival to discharge were bicarbonate (AUROC = 0.622, P < 0.0001), base excess (AUROC = 0.616, P < 0.0001), SIDeffective (AUROC = 0.604, P < 0.0001), SIGsimplified (AUROC = 0.603, P < 0.0001) and pCO2 (AUROC = 0.585, P < 0.0001) (Fig. 1).
Table 2.
Reference intervals, medians, ranges and upper and lower quartiles for the measured acid–base values from the 1024 profiles in the present study
| Parameter | Ref. interval | Minimum | 25% | Median | 75% | Maximum |
|---|---|---|---|---|---|---|
| pH | 7.32–7.38 | 6.808 | 7.306 | 7.362 | 7.404 | 7.560 |
| pCO2 (mmHg) | 38–46 | 11.6 | 30.1 | 35.6 | 41.5 | 73.0 |
| Na+ (mmol/L) | 145–151 | 113.2 | 143.6 | 147.1 | 150.5 | 182.9 |
| K+ (mmol/L) | 3.9–5.1 | 0.97 | 3.76 | 4.10 | 4.46 | 8.9 |
| Ca2+ (mmol/L) | 1.18–1.37 | 0.44 | 1.21 | 1.27 | 1.32 | 2.03 |
| Cl− (mmol/L) | 110–119 | 71 | 109 | 113 | 116 | 139 |
| HCO3 − (mmol/L) | 20–25 | 3 | 16.6 | 19.4 | 22.2 | 44.1 |
| BE (mmol/L) | 0 to −4 | −28.3 | −9.1 | −5.9 | −3.0 | 21.1 |
| Lactate (mmol/L) | <2.0 | 0 | 1.3 | 2.1 | 3.3 | 17 |
| Albumin (g/dL) | 3.1–4.2 | 0.8 | 2.4 | 2.9 | 3.5 | 4.8 |
| Globulin (g/dL) | 1.9–3.6 | 0.5 | 2.0 | 2.5 | 3.0 | 10.4 |
| Phosphate (mg/dL) | 2.9–5.2 | 0.8 | 3.4 | 4.2 | 5.4 | 30.3 |
| BUN (mg/dL) | 10–32 | 2 | 11 | 16 | 27 | 350 |
BE, base excess; BUN, blood urea nitrogen; HCO3 −, bicarbonate.
Table 3.
Medians, ranges, and upper and lower quartiles for the calculated acid–base variables
| Parameter | Minimum | 25% | Median | 75% | Maximum |
|---|---|---|---|---|---|
| AG (mmol/L) | 1.7 | 15.6 | 18.9 | 22.2 | 55.0 |
| AGcorrected (mmol/L) | 3.0 | 16.0 | 19.2 | 22.6 | 55.3 |
| Alb contribution (mmol/L) | 2.2 | 6.7 | 8.1 | 9.8 | 13.5 |
| Phosphate contribution (mmol/L) | 0.5 | 2.0 | 2.4 | 3.1 | 17.6 |
| Atot (mmol/L) | 4.0 | 9.3 | 11.0 | 12.7 | 23.5 |
| SIDapparent (mmol/L) | 6.4 | 32.2 | 35.8 | 39.5 | 65.6 |
| SIDeffective (mmol/L) | 10.6 | 32.4 | 36.0 | 39.6 | 65.6 |
| SIG (mmol/L) | −14.1 | 2.3 | 5.2 | 8.4 | 34.9 |
| SIGsimplified (mmol/L) | −8.2 | 0.9 | 4.2 | 8.1 | 39.8 |
| Water BE effect (mmol/L) | −8.7 | −1.1 | −0.2 | 0.6 | 8.7 |
| Cl− corrected (mmol/L) | 82.4 | 109.7 | 113.5 | 116.7 | 133.8 |
| Cl− effect (mmol/L) | −19.3 | −2.2 | 1.0 | 4.8 | 32.1 |
| Phosphate BE effect (mmol/L) | −15.2 | −0.8 | −0.1 | 0.4 | 1.9 |
| Albumin BE effect (mmol/L) | −4.3 | 0.6 | 2.8 | 4.6 | 10.6 |
| Lactate BE effect (mmol/L) | −17.0 | −3.3 | −2.1 | −1.3 | 0.0 |
| Sum of BE effects (mmol/L) | −20.7 | −2.7 | 0.4 | 3.8 | 40.3 |
| XA− (unmeasured acids) effect on BE (mmol/L) | −41.1 | −9.7 | −6.2 | −3.1 | 12.3 |
| XA− measured (with lactate) (mmol/L) | −10.5 | 5.9 | 8.8 | 11.9 | 38.1 |
| XA− measured (without lactate) (mmol/L) | −0.0 | 8.2 | 11.2 | 14.5 | 41.4 |
AG, anion gap; Atot, total plasma weak acids; BE, base excess; SID, strong ion difference; SIG, strong ion gap; XA−, unmeasured anions.
