Abstract
The aim of the present study was to investigate the impact of carbobenzoxy-Leu-Leu-leucinal (MG-132) on myocardial remodeling in rats with myocardial infarction (MI) and investigate the possible underlying mechanisms. The rat model of MI was established, followed by administration of MG-132 (MG group), pyrrolidine dithiocarbamic acid (PDTC group) or normal saline (MI group) for 28 days. The expression of nuclear factor-κB (NF-κB) p65, interleukin 1β (IL-1β) and matrix metalloproteinase 2 (MMP-2), as well as the total volume of collagen and the ratio of type I/III collagen were then detected. Total collagen, including type I and III collagen, and the ratio of type I/III collagen were significantly increased in MI rats compared with those in the sham group (P<0.01), while it was significantly decreased in the PDTC and MG groups compared with that in the MI group (P<0.01). A similar trend was identified for the expression of NF-κB, IL-1β and MMP-2, which was significantly increased in the MI group compared with that in the sham group (P<0.01), while it was significantly decreased in the MG and PDTC groups compared with that in the MI group (P<0.01). In conclusion, MG-132 was demonstrated to improve post-MI tissue remodeling, and the mechanism may be associated with the inhibition of NF-κB activation and the downregulation of inflammatory cytokines, such as IL-1β.
Keywords: interleukin-1β, myocardial infarction, myocardial remodeling, nuclear factor-κB, proteasome inhibitor
Introduction
The consequences of myocardial infarction (MI) mainly comprise myocardial hypertrophy and myocardial remodeling. The mechanisms of myocardial remodeling following MI are complex, and in recent years, studies have demonstrated that except for mechanical stimulation and neuroendocrine activation, post-MI myocardial remodeling is also associated with the overexpression of inflammatory cytokines. Seropian et al (1) reported that the expression of myocardial interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) in rats was still significantly increased at 20 weeks after MI. Shioi et al (2) found that in hypertrophic left ventricles, the expression of myocardial IL-1β mRNA was increased by 3.9-fold of that in normal ones, which was also correlated with the left ventricular mass index. Zarrouk-Mahjoub et al (3) investigated the roles of inflammatory cytokines in myocardial remodeling and identified that after MI, the excessive and persistent presence of cytokines in post-MI myocardial cells gradually induces their morphological changes and activation of matrix metalloproteinase (MMP), thus further inducing the remodeling process.
The nuclear factor-κB (NF-κB) activation pathway exists in cardiac cells, smooth muscle cells and endothelial cells (4). The transcriptional process of inflammatory cytokines is mainly regulated by NF-κB, which has an important role in the process of myocardial remodeling. Sun et al (5) used the NF-κB inhibitor IMD-0534 to reduce myocardial remodeling and improve cardiac function in MI rats. NF-κB is a transcription factor with the ability to regulate multidirectional gene transcription. It usually forms a dimer with p65/p50 and binds to the inhibitor of NF-κB (I-κB) to thereby form an inactive trimer that then prevails in the cytoplasm (6). The activation of NF-κB is controlled by ubiquitin-proteasome system, and the specific activation process is as follows: First, the Ser32 and 36 residues of I-κB are phosphorylated by certain protein kinases and then ubiquitinated through combining with ubiquitin; when the above complex is degraded by the 26S proteasome, I-κB and NF-κB then dissociate, and NF-κB enters the nucleus so as to initiate the relevant gene transcription (7). When cells are stimulated by ischemia and hypoxia, the activity of the proteasome increases, leading to the degradation of I-κB, the dissociation of NF-κB and I-κB, and the penetration of the activated p65 into the nucleus, followed by upregulation of inflammatory cytokines, including TNF-α, IL-6 and IL-1β. Although NF-κB activates inflammatory cytokines at the transcriptional level, these factors also contribute to the activation of NF-κB, thus initiating inflammatory self-amplification (8). Studies have indicated that ubiquitin-proteasome inhibitors block the activation of NF-κB, which were therefore recommended as novel anti-inflammatory drugs used in diseases including asthma and rheumatoid arthritis (9). Santos et al (10) reported that the proteasome inhibitor MLN519 inhibits the activation of NF-κB, thus reducing the expression of inflammatory cytokines TNF-α, IL-1β and IL-6 and reducing myocardial ischemia-reperfusion injury. Chen et al (11) obtained similar results with carbobenzoxy-Leu-Leu-leucinal (MG-132). However, to the best of our knowledge, no previous study has reported on the application of proteasome inhibitors toward post-MI myocardial remodeling. Previous studies by our group have demonstrated that MG-132 improves myocardial remodeling in MI rats (12), but the underlying mechanisms have remained elusive. In the present study, it was hypothesized that MG-132 may have an important role in post-MI myocardial remodeling by inhibiting the activation of NF-κB and downregulating inflammatory cytokines such as IL-1β; furthermore, the process of remodeling was histologically examined.
