Abstract
The present study investigated the expression and clinical significance of kelch-like ECH-associated protein 1 (Keap1) and nuclear factor erythroid-2-related factor-2 (Nrf2) expression in diffuse large B-cell lymphoma (DLBCL). These proteins were detected by immunohistochemistry in 39 DLBCL cases and 17 cases of reactive lymph node hyperplasia, and their association with the clinicopathological features of DLBCL patients was analyzed. In DLBCL, the percentage of cells with positive staining for Keap1 and Nrf2 was 46.2 and 35.9%, respectively, which was significantly higher than that in reactive lymph node hyperplasia (17.7 and 5.9%, respectively). There was no correlation between Keap1 and Nrf2 expression according to a Spearman rank correlation analysis (r=0.272; P>0.05). Keap1 and Nrf2 expression was associated with the international prognostic index and Ann-Arbor clinical stage (P<0.05), and Keap1 and Nrf2 expression was higher in DLBCL patients with stage III–IV (68.4 and 52.6%, respectively) compared with in those with stage I–II (25.0 and 20.0%, respectively). The aberrant expression of Keap1 and Nrf2 in DLBCL suggests that these factors may have crucial roles in the development and progression of the disease, and may therefore be used as prognostic indicators.
Keywords: immunohistochemical staining, expression diffuse large B-cell lymphoma, kelch-like ECH-associated protein 1, nuclear factor erythroid-2-related factor-2
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an invasive and malignant tumor derived from mature B lymphocytes. It is the most common type of non-Hodgkin lymphoma (NHL), accounting for 30–40% of the morbidity of this disease (1). Although a subset of patients may be successfully treated by chemotherapy, the recurrence rate is high. Therefore, further study to identify novel therapeutic options to treat patients with DLBCL is warranted. Oxidative damage induced by oxidative stress may be associated with the occurrence and development of lymphoma (2,3). Under physiological conditions, the Kelch-like ECH associated protein 1 (Keap1)/nuclear factor erythroid-2-related factor-2 (Nrf2) signaling pathway is a critical system that responds to oxidative stress in vivo (4). It has been indicated that this signaling pathway is associated with the development of conditions including inflammation (5), tumors (6), nerve injury (7), cardiovascular disease (8) and chronic obstructive pulmonary disease (9). Most available studies on Keap1 and Nrf2 expression have mainly focused on lymphoma cell lines, and few have reported on their expression in DLBCL tissues. The present study was performed to characterize Keap1 and Nrf2 expression in DLBCL tissues by immunohistochemical staining, and to explore the role of these proteins in the development and progression of DLBCL. Specifically, the association between the expression of these proteins and clinical features was assessed in an attempt to provide novel approaches for the treatment of DLBCL.
Materials and methods
Patients
The present study included 39 cases of de novo DLBCL, and paraffin-embedded specimens that were histologically confirmed by pathologists of Gansu Provincial Hospital (Lanzhou, China) between October 2012 and November 2016 were utilized. Samples were derived from 21 males and 18 females, whose age ranged from 16 to 83 years of age (median age, 56 years). A total of 17 paraffin-embedded specimens comprising cases of reactive lymph node hyperplasia during the same period, were included in the control group, and the age of these donors varied from 16–70 years of age (median age, 41 years). Diagnoses were confirmed by an experienced pathologist according to the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues in 2016 (10). The complete clinical data were collected for each subject. Patients with the following diseases were excluded: Other malignant tumors, acute infection, cardiovascular diseases, chronic obstructive pulmonary disease, neurodegenerative diseases, liver diseases and ocular diseases, including age-associated macular degeneration, cataracts, diabetic retinopathy and glaucoma (5,7–9,11–13). The present study was approved by the Ethics Committee of Gansu Provincial Hospital and all patients provided written informed consent.
Reagents
Rabbit anti-Keap1 antibody (cat. no. bs-4900R; dilution, 1:400), rabbit anti-Nrf2 antibody (cat. no. bs-1074R; dilution, 1:400), biotin-conjugated goat anti-rabbit immunoglobulin (Ig)G/bio antibody (cat. no. bs-0295G-bio; dilution, 1:100), streptavidin-horseradish peroxidase antibody (cat. no. bs-0437P-HRP; dilution, 1:500) and diaminobenzidine (DAB) staining kit (cat. no. C02-04001) were purchased from Beijing Bioss Biotech Co., Ltd. (Beijing, China).
