Abstract
Dexmedetomidine is a highly-selective α adrenergic receptor agonist, widely used as an anesthesia adjuvant drug in clinic. Effects of dexmedetomidine on autophagy of hippocampal neurons and cognitive dysfunction in aged rats under sevoflurane anesthesia were investigated. Sixty healthy Sprague-Dawley (SD) rats aged 4–6 months of either sex, weighing 150–200 g were selected and randomly divided into control group (n=10), sevoflurane group (n=25) and dexmedetomidine + sevoflurane group (compound group, n=25). The control group was inhaled with 60% O2 for 6 h, the sevoflurane group was inhaled with 3.4–3.6% sevoflurane for 6 h, and the compound group was treated with intraperitoneal injection of 4 µg/kg dexmedetomidine 1 h before the inhalation of sevoflurane. Five rats were taken from each group 1 h before sevoflurane anesthesia (T1), immediately after anesthesia (T2), 12 h after anesthesia (T3) and 24 h after anesthesia (T4), respectively. The expression levels of microtubule-associated protein 1 light-chain 3-I (LC3-I), LC3-II and Beclin-1 were detected via western blotting, and the LC3-II/LC3-I value was calculated. Moreover, Morris water maze test was performed for the five rats in each group 5 weeks after anesthesia to detect the cognitive function; the escape latency, times across platform and swimming time in target quadrant were recorded. The levels of LC3-I, LC3-II and Beclin-1 in hippocampal neurons and LC3-II/LC3-I value in sevoflurane group at T2 were significantly higher than those at T1, and they reached the peak at T3, but were decreased at T4 (P<0.05); the levels of proteins and LC3-II/LC3-I value in compound group at T2 were significantly higher than those at T1 and reached the peak, and remained unchanged at T3-T4; besides, the level of proteins and LC3-II/LC3-I values in compound group at T2-T4 were obviously lower than those in sevoflurane group (P<0.05). In sevoflurane group, the escape latency was prolonged, the times across platform were reduced and the swimming time in target quadrant was shortened compared with those in control group (P<0.05); these parameters were significantly improved in compound group compared with those in sevoflurane group and control group (P<0.05). In conclusion, dexmedetomidine can improve the cognitive dysfunction in aged rats under sevoflurane anesthesia, which is related to the decreased autophagy of hippocampal neurons.
Keywords: dexmedetomidine, sevoflurane, cognitive dysfunction, hippocampal neurons, autophagy
Introduction
Sevoflurane is a kind of commonly-used inhalational general anesthetics. A study has pointed out (1) that sevoflurane can lead to the decline in cognitive function in the recovery period, which may be related to the surgical trauma, anesthesia depth, body inflammation, stress and immune dysfunction. The elderly patients are often complicated with low metabolic function, poor operation and anesthesia tolerance, and many perioperative complications, so the postoperative cognitive dysfunction (POCD) occurs more easily after operation (2). Hippocampus is an important brain area involved in learning and memory ability, and the pathological changes, such as β-amyloid protein deposit and Tau protein phosphorylation, caused by hippocampal neurons are the important mechanisms of Alzheimer's disease (3); therefore, it is assumed that POCD caused by sevoflurane anesthesia may also be related to abnormalities in hippocampal neuronal structure and function. Cell autophagy is an important way for eukaryotes to degrade the misfolded proteins and damaged organelles in the body via lysosomes, and maintain the homeostasis, which is also known as type II programmed death (4). The decreased autophagy is associated with tumorigenesis (4), while the enhanced autophagy is related to the occurrence of neurodegenerative disease (5). Autophagosomes and specific proteins light-chain 3-I (LC3-I), LC3-II and Beclin-1 can reflect the autophagic activity of the body. Dexmedetomidine is a highly-selective α adrenergic receptor agonist, widely used as an anesthesia adjuvant drug in clinic, which is characterized by rapid onset of analgesia and sedation, small side effects, little inhibition on respiration and circulation and high safety (6,7). This study aimed to analyze the effects of dexmedetomidine on autophagy of hippocampal neurons and cognitive dysfunction in aged rats under sevoflurane anesthesia.