Materials and methods
Generation of animal model and specimen collection
A total of 68 Sprague Dawley rats (8 weeks old, male-to-female ratio, 1:1; body weight, 200-250 g) were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China) and used to prepare the MI model. All rats were housed under standard conditions (temperature, 20±1°C; humidity, 60±10%; 12-h light/dark cycle) and had free access to standard rodent chow and water. The experimental processes strictly abided the requirements of the animal experimental guidelines issued by the International Association for the Study of Pain (13,14). The 18 rats in which modeling was successful and who survived for >24 h were randomly divided into the MI group, the MG group and the pyrrolidine dithiocarbamic acid (PDTC) group, with 6 rats in each group. Another 6 rats were set as the sham operation group (SH). The operation procedures in the SH group were identical to those for the MI rats, with the exception that the rats were only threaded but not ligated. The intervention measures in the different groups were as follows: Animals in the MG group were intraperitoneally injected daily with 0.1 mg/kg/day MG-132 (Calbiochem, Shanghai, China) on the second day as described previously (15) until the 28th day; in the PDTC group, animals were intraperitoneally injected with 80 mg/kg/day PDTC (Shanghai Zhen Zhen Biological Science and Technology Co., Ltd., Shanghai, China) on the second day as described previously (16) until the 28th day; in the MI and SH group, animals were intraperitoneally injected the same volume of saline. On day 28, the rats were weighed and then inhaled a lethal concentration of isoflurane (3-5%) in a chamber. The heart was then sampled out before it stopped beating, washed with normal saline and divided into 3 parts along the long axis of the left ventricle. The cardiac base and cardiac apex (non-infarct areas) were frozen in liquid nitrogen at −70°C for reverse-transcription quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. The middle part was cut into 3-mm pieces, fixed in 4% formaldehyde solution for 24 h, embedded in paraffin and cut into 5-µm slices. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Dali University (Dali, China) since the experiment was conducted at Dali University.
Volume fraction of collagens
Five non-overlapping microscopic fields were randomly selected from the non-MI zone of the picrosirius red-stained slices (17) to determine the ratio of collagen (red) vs. the whole area by a biomedical image analysis system (CM2000B, Beijing University of Aeronautics and Astronautics, Beijing, China), and the mean value was used as the total collagen content. Furthermore, the non-MI zone was imaged under a polarized light microscope to calculate the volume fractions of type I collagen (red) and type III collagen (green), as well as to calculate the ratio of type I/III collagen.
Morphological parameters
For each specimen, two hematoxylin and eosin-stained slices were selected to determine various morphometric parameters of cardiomyocytes using the CM-2000B biomedical imaging system (Beijing University of Aeronautics and Astronautics) as described previously (17). From each slice, five fields of view were selected and a computer was used to calculate the morphometric parameters of cardiomyocytes in the field center, including the myocardial cell area, perimeter and mean diameter.
RT-qPCR
The total RNA of rat cardiomyocytes was isolated with TRIzol (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The primers were synthesized by Shanghai Biosune Biotech Co., Ltd. (Shanghai, China) and their sequences are listed in Table I. The RT-qPCR reaction system had a volume of 50 µl, and the two-step process was performed according to the kit instructions (FSQ-101; Toyobo, Tokyo, Japan) and reagents listed in Table II. A cycle for 5 min at 94°C, followed by 31 cycles of 30 sec at 94°C, 30 sec at 50-56°C, 45 sec at 72°C and a final cycle for 5 min at 72°C.
Table I.
Primer sequences used for polymerase chain reaction.
| Gene | Primer sequence (5′-3′) | Product length (bp) |
|---|---|---|
| NF-κB p65 | ||
| Forward | CAGCACATCCAGACAGACACCA | 480 |
| Reverse | GCTGCTAAAAGAATCCTCAAAACC | |
| IL-1β | ||
| Forward | CACCTTCTTTTCCTTCATCTTTG | 381 |
| Reverse | AAGACAAACCGCTTTTCCATC | |
| MMP-2 | ||
| Forward | CTACACCAAGAACTTCCGACTATC | 305 |
| Reverse | CCTCGTACACGGCATCAATC | |
| β-actin | ||
| Forward | CAGCTTCTTCTAGTGCCGTTCC | 219 |
| Reverse | GGAGTCAGGTGTTTCTGGTGGAG |
IL, interleukin; MMP, matrix metalloproteinase; NF, nuclear factor.