Table 4.
Median (25th–75th percentile) measured and calculated acid–base values for survivors and non‐survivors
| Parameter | Ref. interval | Survivors (n = 780) | Non‐survivors (n = 244) | Unadjusted P |
|---|---|---|---|---|
| pH | 7.32–7.38 | 7.36 (7.31–7.41) | 7.36 (7.29–7.40) | 0.261 |
| pCO2 (mmHg) | 38–46 | 36.1 (30.8–42.2) | 33.8 (28.2–39.5) | <0.0001 |
| Na+ (mmol/L) | 145–151 | 147.2 (143.7–150.6) | 146.4 (143.3–150.2) | 0.209 |
| K+ (mmol/L) | 3.9–5.1 | 4.09 (3.78–4.45) | 4.10 (3.66–4.54) | 0.790 |
| Ca2+ (mmol/L) | 1.18–1.37 | 1.28 (1.22–1.33) | 1.25 (1.18–1.30) | <0.0001 |
| Cl− (mmol/L) | 110–119 | 112 (108–116) | 113 (109–118) | 0.079 |
| Corrected Cl− (mmol/L) | 110–119 | 113.2 (109.7–116.4) | 114.3 (109.7–118.1) | 0.021 |
| HCO3 − act (mmol/L) | 20–25 | 19.8 (16.9–22.7) | 18.2 (15.2–20.9) | <0.0001 |
| BEecf (mmol/L) | 0 to −4 | −5.4 (−8.6 to −2.5) | −7.5 (−10.6 to −4.3) | <0.0001 |
| Lactate (mmol/L) | <2.0 | 2 (1.2–3.2) | 2.4 (1.4–3.8) | 0.002 |
| Albumin (g/dL) | 3.1–4.2 | 3.0 (2.5–3.5) | 2.7 (2.1–3.2) | <0.0001 |
| Globulin (g/dL) | 1.9–3.6 | 2.5 (2.0–3.0) | 2.5 (2.0–3.1) | 0.614 |
| Phosphate (mg/dL) | 2.9–5.2 | 4.0 (3.3–5.2) | 4.9 (3.8–6.8) | <0.0001 |
| BUN (mg/dL) | 10–32 | 15.0 (10.0–24.0) | 22.0 (14.0–42.8) | <0.0001 |
| AG (mmol/L) | 13–25 | 18.7 (15.6–21.9) | 19.3 (15.3–23.5) | 0.085 |
| Corrected AG (mmol/L) | 13–25 | 19.1 (16.0–22.2) | 19.8 (15.9–23.9) | 0.045 |
| Atot (mmol/L) | 5.6–11.4* | 11.1 (9.3–12.7) | 10.8 (9.2–12.7) | 0.785 |
| SID apparent (mmol/L) | 31.4–45.7* | 36.2 (32.9–39.7) | 34.6 (30.2–39.4) | 0.001 |
| SID effective (mmol/L) | 29.3–36.8* | 31.0 (27.7–34.2) | 29.0 (25.9–32.2) | <0.0001 |
| SIG (mmol/L) | 3.3–15.3* | 5.1 (2.4–8.2) | 5.5 (2.1–8.7) | 0.482 |
| SIG simplified (mmol/L) | −8.6 to 3.7 | 3.8 (0.6–7.6) | 5.3 (2.1–10.6) | <0.0001 |
| XA− measured (inc. lactate) (mmol/L) | ‐ | 8.7 (6.0–11.7) | 9.0 (5.6–12.2) | 0.583 |
| XA− measured (no lactate) (mmol/L) | ‐ | 11.1 (8.0–14.3) | 11.5 (8.6–15.3) | 0.105 |
Unadjusted P‐values are presented. A Bonferroni correction (n = 24 tests) was applied to determine if these P‐values were statistically significant at P < 0.05. Accordingly, values of P < 0.0021 were considered significant and these are denoted in bold type. Institutional reference intervals were available for the majority of values. Where local intervals were not available, ranges published by (Vanova‐Uhrikova et al. 2017) were used, denoted by *. AG, anion gap; Atot, total plasma weak acids; BEecf, base excess (extracellular fluid); HCO3 − act, actual bicarbonate; SID, strong ion difference; SIG, strong ion gap; XA−, unmeasured anions.