Materials and methods
Animals
Sixty healthy Sprague-Dawley (SD) rats aged 4–6 months of either sex, weighing 150–200 g were purchased from Laboratory Animal Center of Jilin University (certificate no.: SCXK (Ji) 2008–0004). They ate and drank freely at room temperature of 22±2°C and relative humidity of 55±2% to adapt to the environment for 1 week, followed by subsequent experiments. The study was approved by the Animal Ethics Committee of Nanchang University (Nanchang, China).
Research methods
The rats were randomly divided into control group (n=10), sevoflurane group (n=25) and dexmedetomidine + sevoflurane group (compound group, n=25). The control group was inhaled with 60% O2 for 6 h, the sevoflurane group was inhaled with 3.4–3.6% sevoflurane for 6 h, and the compound group was treated with intraperitoneal injection of 4 µg/kg dexmedetomidine 1 h before the inhalation of sevoflurane. Five rats were taken from each group before sevoflurane anesthesia (T1), immediately after anesthesia (T2), 12 h after anesthesia (T3) and 24 h after anesthesia (T4). The expression levels of microtubule-associated protein 1 LC3-I, LC3-II and Beclin-1 were detected via western blotting, and the LC3-II/LC3-I value was calculated. Moreover, Morris water maze test was performed for the five rats in each group 5 weeks after anesthesia to detect the cognitive function; the escape latency, times across platform and swimming time in target quadrant were recorded.
Materials
The rats were placed in a plastic airtight transparent container, then the container was placed in the ZD-600 thermostatic water bath to maintain the temperature of 37°C, and the bottom of the container was covered with calcium lime to absorb the CO2 generated; two side walls of the container were the air inlet and exhaust port; the air inlet was connected to the Vapor 2000 anesthetic gas volatilization pot (Dreager, Lübeck, Germany) for oxygen or sevoflurane delivery (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China), while the exhaust port was connected to the Vamos multifunctional anesthetic gas monitor (Dreager) for monitoring the sevoflurane and CO2 concentrations; the abdomen of rats was connected to the probe of oxygen saturation monitor (Philips Medical Systems B.V., Eindhoven, The Netherlands).
Western blotting
After anesthesia using chloral hydrate via intraperitoneal injection at a dose of 300–350mg/kg, the rats were sacrificed and the brain was removed, the hippocampus tissues were separated and placed in liquid nitrogen at −80°C. After hippocampus tissue homogenate, the centrifugation was performed at 4°C at 10,500 × g for 15 min; the supernatant was taken, and the protein concentration was detected using the bicinchoninic acid (BCA) protein quantification kit (Jiangsu Nanjing Beyotime Technology Co., Ltd., Jiangsu, China). The loading buffer was added, followed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE); the protein was transferred onto the polyvinylidene fluoride (PVDF) membrane. After sealing with blocking buffer at 37°C for 1 h, rabbit polyclonal LC3-I (dilution: 1:2,000; cat. no. ab48394), rabbit polyclonal LC3-II (dilution: 1:2,000; cat. no. ab192890), rabbit polyclonal Beclin-1 (dilution: 1:2,000; cat. no. ab207612) and rabbit polyclonal GAPDH (dilution: 1:500, cat. no. ab37168), purchased from Abcam (Cambridge, MA, USA), were added at 4°C overnight. After washing, the goat anti-mouse polyclonal secondary goat anti-mouse (HRP) IgG antibody (dilution: 1:2,000; cat. no. ab6789; Abcam, Cambridge, MA, USA) was incubated at 37°C for 4 h, followed by washing via phosphate buffered saline (PBS) and ECL development. The results were scanned and saved, and the semi-quantitative analysis was performed using the Lab Works4.5 gel imaging software (Invitrogen; Thermo Fisher Scientific, Inc.).