Table II.
Two-step reverse transcription quantitative polymerase chain reaction components.
| Agents | Volume (µl) |
|---|---|
| ReverTra Ace® | 50 |
| RNase lnhibitor | 50 |
| 5X RT Buffer (25 mM MgCI2) | 200 |
| 10 mM dNTPs | 150 |
| RNase Free H2O | 600 |
| Oligo(dT) 20 (10 pmol/µl) | 250 |
| Random Primer (25 pmol/µl) | 50 |
| Control Primer F (10 pmol/µl) | 50 |
| Control Primer R (10 pmol/µl) | 25 |
| Positive Control RNA (105 copies/µl) | 25 |
| KOD | 50 |
| 10X PCR Buffer for ReverTra | 250 |
| 25 mM MgSO4 | 250 |
The relative production of NF-κB p65, IL-1β and MMP-2 was calculated by QuantityOne 4.6 Image Analysis software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The following thermocycling conditions were used: The integral ratio of the absorbance areas was used to express the DNA content, and the mRNA expression levels were evaluated by the integral ratios of the absorbance areas of the amplified NF-κB p65, IL-1β, MMP-2 and β-actin bands (18).
Western blot analysis
The total myocardial protein was extracted from the rat myocardium using radioimmunoprecipitation assay buffer, followed by determination of the protein content via the bicinchoninic acid method. The protein (40 µg per lane) was then separated by 10% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Thermo Fisher Scientific, Inc.). Following blocking in 5% non-fat milk for 1 h, the membrane was incubated with rabbit anti-mouse NF-κB, IL-1β, MMP-2 and β-actin antibodies (1:750, 1:400, 1:500 and 1:400 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 2 h. The membrane was then incubated with secondary antibody (goat anti-rabbit immunoglobulin G, SA00001-2; ProteinTech Group, Inc., Chicago, IL, USA) at room temperature for 1 h, followed by visualization using a chemoluminescent reagent (Jiangsu Biyuntian Biotechnology Research Institute, Jiangsu, China). Images were captured using a gel imaging system (Bio-Rad Laboratories, Inc.), and then scanned and analyzed.
Immunohistochemistry
Sample preparation was performed according to the instructions of the Goat anti-rabbit SP immunohistochemical kit S (P-0023; Shanghai Dingjie Biotechnology Co., Ltd., Shanghai, China) and NF-κB p65 expression was then observed under high-power vision (magnification, ×400). Samples were embedded, sliced and dewaxed, then xylene I and II were added for 20 min each at room temperature. Subsequently, samples were subjected to rehydration in a gradient alcohol series and were washed with PBS three times for 5 min each time. Samples, were submerged in 3% H2O2 20 µl for 5 min to eliminate the activity of endogenous peroxidase and washed with PBS three times for 5 min each time. Samples were incubated with NF-κB p65 rabbit-anti-mouse IgG (sc-8008, dilution 1:100; Santa Cruz Biotechnology, Inc.) and incubated overnight at 4°C, washed with PBS three times for 5 min each and incubated with secondary antibody labeled with biotin at 37°C for 15 min. Following this, samples were washed with PBS three times for 5 min and incubated with horseradish peroxidase 37°C for 15 min, washed with PBS three times for 5 min, incubated with 3,3′-diaminobenzidine for 3-5 min and counterstained with hematoxylin for 3-5 min at room temperature. Sections were observed using a light microscope (magnification, ×400).
Semi-quantitative analysis was performed according to the method by Wang et al (4), with positive staining appearing as brown granules in the myocardial nuclei. The positive index (PI) was then calculated as PI=number of positive cells/total number of cells in the field ×100%. Five non-overlapping high-power fields were randomly selected from each slice to calculate the average. The observation of IL-1β and MMP-2 followed the same steps; the antibody concentration was 1:100, and positive staining was defined as brown granules appearing in the myocardial cytoplasm or nuclei. Medical image analysis software Image Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used to calculate the integral optical density value.