Figure 1.
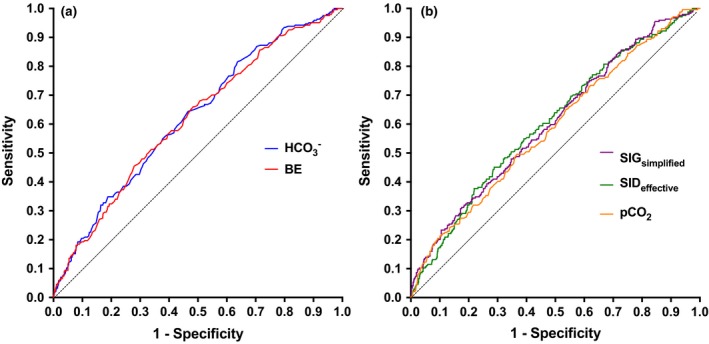
(a) Receiver operating characteristic (ROC) curves for mortality prediction based on bicarbonate and base excess, (b) ROC curves for mortality prediction based on SIG simplified and SID effective and pCO 2. Individual area under the ROC curve (AUROC) values were: bicarbonate (AUROC 0.622, P < 0.0001), base excess (AUROC 0.616, P < 0.0001), SID effective (AUROC 0.604, P < 0.0001), SIG simplified (AUROC 0.603, P < 0.0001), and pCO 2 (AUROC 0.585, P < 0.0001). SID, strong ion difference; SIG, strong ion gap.
The metabolic acid–base status was categorized as normal or abnormal on the basis of reference intervals using eight derived acid–base parameters (HCO3 −, BE, AG, AGcorrected, SIDapparent, SIDeffective, SIG and SIGsimplified). The analysis of contingency tables (summarized in Table 5) suggested that the derived acid–base variables most discriminating for mortality were AG, SIDapparent and SIGsimplified.
Table 5.
Relationships between abnormalities of select measured and calculated acid–base derangements and in‐hospital mortality
| Parameter | RI | OR | P | Sens (%) | Spec (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| HCO3 − (mmol/L) | 20–25 | 1.41 | 0.038 | 35.8 | 71.7 | 80.2 | 25.9 |
| BEecf (mmol/L) | 0 to −4 | 1.59 | 0.017 | 24.9 | 82.8 | 82.2 | 25.6 |
| AG (mmol/L) | 13–25 | 1.63 | 0.003 | 76.9 | 32.8 | 78.5 | 30.8 |
| AGcorrected (mmol/L) | 13–25 | 1.60 | 0.005 | 77.3 | 32.0 | 78.4 | 30.6 |
| SIDapparent (mmol/L) | 31.4–45.7* | 2.02 | <0.0001 | 79.6 | 34.1 | 79.6 | 34.1 |
| SIDeffective (mmol/L) | 29.3–36.8* | 1.46 | 0.010 | 52.4 | 57.0 | 79.6 | 27.3 |
| SIG (mmol/L) | 3.3–15.3* | 1.15 | 0.383 | 63.2 | 40.2 | 77.2 | 25.5 |
| SIGsimplified (mmol/L) | −8.6 to 13.7* | 2.41 | <0.0001 | 92.7 | 16.0 | 77.9 | 40.6 |
Institutional reference intervals were available for the majority of values. Where local intervals were not available, ranges published by Vanova‐Uhrikova et al. (2017) were used, denoted by *. P‐values < 0.05 were considered significant and are denoted in bold type. AG, anion gap; BEecf, base excess (extracellular fluid); HCO3 −act, actual bicarbonate; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value; RI, reference interval; Sens, sensitivity; Spec, specificity; SID, strong ion difference; SIG, strong ion gap; XA−, unmeasured anions; Z, Z‐statistic.