Morris water maze test
Morris water maze test included the place navigation and space exploration. In place navigation test, the platform was placed in the third quadrant, and the rats stood on the platform for 30 sec; the water was poured into the pool wall from different quadrants, and the time of finding and climbing on platform within 60 sec (escape latency) was recorded. After finding the platform, the rats stood for 30 sec and rested. If the rats failed to find the platform within 60 sec, they were led onto the platform to stay for 15 sec, and the escape latency was recorded as 60 sec. The test was repeated 4 times per day for 5 days, and the average was taken. In space exploratory test, the platform was removed, and the rat was put into the water optionally from the same entry point; the swimming trajectory within 60 sec was shot using a camera, the times across platform and the swimming time in original quadrant (target quadrant) were recorded.
Statistical analysis
Statistical analysis was performed using Statistical Product and Service Solutions (SPSS) 20.0 software (IBM Corp., Armonk, NY, USA). Measurement data are presented as mean ± standard deviation; one-way analysis of variance (ANOVA) was used for the comparison among groups, and least-significant difference (LSD) t-test was used for the pairwise comparison. The repeated measures analysis of variance (ANOVA) was used for the data comparisons at different time points. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of autophagy-associated proteins at different time points
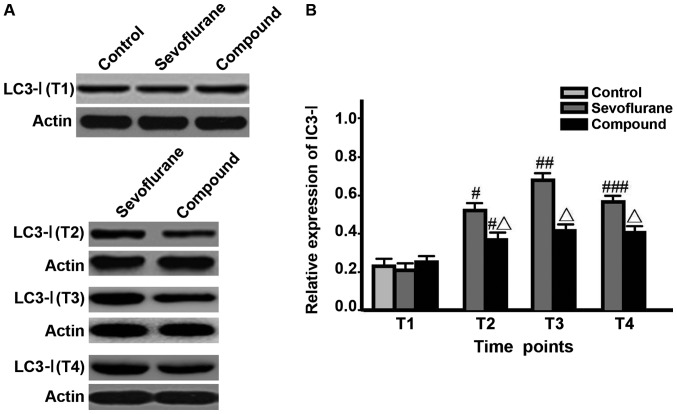
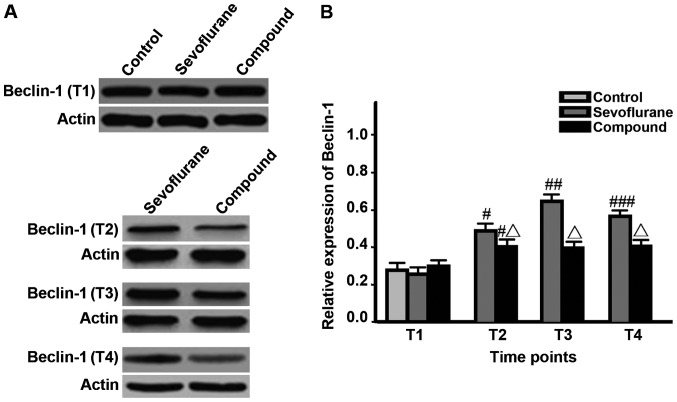
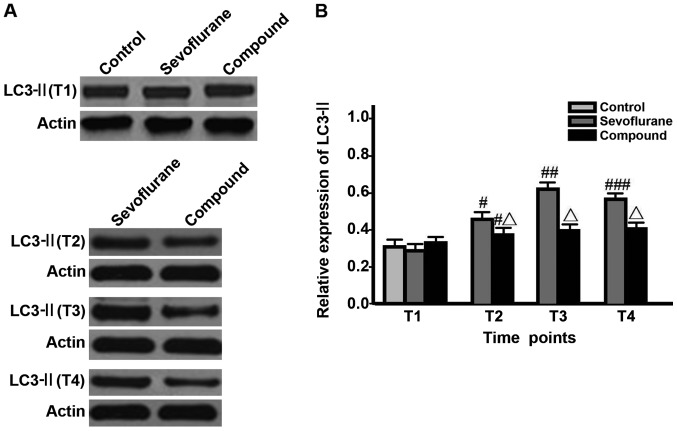
The levels of LC3-I, LC3-II and Beclin-1 in hippocampal neurons and LC3-II/LC3-I value in sevoflurane group at T2 were significantly higher than those at T1, and the value was the highest at T3, but were decreased at T4 (P<0.05); the levels of proteins and LC3-II/LC3-I value in compound group at T2 were significantly higher than those at T1 and reached the peak, and remained unchanged at T3-T4; besides, the levels of proteins and LC3-II/LC3-I values in compound group at T2-T4 were significantly lower than those in sevoflurane group (P<0.05) (Figs. 1–3, Table I).