Statistical analysis
All the data were analyzed using the SPSS12.0 statistical package (SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ± standard deviation. Comparison between two groups was performed using the Student's t-test. Multigroup comparisons were performed using one-way analysis of variance followed by Student-Newman-Keuls post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Volume fraction of collagens by sirius red staining
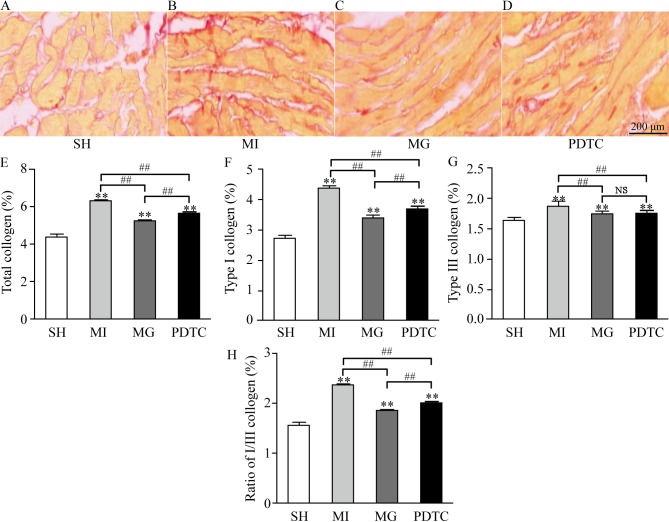
As indicated in Fig. 1, collagen was deposited differently in the different groups. In the sham group, only a small amount of collagen was deposited in the non-infarct area, while the amount of collagen deposited in the MI group was large. In comparison with that in the MI group, the volume of collagen deposition in the MG group was decreased after treatment with MG-132, and there were significant differences between the PDTC group and the MI group. Compared with the SH group, total collagen, type I collagen, type III collagen and the ratio of I/III collagen in the MI, MG and PDTC groups were significantly increased (P<0.01). This was more notable in the MI group; however, the above indexes in the MG group and the PDTC group were significantly lower compared with those in MI group (P<0.01), suggesting that MG-132 and PDTC improved the collagen remodeling in non-infarcted area after myocardial infarction in rats. In comparison with that in the PDTC group, total collagen, type I collagen and the ratio of I/III were significantly decreased in the MG group (P<0.01). However, there was no significant difference between the MG and PDTC groups with regard to type III collagen.
Figure 1.
Volume fraction of collagens in different groups. (A-D) Pathological images for (A) SH, (B) MI, (C) MG and (D) PDTC groups by Masson Trichrome Staining (scale bar, 200 µm). (E) Total collagen; (F) type I collagen; (G) type III collagen; (H) ratio of type I/III collagen. **P<0.01 vs. SH; ##P<0.01 as indicated. Groups: MI, myocardial infarction model group treated with saline for 28 days; SH, sham; MG, model group treated with MG-132 for 28 days; PDTC, model group treated with pyrrolidine dithiocarbamic acid for 28 days. NS, not significant.
Morphological parameters
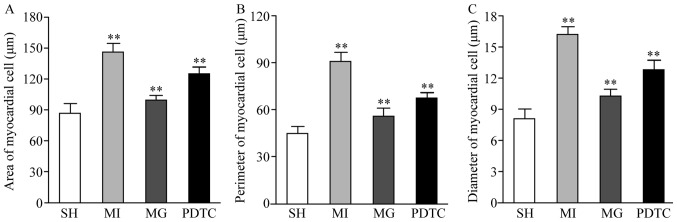
The cell surface area, perimeter and diameter in the non-infraction zone were significantly increased in the MI group compared with those in the SH group, while they were reduced in the PDTC group and further decreased in the MG group compared with those in the MI group. Significant differences were determined between each pair of groups (P<0.01; Fig. 2).
Figure 2.
(A) Cell surface area, (B) perimeter and (C) diameter in non-infarction zone. **P<0.01 for comparison between either two groups. Groups: MI, myocardial infarction model group treated with saline for 28 days; SH, sham; MG, model group treated with MG-132 for 28 days; PDTC, model group treated with pyrrolidine dithiocarbamic acid for 28 days.