Discussion
Acid–base analysis is an important part of the management of critically ill patients. In this study, we leveraged a large electronic database to identify contemporaneous acid–base, electrolyte and metabolite profiles from 1024 dogs. This approach enabled complex analysis of acid–base abnormalities to be performed on samples collected early in the course of patient management and identified multiple acid–base abnormalities. Although several variables were associated with outcome, none of the calculated acid–base parameters was strongly discriminant for the prediction of mortality in this study. Contrary to our hypothesis, the indices derived from quantitative acid–base analyses were not more accurate predictors of outcome than the calculated bicarbonate or base excess parameters.
Recent studies evaluating various approaches to acid–base analysis in dogs have shown that the semi‐quantitative and Stewart approaches are more sensitive than the traditional Henderson–Hasselbach approach for detecting abnormalities (Hopper et al. 2014a). The present study suggests that although additional insight into patient status may be provided by these more complex analyses, they may not provide better prognostic information than traditional approaches. This does not mean that a more complex acid–base analysis does not enable clinicians to better identify the underlying causes of acid–base abnormalities or potentially provide the information necessary to alter or improve care. Rather, data from this study suggest that the presence of unmeasured anions or cations at hospital admission is not associated with mortality.
Statistically significant differences in the concentrations of calcium, bicarbonate, lactate, albumin, phosphate, urea nitrogen, and in the pCO2, SIDeffective, and SIGsimplified, values between survivors and non‐survivors were identified. However, while the differences in these parameters between groups were statistically significant, these differences are unlikely to be clinically useful. The degree of overlap between the values for the two populations was such that no single value would enable clinical discrimination for an individual patient. Consistent with this, although AUROC values were approximately 0.6 (and significantly greater than 0.5) for several parameters, ROC curve analysis did not yield useful cut‐offs to predict survival with good sensitivity or specificity. This may have resulted from the heterogeneity of the disease processes in the dogs in this study. Stronger associations may exist between acid–base abnormalities and outcome in specific conditions, such as diabetic ketoacidosis or acute kidney injury, where the acid–base disturbances may better reflect the severity of the primary disorder (Hume et al. 2006; Segev et al. 2008; Lee et al. 2011; Malek et al. 2013; Trotman et al. 2013). Serial evaluation of acid–base status in response to therapeutic intervention may be required to determine the optimal pattern or patterns of abnormalities for the prediction of outcome in individual patients.
The ability to accurately prognosticate patients presenting to an emergency room is an important aspect of client communication. Identifying high‐risk patients may also assist in the prioritization of care. Often, a definitive diagnosis cannot be established early in the course of a patient's episode of care, and the ability of point‐of‐care testing to help prognosticate, regardless of the underlying disease process, would be valuable. The limited associations between acid–base abnormalities and outcome identified in this study suggest that these parameters may not meet this need. The complexity of acid–base derangements and their susceptibility to change following therapeutic interventions may limit their use for prognostication. This seems to be the case with traditional acid–base analysis, as well as the more descriptive Stewart and semi‐quantitative approaches.
The results of the present investigation are consistent with previous studies that suggest single time point observations of acid–base variables are of limited value in veterinary (Hume et al. 2006) and human medicine (Nguyen et al. 2004). However, both of those studies involved a limited number of patients (127 dogs and 111 people) and were studies of patients with a single disease process. The present study sought to extend our understanding in this area by determining if acid–base analysis was prognostic in a larger group of patients with heterogeneous disease processes. Although blood lactate concentrations have been associated with outcome in some specific populations (de Papp et al. 1999), it is recognized that serial evaluation may provide additional discriminant power (Holahan et al. 2010; Mooney et al. 2014; Cortellini et al. 2015). In the present study, which enrolled a heterogeneous group of dogs, lactate measurement was significantly greater in non‐survivors compared with survivors. The absolute difference between survivors and non‐survivors was small, however, which may reflect the limited illness severity in the cohort of dogs studied here and the heterogeneity in the nature and extent of the underlying disease processes.