Immunohistochemical staining
All specimens were fixed in 10% neutral formalin, embedded in paraffin, sliced to a thickness of 4 µm and stained. First, the slides were baked at 60°C for 2 h. Subsequently, the paraffin sections were dewaxed three times using xylene (15 min each time) and hydrated in a descending series of ethanol. Antigen retrieval was performed by boiling samples in 0.01 M citrate buffer (pH 6.0) at 95°C for 15 min, followed by cooling to room temperature. The paraffin sections were incubated at room temperature in 3% hydrogen peroxide solution for 20 min to eliminate endogenous peroxidase activity. Samples were then incubated in 10% normal goat serum at 37°C for 20 min, and the primary antibody (anti-Keap1 or anti-Nrf2) was then added, followed by incubation at 4°C overnight. Following the addition of the antibody (biotin-conjugated goat anti-rabbit IgG secondary antibody), sections were incubated at 37°C for 20 min. An antibody (streptavidin-horseradish peroxidase tertiary antibody) was added to the sections at 37°C for 20 min. After each completed step, the slides were washed three times with PBS for 5 min each. DAB chromogenic reagent was added, and samples were counterstained with hematoxylin, hydrated in an ascending series of ethanol, cleared in xylene and mounted. Samples in which the primary antibody (anti-Keap1 or anti-Nrf2) was replaced with PBS served as a negative control.
Evaluation of staining
Keap1 and Nrf2 staining was evaluated and analyzed by two senior doctors in the pathology department (Dr Yamei Dang and Dr Fenghui Zhao; Gansu Provincial Hospital, Lanzhou, China) under low magnification (×100), high magnification (×400) and oil immersion microscopy (×1,000). If there was a disagreement, the result of staining was evaluated and analyzed by a third doctor. If two doctors had the same result, that was considered the final result. All pathologists were unaware of the clinical data. The staining results were evaluated as follows (14–16): The percentage of positively stained cells were as follows: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100% of cells stained. Sections were also scored on the basis of staining intensity: 0, negative or no staining; 1, pale yellow, weak staining intensity; 2, yellow, moderate staining intensity; and 3, brown, strong staining intensity. The semi-quantitative score was based on the multiplication of positive cell percentage and positive cell staining intensity score. Staining results were divided into four grades based on the scores regarding the percentage of positively stained cells and staining intensity. Results were presented as follows: 0 (−, negative), 1–4 (+, weakly positive), 5–8 (++, moderately positive) and 9–12 (+++, strongly positive). Samples rated as (+) - (+++) were regarded as positive.
Statistical analysis
Data was analyzed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The positive rates between different groups were compared by performing a χ2 test [n≥40; theoretical frequency (T) ≥5], continuity correction of χ2 test (n≥40, 1≤T<5) and Fisher's exact test (n<40 or T<1). Spearman's rank correlation analysis was used to assess the correlation between Keap1 and Nrf2. P<0.05 was considered to indicate a statistically significant difference.