Effects of MG-132 and PDTC on NF-κB, IL-1β and MMP-2 after MI
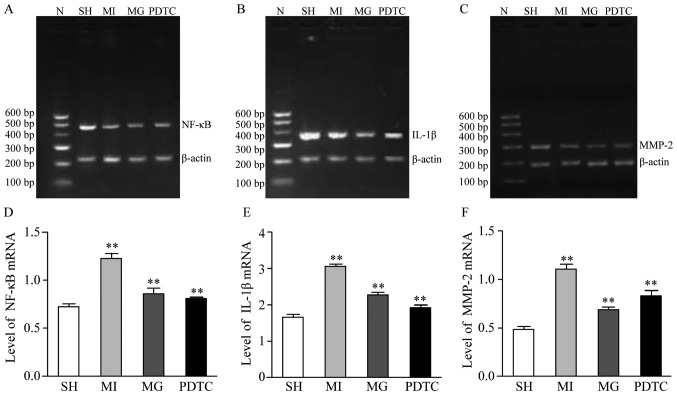
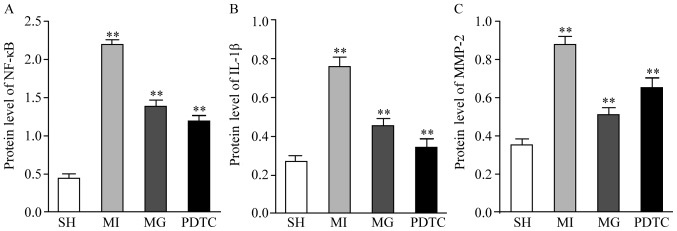
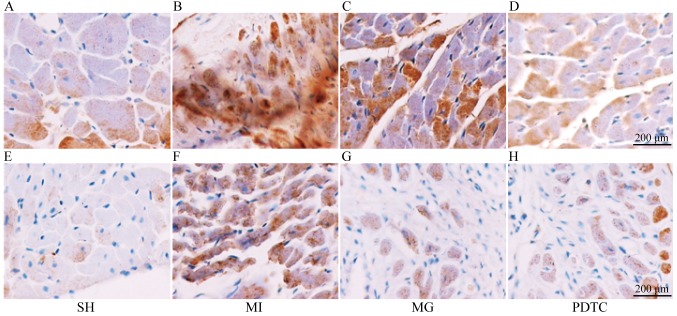
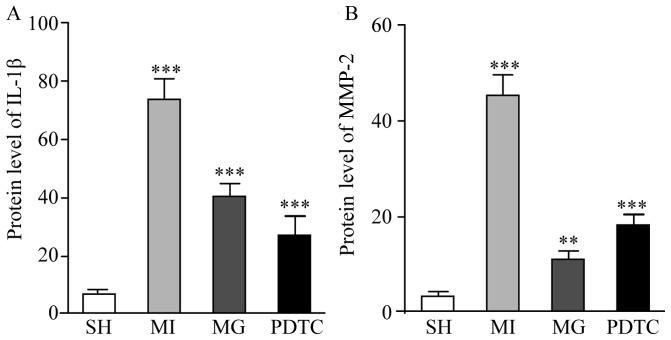
The mRNA and protein levels of NF-κB, IL-1β and MMP-2 were determined by RT-qPCR (Fig. 3) and western blot analysis (Fig. 4). Furthermore, immunohistochemistry was performed to indicate these effects (Figs. 5 and 6; data not shown for NF-κB). The results indicated that NF-κB, IL-1β and MMP-2 displayed the greatest increase in the MI group compared with that in the SH group, while these increases were significantly blunted by treatment with PDTC or MG-132. Treatment with PDTC caused the greatest decrease in NF-κB and IL-1β and MG-132 caused the greatest decrease in MMP-2. Immunohistochemistry indicated that NF-κB was expressed in the nuclei (data not shown), IL-1β was expressed in the cytoplasm and MMP-2 was expressed in the nuclei as well as the cytoplasm. The results of the semi-quantitative analysis of the immunohistochemical images were in accordance with the western blot results (Fig. 6).
Figure 3.
mRNA expression of NF-κB, IL-1β and MMP-2 in non-infarction zone in different groups. (A-C) Representative gels of the amplimers of (A) NF-κB, (B) IL-1β and (C) MMP-2. (D-F) Quantified expression levels of (D) NF-κB, (E) IL-1β and (F) MMP-2. **P<0.01 for comparison between either two groups. Groups: MI, myocardial infarction model group treated with saline for 28 days; SH, sham; MG, model group treated with MG-132 for 28 days; PDTC, model group treated with pyrrolidine dithiocarbamic acid for 28 days; N, marker; IL, interleukin; MMP, matrix metalloproteinase; NF, nuclear factor.
Figure 4.
Protein expression of (A) NF-κB, (B) IL-1β and (C) MMP-2 in the non-infarction zone in different groups. **P<0.01 for comparison between either two groups. Groups: MI, myocardial infarction model group treated with saline for 28 days; SH, sham; MG, model group treated with MG-132 for 28 days; PDTC, model group treated with pyrrolidine dithiocarbamic acid for 28 days; IL, interleukin; MMP, matrix metalloproteinase; NF, nuclear factor.
Figure 5.