In the present study, it was not possible to determine if the associations between acid–base and mortality were independent of illness severity. As such, the associations observed may represent epiphenomena associated with illness severity. In similar studies of human ICU patients, the availability of illness‐severity scores such as the SOFA or the APACHE II score enable the level of disease to be accounted for in multivariable analyses (Dubin et al. 2007; Boniatti et al. 2009). It was not possible to calculate any of the established veterinary illness‐severity scores such as SPI2 or the canine APPLE score in the present study due to a lack of availability of the necessary data. Future studies gathering data prospectively might be able to overcome this issue and confirm that acid–base disturbances are independently associated with outcome. At this time, we can only speculate that this is the case.
The datasets evaluated in this study did not include more than one profile from a single patient care episode, although some patients were included more than once on separate occasions. It is possible that such repeated inclusion might have biased the results, by increasing the strength of the outcome signal (towards survival or non‐survival) from individual patients. It is unlikely that such an effect would have affected the comparisons between analysis systems, however. It was also not possible to eliminate the impact of therapy on the measured acid–base abnormalities or on outcome. Patients may have received therapy including fluid administration before or after the electrolyte profile was recorded. This is important because administration of normal saline – an acidifying fluid due to the associated hyperchloremia – has been associated with an increased risk of AKI and death in people (Yunos et al. 2012; Raghunathan et al. 2014; Shaw et al. 2014). For these therapies conducted at admission to have impacted outcome at hospital discharge, however, their effect would have needed to persist throughout the hospital stay in order to affect the identified associations with outcome. The impact of euthanasia is difficult to address in all veterinary studies incorporating mortality as an outcome measure. Due to the small number of patients that died (i.e. were not euthanized), it was not feasible to analyse them as a separate group.
The blood‐gas analyses, lactate measurements and serum biochemistry testing were performed on different machines. As a result of this, and owing to the retrospective nature of this study, it was not possible to determine with 100% certainty that the blood samples for these separate analyses were drawn simultaneously, and hence, we cannot be certain that no therapeutic interventions occurred before all of the blood samples were obtained. The standard operating procedure in the institution emergency room, where the majority of samples were obtained, is to obtain blood samples for all of these analyses simultaneously at the time of catheter placement. As such, it is unlikely that therapeutic interventions that would alter acid–base balance occurred between collection of samples for these distinct analyses.
The reference intervals for the blood‐gas analyser used in this study were generated in 2007 using 20 healthy dogs. Since that time, guidelines for reference interval generation have been published by the American Society of Veterinary Clinical Pathology (Friedrichs et al. 2012). These guidelines recommend using at least 40 healthy animals for construction of a reference interval. It is possible that generating a reference interval with a larger number of dogs would alter the interpretation of some of the results in the present study. However, this would be a systematic error that should not affect between group comparisons and hence it is unlikely that a different reference interval would alter the key findings of the present study.
Despite the counter‐intuitive results of the present study, the findings do have implications for veterinary clinical practice. Acid–base analysis using strong ion approaches may enhance the clinician's understanding of the patient's disease, but a more straightforward approach may offer similar levels of prognostic information. It is not clear if seeking to manage the acid–base disturbance directly will improve outcome and clinicians should consider if attempting to normalize the pH artificially is likely to improve the situation. For instance, in patients with acidosis due to diabetic ketosis, bicarbonate therapy may be detrimental (Okuda et al. 1996). A better solution may be to identify and manage the underlying disease causing the acid–base disturbance. Given the retrospective nature of this study, it is only possible to speculate about the causes of the associations identified between acid–base disturbances and outcome. Marked alterations in pH, irrespective of cause will detrimentally affect the cellular processes that underpin tissue and organ function, by affecting the function of enzyme systems, ion channels, and the concentrations of electrolytes.
Conclusion
In summary, this study suggests that both the conventional and strong ion approaches to acid–base analysis identified multiple disorders in this population of dogs with heterogeneous disease processes. The acid–base disturbances as identified by both the traditional and strong ion approaches were associated with mortality in the dogs evaluated in this study, albeit to a limited extent. Contrary to our hypothesis, the accuracy of outcome prediction was not improved by performing the more complex strong ion analyses, compared with evaluation of traditional acid–base parameters. Future studies should focus on confirming these associations in a prospective manner accounting for disease severity.
Source of funding
This research received no specific funding support.
Conflict of interest
The authors declare that they have no interest or relationship, financial or otherwise that would influence the reporting of this study.