Figure 1.
Detection of the LC3-I expression levels at different time points via western blotting (#P<0.05, comparisons of sevoflurane group and compound group at T2 with the same groups at T1; ##P<0.05, comparison between sevoflurane group at T3 and the same group at T2; ###P<0.05, comparison between sevoflurane group at T4 and the same group at T3; △P<0.05, comparisons between compound group at T2-T4 and sevoflurane group in the same time).
Figure 3.
Detection of the Beclin-1 expression levels in different time via western blotting (#P<0.05, comparisons of sevoflurane group and compound group at T2 with the same groups at T1; ##P<0.05, comparison between sevoflurane group at T3 and the same group at T2; ###P<0.05, comparison between sevoflurane group at T4 and the same group at T3; △P<0.05, comparisons between compound group at T2-T4 and sevoflurane group at the same time points).
Table I.
Comparison of LC3-II/LC3-I values at different time points.
| Groups | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Control | 1.05±0.26 | – | – | – |
| Sevoflurane | 1.03±0.23 | 0.89±0.13a | 0.92±0.15b | 0.77±0.14c |
| Compound | 1.07±0.28 | 0.67±0.14a,d | 0.68±0.16d | 0.66±0.12d |
P<0.05, comparison of sevoflurane group and compound group at T2 with the same groups at T1
P<0.05, comparison between sevoflurane group at T3 and the same group at T2
P<0.05, comparison between sevoflurane group at T4 and the same group at T3
P<0.05, comparisons between compound group at T2-T4 and sevoflurane group at the same time points.
Escape latency, times across platform and swimming time in target quadrant of rats
In sevoflurane group, the escape latency was prolonged, the times across platform were reduced and the swimming time in target quadrant was shortened compared with those in control group (P<0.05); these parameters were significantly improved in compound group compared with those in sevoflurane group and control group (P<0.05) (Table II).
Table II.
Escape latency, times across platform and swimming time in target quadrant of rats.
| Groups | Escape latency (s) | Times across platform (time) | quadrant (s) |
|---|---|---|---|
| Control | 26.7±4.5 | 2.3±0.3 | 14.6±2.3 |
| Sevoflurane | 33.5±4.9a | 1.6±0.3a | 10.4±2.1a |
| Compound | 28.2±4.6b | 2.1±0.4b | 13.2±2.4b |
P<0.05, comparison between sevoflurane group and control group
P<0.05, comparison between compound group and sevoflurane group.
Discussion
The median effective dose of dexmedetomidine analgesic on rats is 1 µg/kg (8). Dexmedetomidine improves the occurrence of cognitive dysfunction after sevoflurane anesthesia, which may be related to the clearance of damaged mitochondria, delayed apoptosis, inhibition of cytochrome c release, reduction of Caspase family activation, and reduction of apoptosis (9).
In this study, sevoflurane was used on anesthetized aged SD rats, and the effects of dexmedetomidine on hippocampal neurons autophagy and cognitive impairment in old rats were studied.
Morris water maze test is a common method to evaluate the spatial learning and memory ability of rodents (10). The results are reliable and repeatable. The study concluded that compared with rats in the control group, the rats in sevoflurane group had longer escape latency; they crossed the platform fewer times, and swam for a shorter period of time in the target quadrant. The condition of rats in the composite group was significantly better compared with that in sevoflurane group. Therefore, we believe that dexmedetomidine can improve the cognitive dysfunction of sevoflurane anesthesia in aged rats.