Immunohistochemical staining for IL-1β and MMP-2. (A-D) Staining for IL-1β in the (A) SH, (B) MI, (C) MG and (D) PDTC groups. (E-H) Staining for MMP-2 in the (E) SH, (F) MI, (G) MG and (H) PDTC groups (scale bar, 200 µm). Groups: MI, myocardial infarction model group treated with saline for 28 days; SH, sham; MG, model group treated with MG-132 for 28 days; PDTC, model group treated with pyrrolidine dithiocarbamic acid for 28 days; IL, interleukin; MMP, matrix metalloproteinase; NF, nuclear factor.
Figure 6.
Semi-quantitative evaluation of (A) IL-1β and (B) MMP-2 expression from immunohistochemical staining images in different groups. **P<0.01, ***P<0.001 for comparison between either two groups. Groups: MI, myocardial infarction model group treated with saline for 28 days; SH, sham; MG, model group treated with MG-132 for 28 days; PDTC, model group treated with pyrrolidine dithiocarbamic acid for 28 days; IL, interleukin; MMP, matrix metalloproteinase; NF, nuclear factor.
Discussion
Post-MI myocardial remodeling is associated with the upregulation of inflammatory cytokines, while intervention with ubiquitin proteasome inhibitors blocks the activation of NF-κB and leads to downregulation of the inflammatory cytokines. It has been reported that short- and long-term treatment with MG-132 significantly attenuated hypertension-induced cardiac remodeling and dysfunction, which may be mediated by the NF-κB/transforming growth factor β1 signaling pathway (16). The present study investigated the effects of MG-132 on myocardial remodeling in comparison with the MI, SH and PDTC groups. Morphological changes and collagen remodeling of myocardial cells were observed at 28 days after MI, and the mRNA and protein expression of NF-κB, IL-1β and MMP-2 were also determined.
The present study demonstrated that on day 28 after MI, the surface area, perimeter and diameter of non-infarcted cardiomyocytes in the MI group were increased compared with those in the SH group, indicating that myocardial hypertrophy occurred in the non-infarcted zone after MI. The application of MG-132 reduced the surface area, perimeter and diameter of cardiomyocytes, indicating that MG-132 reduced the degree of myocardial hypertrophy in the non-MI zone. Studies using a model of myocardial hypertrophy revealed that the proteasome inhibitors MG-132 and MG-262 reduce the size of hypertrophic cardiomyocytes by 2-fold and reduce the expression of myocardial hypertrophy-associated α-myosin heavy chain, myosin light chain 2 and α-actin (19). The present study demonstrated that in vivo application of MG-132 inhibited post-MI myocardial remodeling, thus improving post-MI myocardial hypertrophy in the non-MI zone.
Post-MI myocardial remodeling includes myocardial parenchymal and interstitial remodeling, while the former appears as myocardial hypertrophy and the latter is mainly caused by myocardial interstitial fibrosis through increased collagen deposition; changes of myocardial collagens are the driving factor of interstitial remodeling. The present study used picrosirius red staining and microscopically observed the deposition of total collagen, type I collagen and type III collagen in the non-MI zone. The results demonstrated that the MI group exhibited a significant increase in collagen deposition as well as the quantified content of total collagen, type I collagen and type III collagen. Furthermore, the ratio of type I/III collagen was also increased, indicating that collagen remodeling occurs after MI. The deposition of total collagen, type I collagen and type I/III collagen in the MG group was significantly decreased compared with that in the MI group, and the ratio of type I vs. III collagen was decreased, consistent with the results of Meiners et al (20), who used a rat model of spontaneous hypertensive to demonstrate that MG-132 improves myocardial collagen remodeling in the MI model.