Results
Keap1 and Nrf2 expression in reactive lymph node hyperplasia and DLBCL tissues
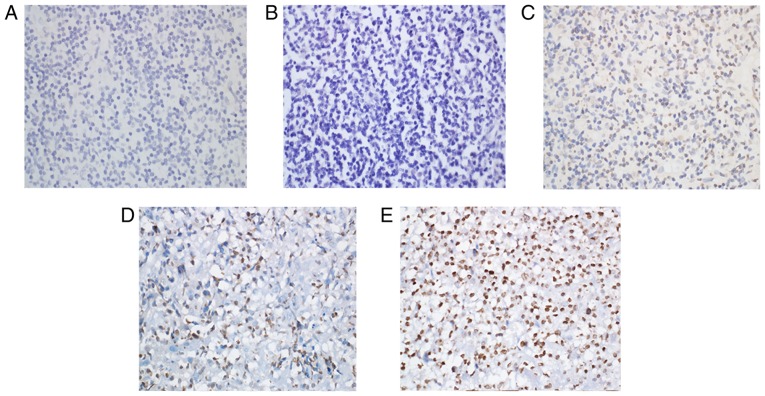
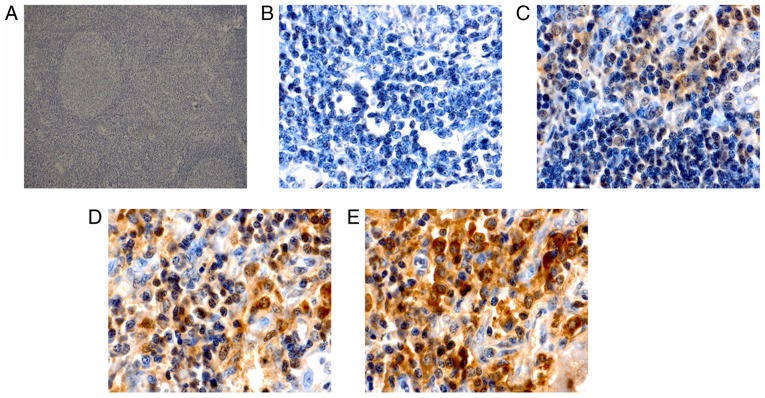
Immunohistochemical staining for Keap1 was observed as yellow or brown granules that were predominantly located in the cytoplasm, with a few situated in the nucleus (Fig. 1). However, Nrf2 staining presented as yellow or brown granules, which were mainly located in the cellular nuclei, with little staining was detected in the cytoplasm (Fig. 2). In the reactive lymph node hyperplasia samples, Nrf2 staining for 1 case was positive in the nuclei of the follicle cells, while it was negative for the other cases. In 3 cases, Keap1 staining was scattered in the cytoplasm of the follicle cells, plasmacytes and histiocytes. In most DLBCL tissues, a strong expression of Keap1 was observed, with a diffuse distribution in the cytoplasm of heterotypic malignant lymphocytes (Fig. 1E). Furthermore, a strong expression of Nrf2 was mainly observed as a diffuse distribution in the nuclei of heterotypic malignant lymphocytes (Fig. 2E). In addition, a weak or moderate expression of Keap1 and Nrf2 was observed in malignant lymphocytes (Fig. 1C and D; Fig. 2C and D). The Keap1 positivity rate in DLBCL tissues was 46.2%, which was significantly higher than that of reactive lymph node hyperplasia (17.7%; P<0.05; Table I). Furthermore, statistical analysis indicated that the positivity rate of Nrf2 was 35.9% in DLBCL tissues and only 5.9% in reactive lymph node hyperplasia, and this difference was statistically significant (P<0.05; Table I).
Figure 1.
Representative immunohistochemical images with staining for Keap1. (A) Reactive lymph node hyperplasia tissue with negative staining for Keap1. (B-E) diffuse large B-cell lymphoma tissues with (B) negative staining, (C) weakly positive staining (+), (D) moderately positive staining (++) and (E) strongly positive staining (+++) for Keap1. Magnification, ×400. Keap1, kelch-like ECH-associated protein 1.
Figure 2.
Representative immunohistochemical images with staining for Nrf2. (A) Reactive lymph node hyperplasia tissue (magnification, ×100) with negative staining for Nrf2; (B-E) diffuse large B-cell lymphoma tissues with (magnification, ×1,000) (B) negative staining, (C) weakly positive staining (+), (D) moderately positive staining (++) and (E) strongly positive staining (+++) for Nrf2. Nrf2, nuclear factor erythroid-2-related factor-2.
Table I.
Keap1 and Nrf2 expression in DLBCL tissues and reactive lymph node hyperplasia.
| Keap1 | Nrf2 | ||||||
|---|---|---|---|---|---|---|---|
| Group | n | Positives | χ2 | P-value | Positives | χ2 | P-value |
| DLBCL tissues | 39 | 18 (46.2) | 4.105 | 0.043a | 14 (35.9) | 4.016 | 0.045b |
| Reactive lymph node hyperplasia | 17 | 3 (17.7) | 1 (5.9) | ||||
P<0.05, χ2 test (n>40; T>5)
P<0.05, χ2 test (n>40; T=4.55). Values are expressed as n (%). DLBCL, diffuse large B-cell lymphoma; Nrf2, nuclear factor erythroid-2-related factor-2; Keap1, kelch-like ECH-associated protein 1; T, theoretical frequency.