Ethics statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this study represents a retrospective analysis of electrolyte and acid–base data collected as part of clinician‐driven care provided to patients treated at the institution hospital. No client or patient identifying information is presented.
Contributions
EZ collected and analysed data and co‐wrote the manuscript; DJF collected and analysed data, and edited the manuscript; RG conceived the study, collected and analysed data, and co‐wrote the manuscript.
Supporting information
Table S1. A summary of the final diagnoses (n = 1676) for the dogs reported in the present study. Some dogs had more than one final diagnosis recorded, while an open diagnosis was recorded for 48 dogs.
Acknowledgements
There are no acknowledgements to declare.
References
- Benjamin E., Oropello J.M., Abalos A.M., Hannon E.M., Wang J.K., Fischer E. & Iberti T.J. (1994) Effects of acid‐base correction on hemodynamics, oxygen dynamics, and resuscitability in severe canine hemorrhagic shock. Critical Care Medicine 22, 1616–1623. [PubMed] [Google Scholar]
- Boag A.K., Coe R.J., Martinez T.A. & Hughes D. (2005) Acid‐base and electrolyte abnormalities in dogs with gastrointestinal foreign bodies. Journal of Veterinary Internal Medicine 19, 816–821. [DOI] [PubMed] [Google Scholar]
- Boniatti M.M., Cardoso P.R., Castilho R.K. & Vieira S.R. (2009) Acid‐base disorders evaluation in critically ill patients: we can improve our diagnostic ability. Intensive Care Medicine 35, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Chen X.F., Ye J.L. & Zhu Z.Y. (2013) The use of sodium bicarbonate in stages in treating hypoperfusion induced lactic acidemia in septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25, 24–27. [DOI] [PubMed] [Google Scholar]
- Constable P.D. (2000) Clinical assessment of acid‐base status: comparison of the Henderson‐Hasselbalch and strong ion approaches. Veterinary Clinical Pathology 29, 115–128. [DOI] [PubMed] [Google Scholar]
- Constable P.D. & Stampfli H.R. (2005) Experimental determination of net protein charge and A(tot) and K(a) of nonvolatile buffers in canine plasma. Journal of Veterinary Internal Medicine 19, 507–514. [PubMed] [Google Scholar]
- Constable P.D., Hinchcliff K.W. & Muir W.W. 3rd (1998) Comparison of anion gap and strong ion gap as predictors of unmeasured strong ion concentration in plasma and serum from horses. American Journal of Veterinary Research 59, 881–887. [PubMed] [Google Scholar]
- Corey H.E. (2003) Stewart and beyond: new models of acid‐base balance. Kidney International 64, 777–787. [DOI] [PubMed] [Google Scholar]
- Cortellini S., Seth M. & Kellett‐Gregory L.M. (2015) Plasma lactate concentrations in septic peritonitis: a retrospective study of 83 dogs (2007‐2012). Journal of Veterinary Emergency and Critical Care 25, 388–395. [DOI] [PubMed] [Google Scholar]
- Dibartola S.P. (1992) Fluid Therapy in Small Animal Practice. 1st edn W B Saunders Co; Philadelphia. [Google Scholar]
- Dimeski G., Badrick T. & John A.S. (2010) Ion selective electrodes (ISEs) and interferences–a review. Clinica Chimica Acta 411, 309–317. [DOI] [PubMed] [Google Scholar]
- Dubin A., Menises M.M., Masevicius F.D., Moseinco M.C., Kutscherauer D.O., Ventrice E. et al (2007) Comparison of three different methods of evaluation of metabolic acid‐base disorders. Critical Care Medicine 35, 1264–1270. [DOI] [PubMed] [Google Scholar]
- Durocher L.L., Hinchcliff K.W., DiBartola S.P. & Johnson S.E. (2008) Acid‐base and hormonal abnormalities in dogs with naturally occurring diabetes mellitus. Journal of the American Veterinary Medical Association 232, 1310–1320. [DOI] [PubMed] [Google Scholar]
- Fencl V. & Leith D.E. (1993) Stewart's quantitative acid‐base chemistry: applications in biology and medicine. Respiratory Physiology 91, 1–16. [DOI] [PubMed] [Google Scholar]