In order to confirm whether MG-132 exerted its effect of improving post-MI myocardial remodeling through inhibiting the activation of NF-κB and downregulating the expression of inflammatory cytokines, the present study detected the expression of NF-κB, IL-1β and MMP-2 in cardiomyocytes at the mRNA and protein level. The immunohistochemical results indicated that NF-κB in the MI group was mainly expressed in the nuclei (data not shown), indicating that NF-κB was continuously activated after MI, consistent with the results of Wang et al (21). Furthermore, the RT-qPCR results of the present study demonstrated that the mRNA expression of NF-κB was increased after MI, and the protein expression of NF-κB p65 was also confirmed to be increased by western blot analysis. Activation of NF-κB increases the expression of inflammatory cytokines, which then participate in cardiomyocyte hypertrophy and collagen remodeling through a series of signaling pathways. In the present study, the gene and protein levels of IL-1β in the MI group were upregulated, indicating that inflammatory cytokines are upregulated in the process of post-MI myocardial remodeling, consistent with the results of a previous study (19). IL-1β may initiate the c-Jun-N-terminal kinase/stress activated protein kinase pathway and activate mitogen-activated protein kinase signaling, thus leading to myocardial hypertrophy (20). IL-1β also inhibits collagen synthesis by myocardial fibroblasts, increases the activity of MMPs and promotes interstitial collagen remodeling (20). After application of MG-132, NF-κB and its downstream inflammatory cytokines (such as IL-1β) were decreased, and the trend in the expression of IL-1β was consistent with that of NF-κB, thus further confirming that MG-132 inhibited the activation of NF-κB and decreased the expression of inflammatory cytokines. In parallel with the decrease in IL-1β, myocardial hypertrophy in the non-MI zone was improved, consistent with the results of previous studies (17). The present study also demonstrated that after MG-132 was applied, the expression of MMP-2 reduced in parallel with the decrease of NF-κB, IL-1β and the deposition of total collagen, type I collagen and type I/III collagen in the non-MI zone was significantly reduced, indicating that this pathway improves collagen remodeling in the non-MI zone. While previous studies reported that MG-132 attenuated myocardial hypertrophy and collagen remodeling by decreasing the expression of IL-1β, no direct evidence regarding inhibition of NF-κB was ever provided (4,11). In the present study, by measuring the gene and protein expression of NF-κB and its downstream products IL-1β and MMP-2 and analyzing their association with myocardial remodeling in the non-MI zone, it was confirmed that the mechanisms by which MG-132 inhibited post-MI myocardial remodeling included the reduction of NF-κB activation and the downregulation of inflammatory factors. MG-132 may inhibit NF-κB through blocking the activity of the 20 s proteasome (21,22), so that the degradation of I-κB is reduced, the combination of NF-κB with I-κB is increased, and the nuclear translocation of NF-κB is blocked to thereby inhibit the activation of NF-κB.
The effects of MG-132 in improving post-MI myocardial remodeling may proceed via additional mechanisms. The present study compared MG-132 with PDTC, a specific inhibitor of NF-κB. The results indicated that the expression of NF-κB, IL-1β and MMP-2 in the PDTC group were decreased at the mRNA and protein level, and morphological parameters including the cell surface area, perimeter, diameter, and collagen deposition were improved, which further confirmed that inhibiting the activation of NF-κB improves post-MI myocardial remodeling. However, the comparison between MG-132 and PDTC revealed that although PDTC exhibited stronger effects in inhibiting the activation of NF-κB and downregulating IL-1 than MG-132, its effects in myocardial morphological changes, improving collagen deposition and downregulating MMP-2 were less pronounced than those of MG-132. This difference may be due to the dosage of MG-132 and PDTC, which requires further study. However, in addition to inhibiting the activation of NF-κB and downregulating the expression of inflammatory proteins, MG-132 may have further mechanisms of action, including dopaminergic neurons (23), nuclear factor erythroid 2-related factor 2 (24) and it also could induce apoptosis (25). Since MG-132 has not been introduced for clinical use, additional experiments and clinical research are needed in the future.
Acknowledgements
Not applicable.
Funding
This work was funded by the Doctoral Research Foundation of Dali University (grant no. KYBS201212).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XW, ZC and KL collaborated to design the study. HL and SK were responsible for data analysis. YY performed RT-qPCR experiments. ZC and YD performed western blot analysis and immunohistochemistry analysis. All authors collaborated to interpret results and develop the manuscript.