Association between Keap1 or Nrf2 expression and clinicopathological features in DLBCL
In DLBCL tissues, the expression levels of Keap1 and Nrf2 were associated to the international prognostic index (IPI) and Ann-Arbor clinical stage (P<0.05), and Keap1 and Nrf2 expression was higher in DLBCL patients with stage III–IV (68.4 and 52.6%, respectively) compared with those with stage I–II (25.0 and 20.0%, respectively). However, Keap1 or Nrf2 expression was not associated with patient age, sex and lactate dehydrogenase levels (P>0.05; Table II).
Table II.
Association between Keap1 or Nrf2 expression and clinicopathological features of patients with diffuse large B-cell lymphoma.
| Keap1 expression | Nrf2 expression | ||||
|---|---|---|---|---|---|
| Variable | n | Positives | P-valuea | Positives | P-valuea |
| Age (years) | 1.000 | 0.740 | |||
| ≤50 | 15 | 7 (46.7) | 6 (40.0) | ||
| >50 | 24 | 11 (45.8) | 8 (33.3) | ||
| Sex | 0.752 | 0.180 | |||
| Male | 21 | 9 (42.9) | 10 (47.6) | ||
| Female | 18 | 9 (50.0) | 4 (22.2) | ||
| LDH (U/l) | 0.192 | 0.740 | |||
| ≤250 | 16 | 5 (31.3) | 5 (31.3) | ||
| >250 | 23 | 13 (56.5) | 9 (39.1) | ||
| Clinical stage | 0.010 | 0.048 | |||
| I–II | 20 | 5 (25.0) | 4 (20.0) | ||
| III–IV | 19 | 13 (68.4) | 10 (52.6) | ||
| IPI | 0.025 | 0.043 | |||
| 0–2 | 23 | 7 (30.4) | 5 (21.7) | ||
| 3–5 | 16 | 11 (68.8) | 9 (56.3) | ||
Fisher's exact test (n=39). Values are expressed as n (%). Nrf2, nuclear factor erythroid-2-related factor-2; Keap1, kelch-like ECH-associated protein 1; LDH, lactate dehydrogenase; IPI, international prognostic index.
Correlation between Keap1 and Nrf2 expression in DLBCL tissues
Out of 14 cases of DLBCL with expression of Nrf2, expression of Keap1 was detected in 9 cases. Statistical analysis indicated that Nrf2 expression was not correlated with Keap1 expression in DLBCL tissues (r=0.272, P=0.094; Table III).
Table III.
Correlation between Keap1 and Nrf2 expression in diffuse large B-cell lymphoma tissues.
| Nrf2 expression (n) | ||||
|---|---|---|---|---|
| Keap1 expression (n) | Positives (%) | Negatives (%) | r-value | P-value |
| Positives | 9 (23.1) | 9 (23.1) | 0.272 | 0.094 |
| Negatives | 5 (12.8) | 16 (41.0) | ||
Nrf2, nuclear factor erythroid-2-related factor-2; Keap1, kelch-like ECH-associated protein 1. Samples rated as (+) to (+++) were regarded as positive. Samples rated as (−) was regarded as negative. There were a total of 39 cases.