- Fencl V., Jabor A., Kazda A. & Figge J. (2000) Diagnosis of metabolic acid‐base disturbances in critically ill patients. American Journal of Respiratory and Critical Care Medicine 162, 2246–2251. [DOI] [PubMed] [Google Scholar]
- Friedrichs K.R., Harr K.E., Freeman K.P., Szladovits B., Walton R.M., Barnhart K.F. et al (2012) ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Veterinary Clinical Pathology 41, 441–453. [DOI] [PubMed] [Google Scholar]
- Goggs R., De Rosa S. & Fletcher D.J. (2017) Electrolyte disturbances are associated with non‐survival in dogs – A multivariable analysis. Frontiers in Veterinary Science 4, 135 10.3389/fvets.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan M.L., Brown A.J. & Drobatz K.J. (2010) The association of blood lactate concentration with outcome in dogs with idiopathic immune‐mediated hemolytic anemia: 173 cases (2003‐2006). Journal of Veterinary Emergency and Critical Care 20, 413–420. [DOI] [PubMed] [Google Scholar]
- Hopper K., Epstein S.E., Kass P.H. & Mellema M.S. (2014a) Evaluation of acid‐base disorders in dogs and cats presenting to an emergency room. Part 1: comparison of three methods of acid‐base analysis. Journal of Veterinary Emergency and Critical Care 24, 493–501. [DOI] [PubMed] [Google Scholar]
- Hopper K., Epstein S.E., Kass P.H. & Mellema M.S. (2014b) Evaluation of acid‐base disorders in dogs and cats presenting to an emergency room. Part 2: comparison of anion gap, strong ion gap, and semiquantitative analysis. Journal of Veterinary Emergency and Critical Care 24, 502–508. [DOI] [PubMed] [Google Scholar]
- Hume D.Z., Drobatz K.J. & Hess R.S. (2006) Outcome of dogs with diabetic ketoacidosis: 127 dogs (1993‐2003). Journal of Veterinary Internal Medicine 20, 547–555. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Chang C.C., Chan J.P., Hsu W.L., Lin K.W. & Wong M.L. (2011) Prognosis of acute kidney injury in dogs using RIFLE (Risk, Injury, Failure, Loss and End‐stage renal failure)‐like criteria. Veterinary Record 168, 264 10.1136/vr.c6234. [DOI] [PubMed] [Google Scholar]
- Malek S., Sinclair E., Hosgood G., Moens N.M., Baily T. & Boston S.E. (2013) Clinical findings and prognostic factors for dogs undergoing cholecystectomy for gall bladder mucocele. Veterinary Surgery 42, 418–426. [DOI] [PubMed] [Google Scholar]
- Meltesen H.S. & Bohn A.A. (2012) Using corrected serum chloride and predicted bicarbonate concentrations to interpret acid‐base status in dogs. Veterinary Clinical Pathology 41, 509–517. [DOI] [PubMed] [Google Scholar]
- Mooney E., Raw C. & Hughes D. (2014) Plasma lactate concentration as a prognostic biomarker in dogs with gastric dilation and volvulus. Topics in Companion Animal Medicine 29, 71–76. [DOI] [PubMed] [Google Scholar]
- deMorais H.A. , Leisewitz A.L. (2012) Mixed Acid‐Base Disorders In: Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. 4th edn (ed S.P. DiBartola), pp 302–315. Elsevier Saunders: St. Louis, MO: [Google Scholar]
- National Committee for Clinical Laboratory Standards . (1994). Definitions of quantities and conventions related to blood pH and gas analysis; approved standard. NCCLS Document C12‐A 14,
- Nguyen H.B., Rivers E.P., Knoblich B.P., Jacobsen G., Muzzin A., Ressler J.A. & Tomlanovich M.C. (2004) Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Critical Care Medicine 32, 1637–1642. [DOI] [PubMed] [Google Scholar]
- Okuda Y., Adrogue H.J., Field J.B., Nohara H. & Yamashita K. (1996) Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. Journal of Clinical Endocrinology and Metabolism 81, 314–320. [DOI] [PubMed] [Google Scholar]
- de Papp E., Drobatz K.J. & Hughes D. (1999) Plasma lactate concentration as a predictor of gastric necrosis and survival among dogs with gastric dilatation‐volvulus: 102 cases (1995‐1998). Journal of the American Veterinary Medical Association 215, 49–52. [PubMed] [Google Scholar]