Ethics approval and consent to participate
The experimental animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Dali University (Dali, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. 1997;81:664–671. doi: 10.1161/01.RES.81.5.664. [DOI] [PubMed] [Google Scholar]
- 3.Zarrouk-Mahjoub S, Zaghdoudi M, Amira Z, Chebi H, Khabouchi N, Finsterer J, Mechmeche R, Ghazouani E. Pro- and anti-inflammatory cytokines in post-infarction left ventricular remodeling. Int J Cardiol. 2016;221:632–636. doi: 10.1016/j.ijcard.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/bj20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun B, Xia Q, Gao Z. Total flavones of choerospondias axillaris attenuate cardiac dysfunction and myocardial interstitial fibrosis by modulating NF-kB signaling pathway. Cardiovasc Toxicol. 2015;15:283–289. doi: 10.1007/s12012-014-9298-3. [DOI] [PubMed] [Google Scholar]
- 6.Xiao C, Ghosh S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol. 2005;560:41–45. doi: 10.1007/0-387-24180-9_5. [DOI] [PubMed] [Google Scholar]
- 7.Zandi E, Karin M. Bridging the gap: Composition, regulation, and physiological function of the IkappaB kinase complex. Mol Cell Biol. 1999;19:4547–4551. doi: 10.1128/MCB.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner H, Mohacsi P, Fuller-Bicer GA, Rieben R, Meier B, Hess O, Hullin R. Cytokine activation and disease progression in patients with stable moderate chronic heart failure. J Heart Lung Transplant. 2007;26:622–629. doi: 10.1016/j.healun.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: A new anti-inflammatory strategy. J Mol Med (Berl) 2003;81:235–245. doi: 10.1007/s00109-003-0422-2. [DOI] [PubMed] [Google Scholar]
- 10.Santos DG, Resende MF, Mill JG, Mansur AJ, Krieger JE, Pereira AC. Nuclear Factor (NF) kappaB polymorphism is associated with heart function in patients with heart failure. BMC Med Genet. 2010;11:89. doi: 10.1186/1471-2350-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen FT, Yang CM, Yang CH. The protective effects of the proteasome inhibitor bortezomib (velcade) on ischemia-reperfusion injury in the rat retina. PLoS One. 2013;8:e64262. doi: 10.1371/journal.pone.0064262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Chen B, Liu D, Yang Y, Xiong Z, Zeng J, Dong Y. MG132 treatment attenuates cardiac remodeling and dysfunction following aortic banding in rats via the NF-κB/TGFβ1 pathway. Biochem Pharmacol. 2011;81:1228–1236. doi: 10.1016/j.bcp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 14.Jin JL, Lv RG, Guo J, Liu XH, Liang YW, Wei JR, Wang L. Improvement of left ventricular remodelling by inhibition of NF-κB in a rat model of myocardial infarction. Heart Lung Circ. 2016;25:1007–1012. doi: 10.1016/j.hlc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZR, Wu XH, Luo KL, He Q, Xiang YL. Proteasome inhibitor MG-132 improves myocadial hypertrophy after myocardial infarction in rats. Basic Clin Med. 2012;32:1326–1331. (In Chinese) [Google Scholar]
- 16.Snyder JG, Prewitt R, Campsen J, Britt LD. PDTC and Mg132, inhibitors of NF-kappaB, block endotoxin induced vasodilation of isolated rat skeletal muscle arterioles. Shock. 2002;17:304–307. doi: 10.1097/00024382-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, Changotade S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 2014;62:751–758. doi: 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Li SN, Huang S, Xu B. Inhibitor of ubiquitin proteasome system suppress Calcineurin-dependent cardiomyocyte hypertrophy. Chin J Arteriosclerosis. 2014;22:774–778. (In Chinese) [Google Scholar]
- 20.Meiners S, Hocher B, Weller A, Laule M, Stangl V, Guenther C, Godes M, Mrozikiewicz A, Baumann G, Stangl K. Downregulation of matrix metalloproteinases and collagens and suppression of cardiac fibrosis by inhibition of the proteasome. Hypertension. 2004;44:471–477. doi: 10.1161/01.HYP.0000142772.71367.65. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Suo F, Liu J, Hu H, Xue M, Cheng W, Xuan Y, Yan S. Myocardial infarction induces sympathetic hyperinnervation via a nuclear factor-κB-dependent pathway in rabbit hearts. Neurosci Lett. 2013;535:128–133. doi: 10.1016/j.neulet.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Liao YH, Cheng X, Ge H, Guo H, Wang M. Effects of carvedilol on cardiac cytokines expression and remodeling in rat with acute myocardial infarction. Int J Cardiol. 2006;111:247–255. doi: 10.1016/j.ijcard.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 23.Wójcik S, Spodnik JH, Spodnik E, Dziewiątkowski J, Moryś J. Nigrostriatal pathway degeneration in rats after intraperitoneal administration of proteasome inhibitor MG-132. Folia Neuropathol. 2014;52:41–55. doi: 10.5114/fn.2014.41743. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Sun W, Du B, Miao X, Bai Y, Xin Y, Tan Y, Cui W, Liu B, Cui T, et al. Therapeutic effect of MG-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: Roles of Nrf2 and NF-κB. Am J Physiol Heart Circ Physiol. 2013;304:H567–H578. doi: 10.1152/ajpheart.00650.2012. [DOI] [PubMed] [Google Scholar]
- 25.Chen SF, Chen HY, Liu XB, Zhang YX, Liu W, Wang WH, Zhang B, Wang LX. Apoptotic effect of MG-132 on human tongue squamous cell carcinoma. Biomed Pharmacother. 2011;65:322–327. doi: 10.1016/j.biopha.2011.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.