Discussion
The present study provided the first evidence of high expression of Keap1 and Nrf2 proteins in DLBCL tissues, to the best of our knowledge. The Keap1-Nrf2/antioxidant response element (ARE) signaling pathway is a key endogenous antioxidant stress response pathway and induces endogenous antioxidant reactions. This pathway mainly comprises three parts, namely Keap1, Nrf2 and ARE (17). Keap1 is aptly named because it is similar to the Drosophila actin binding protein Kelch, which is a polypeptide containing 624 amino acids (18). Nrf2 is a basic leucine zipper transcription factor and belongs to the Cap'n'Collar subfamily, which was first reported by Moi et al (19) in 1994. Keap1 is a large cytoplasmic chaperone of Nrf2 that has a negative role in the regulation of Nrf2 transcriptional activity. Under oxidative stress from endogenous and exogenous sources, Nrf2 dissociates from Keap1, translocates to the nucleus, and transactivates a series of downstream antioxidant enzymes and phase-II detoxification enzymes to combat oxidative stress (11). Therefore, the expression of Nrf2 and Keap1 indicates that Nrf2 dissociates from Keap1 and in turn has been activated. Relevant studies report that Nrf2 has opposing actions in humans (5,20). Furthermore, the present study suggests that Nrf2 does not only protect normal cells, but also cancer cells from oxidative damage, thereby promoting their growth and survival (21).
Previous studies have demonstrated that Keap1 and Nrf2 are aberrantly expressed in solid tumors. Kawasaki et al (22) reported that abnormal expression of Nrf2 is closely associated with clinical characteristics of gastric cancer, including lymph node metastasis and clinical stage. Nrf2 expression may also be associated with resistance to chemotherapy. In addition, Isohookana et al (23) suggested that Keap1 overexpression may be a promising prognostic biomarker for pancreatic cancer. Shibata et al (24) utilized resequencing analysis to demonstrate that esophageal squamous cancer is associated with an Nrf2 gene mutation, which is linked with poor prognosis and recurrence. Furthermore, Nrf2 mutations were identified to be mainly located in the Keap1 binding domain. Another study by Shibata et al (25) indicated that Keap1 gene mutations in gallbladder cancer may result in Nrf2 overexpression. These authors speculated that this may be the molecular mechanism of chemotherapy resistance.
At present, little is known regarding Keap1 and Nrf2 expression in lymphoma tissues. In the present study, Keap1 and Nrf2 expression was detected in DLBCL tissues by immunohistochemistry. The results indicated that Keap1 and Nrf2 expression was higher in DLBCL tissues than that in reactive lymph node hyperplasia tissues, and their expression was correlated with the IPI and Ann-Arbor clinical stage (P<0.05). In addition, and Keap1 and Nrf2 expression was higher in patients with stage III/IV DLBCL. In addition, no correlation was identified between Nrf2 and Keap1 expression in DLBCL tissues. The reason for this remains elusive but it may be due to Keap1 mutations, which alter the structure of Keap1 such that the degradation of Nrf2 is impaired (26). The present results suggest that Keap1 and Nrf2 may have crucial roles in disease development, and hence may be used as prognostic indicators.
Studies have been found that, in many other cancers, Nrf2 may be closely linked to chemotherapy resistance, promote tumor growth and lead to poor prognosis, including lung cancer (27), pancreatic carcinoma (23,28), breast cancer (29,30), head and neck cancer (31), prostate cancer (32), ovarian carcinoma (33,34) and cervical cancer (35). In addition, in hematopoietic malignancies, only few studies have reported on Nrf2 expression in lymphoma cell lines. Zha et al (36) reported that in the Raji cell line, treatment with disulfiram (DS) and DS/Cu causes excessive production of reactive oxygen species such that Nrf2 expression is inhibited, which subsequently promotes apoptosis in transplanted tumors in nude mice. It has also been indicated that inhibition of Nrf2 expression may promote apoptosis of lymphoma cells. A study by Chen et al (37) on the relapse of mantle cell lymphoma compared Nrf2 expression between bortezomib-sensitive cell lines (Jeko and SP53) and resistant cell lines (Mino and Rec-1) after bortezomib treatment. The results indicated that Nrf2 expression was upregulated in bortezomib-resistant cell lines, whereas it was decreased or not significantly changed in sensitive cell lines. This suggested that elevated Nrf2 expression may be associated with bortezomib resistance in mantle cell lymphoma. These studies indicated that elevated Keap1 and Nrf2 expression may be associated with chemotherapy resistance and replace of lymphoma, but the underlying mechanisms remain to be elucidated.