- Raghunathan K., Shaw A., Nathanson B., Stürmer T., Brookhart A., Stefan M.S. et al (2014) Association between the choice of IV crystalloid and in‐hospital mortality among critically ill adults with sepsis. Critical Care Medicine 42, 1585–1591. [DOI] [PubMed] [Google Scholar]
- Rossmeisl J.H. Jr, Zimmerman K., Inzana K.D. & Higgins M.A. (2006) Assessment of the use of plasma and serum chloride concentrations as indirect predictors of serum bromide concentrations in dogs with idiopathic epilepsy. Veterinary Clinical Pathology 35, 426–433. [DOI] [PubMed] [Google Scholar]
- Russell K.E., Hansen B.D. & Stevens J.B. (1996) Strong ion difference approach to acid‐base imbalances with clinical applications to dogs and cats. Veterinary Clinics of North America Small Animal Practice 26, 1185–1201. [DOI] [PubMed] [Google Scholar]
- Segev G., Kass P.H., Francey T. & Cowgill L.D. (2008) A novel clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. Journal of Veterinary Internal Medicine 22, 301–308. [DOI] [PubMed] [Google Scholar]
- Shapiro B.A., Harrison R.A., Cane R.D. & Kozlowski‐Templin R. (1989) Clinical Application of Blood Gases. 4th edn Year Book Medical Publishers; Chicago, IL. [Google Scholar]
- Shaw A.D., Raghunathan K., Peyerl F.W., Munson S.H., Paluszkiewicz S.M. & Schermer C.R. (2014) Association between intravenous chloride load during resuscitation and in‐hospital mortality among patients with SIRS. Intensive Care Medicine 40, 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegling‐Vlitakis C., Kohn B., Kellermeier C., Schmitz R. & Hartmann H. (2007) Qualification of the Stewart variables for the assessment of the acid‐base status in healthy dogs and dogs with different diseases. Berliner und Münchener Tierärztliche Wochenschrift 120, 148–155. [PubMed] [Google Scholar]
- Slawuta P. & Glinska‐Suchocka K. (2012) Comparison of the utility of the classic model (the Henderson‐Hasselbach equation) and the Stewart model (Strong Ion Approach) for the diagnostics of acid‐base balance disorders in dogs with right sided heart failure. Polish Journal of Veterinary Science 15, 119–124. [DOI] [PubMed] [Google Scholar]
- Slawuta P., Nicpon J. & Skrzypczak P. (2010) Contemporary approach to acid‐base balance and its disorders in dogs and cats. Polish Journal of Veterinary Science 13, 561–567. [PubMed] [Google Scholar]
- Stewart P.A. (1983) Modern quantitative acid‐base chemistry. Canadian Journal of Physiology and Pharmacology 61, 1444–1461. [DOI] [PubMed] [Google Scholar]
- Torrente C., Manzanilla E.G. & de Gopegui R.R. (2014) A comparison of traditional and quantitative analysis of acid‐base imbalances in hypoalbuminemic dogs. Journal of Veterinary Emergency and Critical Care 24, 509–518. [DOI] [PubMed] [Google Scholar]
- Trotman T.K., Drobatz K.J. & Hess R.S. (2013) Retrospective evaluation of hyperosmolar hyperglycemia in 66 dogs (1993‐2008). Journal of Veterinary Emergency and Critical Care 23, 557–564. [DOI] [PubMed] [Google Scholar]
- Vanova‐Uhrikova I., Rauserova‐Lexmaulova L., Rehakova K., Scheer P. & Doubek J. (2017) Determination of reference intervals of acid‐base parameters in clinically healthy dogs. Journal of Veterinary Emergency and Critical Care 27, 325–332. [DOI] [PubMed] [Google Scholar]
- Youden W.J. (1950) Index for rating diagnostic tests. Cancer 3, 32–35. [DOI] [PubMed] [Google Scholar]
- Yunos N.M., Bellomo R., Hegarty C., Story D., Ho L. & Bailey M. (2012) Association between a chloride‐liberal vs chloride‐restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308, 1566–1572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. A summary of the final diagnoses (n = 1676) for the dogs reported in the present study. Some dogs had more than one final diagnosis recorded, while an open diagnosis was recorded for 48 dogs.