According to the results of the present study, Keap1 and Nrf2 may have crucial roles in the development of DLBCL and are associated with patient prognosis. However, the present study has certain limitations: According to the 2016 revision of the WHO classification of lymphoid neoplasms, DLBCL comprises numerous subtypes. Therefore, in the present study, different subtypes of DLBCL cases were collected to increase the sample size and minimize the error of the final statistical results. In addition, the present study examined a relatively small number of cases, none of the patients were followed up, and the median survival was unknown. Therefore, further experiments are required that include the examination of more samples and follow-up analyses for the same or even different subtypes of DLBCL patients. Furthermore, cell and animal experiments will be performed in the future to explore the specific molecular mechanisms through which Keap1 and Nrf2 regulate DLBCL and further determine whether Keap1 and Nrf2 expression is associated with chemotherapy resistance in DLBCL.
Acknowledgements
The authors would like to thank two pathologists, Dr Yamei Dang and Dr Fenghui Zhao in the Department of Pathology of Gansu Provincial Hospital (Lanzhou, China), for their guidance during the evaluation of staining.
Glossary
Abbreviations
- Keap1
kelch-like ECH-associated protein 1
- Nrf2
nuclear factor erythroid-2-related factor-2
- DLBCL
diffuse large B-cell lymphoma
- IPI
international prognostic index
- ARE
antioxidant response element
Funding
This study was supported by National Natural Science Foundation of China (grant nos. 81560498, 81260342 and 30960438).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HL conceived and designed the experiments. XY, YZ, LX, JZ, YQ and QJ performed the experiments. XY, LX and JZ analyzed the data. XY drafted the manuscript, and HL and XY revised the manuscript. The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work.
Ethical approval and consent to participate
The present study was approved by the Ethics Committee of Gansu Provincial Hospital (Lanzhou, China) and all patients provided written informed consent.
Consent for publication
The patient provided written informed consent for the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing interests regarding this study.
References
- 1.Menon MP, Pittaluga S, Jaffe ES. The histological and biological spectrum of diffuse large B-cell lymphoma in the World Health Organization classification. Cancer J. 2012;18:411–420. doi: 10.1097/PPO.0b013e31826aee97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafson HL, Yao S, Goldman BH, Lee K, Spier CM, LeBlanc ML, Rimsza LM, Cerhan JR, Habermann TM, Link BK, et al. Genetic polymorphisms in oxidative stress-related genes are associated with outcomes following treatment for aggressive B-cell non-Hodgkin lymphoma. Am J Hematol. 2014;89:639–645. doi: 10.1002/ajh.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peroja P, Pasanen AK, Haapasaari KM, Jantunen E, Soini Y, Turpeenniemi-Hujanen T, Bloigu R, Lilja L, Kuittinen O, Karihtala P. Oxidative stress and redox state-regulating enzymes have prognostic relevance in diffuse large B-cell lymphoma. Exp Hematol Oncol. 2012;1:2. doi: 10.1186/2162-3619-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uruno A, Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25:153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki H, Tanji K, Wakabayashi K, Matsuura S, Itoh K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol Int. 2015;65:210–219. doi: 10.1111/pin.12261. [DOI] [PubMed] [Google Scholar]
- 8.Barančík M, Grešová L, Barteková M, Dovinová I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol Res. 2016;65(Suppl 1):S1–S10. doi: 10.33549/physiolres.933403. [DOI] [PubMed] [Google Scholar]
- 9.Sandford AJ, Malhotra D, Boezen HM, Siedlinski M, Postma DS, Wong V, Akhabir L, He JQ, Connett JE, Anthonisen NR, et al. NFE2L2 pathway polymorphisms and lung function decline in chronic obstructive pulmonary disease. Physiol Genomics. 2012;44:754–763. doi: 10.1152/physiolgenomics.00027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taguchi K, Yamamoto M. The KEAP1-NRF2 system in cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng ML, Lu YF, Chen H, Shen ZY, Liu J. Liver expression of Nrf2-related genes in different liver diseases. Hepatobiliary Pancreat Dis Int. 2015;14:485–491. doi: 10.1016/S1499-3872(15)60425-8. [DOI] [PubMed] [Google Scholar]
- 13.Batliwala S, Xavier C, Liu Y, Wu H, Pang IH. Involvement of Nrf2 in ocular diseases. Oxid Med Cell Longev. 2017;2017:1703810. doi: 10.1155/2017/1703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasanen AK, Kuitunen H, Haapasaari KM, Karihtala P, Kyllönen H, Soini Y, Turpeenniemi-Hujanen T, Kuittinen O. Expression and prognostic evaluation of oxidative stress markers in an immunohistochemical study of B-cell derived lymphomas. Leuk Lymphoma. 2012;53:624–631. doi: 10.3109/10428194.2011.624226. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Wang X, Wu W, Dang H, Wang B. Expression of the Nrf2 and Keap1 proteins and their clinical significance in osteosarcoma. Biochem Biophys Res Commun. 2016;473:42–46. doi: 10.1016/j.bbrc.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 16.Soini Y, Eskelinen M, Juvonen P, Kärjä V, Haapasaari KM, Saarela A, Karihtala P. Nuclear Nrf2 expression is related to a poor survival in pancreatic adenocarcinoma. Pathol Res Pract. 2014;210:35–39. doi: 10.1016/j.prp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: An insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Zhang C, Zhang L, Yang Q, Zhou S, Wen Q, Wang J. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer. 2015;15:531. doi: 10.1186/s12885-015-1541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Ishigami S, Arigami T, Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H, Nakajo A, et al. Clinicopathological significance of nuclear factor (erythroid-2)- related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer. 2015;15:5. doi: 10.1186/s12885-015-1008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isohookana J, Haapasaari KM, Soini Y, Karihtala P. Keap1 expression has independent prognostic value in pancreatic adenocarcinomas. Diagn Pathol. 2015;10:28. doi: 10.1186/s13000-015-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata T, Kokubu A, Saito S, Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y, Kushima R, Kiyono T, Yamamoto M. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. 1368. e1-4. [DOI] [PubMed] [Google Scholar]
- 26.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genrich G, Kruppa M, Lenk L, Helm O, Broich A, Freitag-Wolf S, Rocken C, Sipos B, Schäfer H, Sebens S. The anti-oxidative transcription factor Nuclear factor E2 related factor-2 (Nrf2) counteracts TGF-β1 mediated growth inhibition of pancreatic ductal epithelial cells -Nrf2 as determinant of pro-tumorigenic functions of TGF-β1. BMC Cancer. 2016;16:155. doi: 10.1186/s12885-016-2191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 30.Khatri R, Shah P, Guha R, Rassool FV, Tomkinson AE, Brodie A, Jaiswal AK. Aromatase inhibitor-mediated downregulation of INrf2 (Keap1) leads to increased Nrf2 and resistance in breast cancer. Mol Cancer Ther. 2015;14:1728–1737. doi: 10.1158/1535-7163.MCT-14-0672. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Stacy DR, Ely K, Massion PP, Yarbrough WG, Hallahan DE, Sekhar KR, Freeman ML. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–818. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- 32.Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- 33.Zembutsu H. Keap1-Nrf2 pathway and drug resistance in epithelial ovarian cancer. Pharmacogenomics. 2011;12:1516–1517. [PubMed] [Google Scholar]
- 34.Shim GS, Manandhar S, Shin DH, Kim TH, Kwak MK. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic Biol Med. 2009;47:1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Ma JQ, Tuersun H, Jiao SJ, Zheng JH, Xiao JB, Hasim A. Functional role of NRF2 in cervical carcinogenesis. PLoS One. 2015;10:e0133876. doi: 10.1371/journal.pone.0133876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zha J, Chen F, Dong H, Shi P, Yao Y, Zhang Y, Li R, Wang S, Li P, Wang W, Xu B. Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J Transl Med. 2014;12:163. doi: 10.1186/1479-5876-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Pittman EF, Romaguera J, Fayad L, Wang M, Neelapu SS, McLaughlin P, Kwak L, McCarty N. Nuclear translocation of B-cell-specific transcription factor, BACH2, modulates ROS mediated cytotoxic responses in mantle cell lymphoma. PLoS One. 2013;8:e69126. doi: 10.1371/journal.pone.0069126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.