Liu et al (11) showed that the sevoflurane anesthesia-induced POCD in aged rats is related to the β-amyloid deposit and Tau protein phosphorylation of hippocampal neurons. Satomoto et al (12) pointed out that the sevoflurane anesthesia can cause behavioral changes and cognitive abnormalities in young rats. Autophagy is a defense behavior of the body against the environmental stimulus, and the autophagosomes and specific proteins LC3-I, LC3-II and Beclin-1 can reflect the autophagic activity of the body (13). LC3-I can bind with the phosphatidylethanolamin on the surface of autophagy membrane after the ubiquitin-like modification, forming LC3-II (13), both of which are two protein products in the autophagy process, and the LC3-II/LC3-I value is better in reflecting the stability of autophagy. Beclin-1 is a specific gene involved in autophagy regulation in mammals.
The downregulation of Beclin-1 expression indicates the decline in autophagy, which is related to the anti-apoptotic protein Bcl-2 (14). Beclin-1 contains the BH3 structural domain, which binds Beclin-1 and Bcl-2 to inhibit Beclin-1-dependent autophagy-activated pathway through the interaction with Bcl-2 surface BH3. However, the combination of Beclin-1 and Bcl-2 does not affect the fact that Bcl-2 binds to the pro-apoptotic protein Bax, playing an anti-apoptotic effect, through the structural domain (15). The results of this study showed that the levels of LC3-I, LC3-II and Beclin-1 in hippocampal neurons and LC3-II/LC3-I value in sevoflurane group at T2 were significantly higher than those at T1, and they reached the peak at T3, but decreased at T4; the level of proteins and LC3-II/LC3-I values in compound group at T2-T4 were obviously lower than those in sevoflurane group. Therefore, it is believed that sevoflurane anesthesia can up-regulate the autophagic activity of hippocampal neurons, and the anesthesia combined with dexmedetomidine can reduce the autophagic activity.
This study showed that the mechanism of sevoflurane anesthesia leading to the cognitive decline of rats was related to the increased autophagy of hippocampal neurons, providing an important reference basis for the clinical diagnosis and prevention of POCD. At the same time, the dexmedetomidine-assisted anesthesia can improve the cognitive function, providing an important idea for the application of dexmedetomidine.
Figure 2.
Detection of the LC3-II expression levels in different time via western blotting (#P<0.05, comparisons of sevoflurane group and compound group at T2 with the same groups at T1; ##P<0.05, comparison between sevoflurane group at T3 and the same group at T2; ###P<0.05, comparison between sevoflurane group at T4 and the same group at T3; △P<0.05, comparisons between compound group at T2-T4 and sevoflurane group at the same time points).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
CY conceived and designed the study, and drafted the manuscript. CY, ZF and XL collected, analyzed and interpreted the experimental data. ZF revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Animal Ethics Committee of Nanchang University (Nanchang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tao G, Zhang J, Zhang L, Dong Y, Yu B, Crosby G, Culley DJ, Zhang Y, Xie Z. Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3β activation in young mice. Anesthesiology. 2014;121:510–527. doi: 10.1097/ALN.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111:119–125. doi: 10.3238/arztebl.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120:4081–4091. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittington RA, Virág L, Gratuze M, Petry FR, Noël A, Poitras I, Truchetti G, Marcouiller F, Papon MA, El Khoury N, et al. Dexmedetomidine increases tau phosphorylation under normothermic conditions in vivo and in vitro. Neurobiol Aging. 2015;36:2414–2428. doi: 10.1016/j.neurobiolaging.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni J, Wei J, Yao Y, Jiang X, Luo L, Luo D. Effect of dexmedetomidine on preventing postoperative agitation in children: A meta-analysis. PLoS One. 2015;10:e0128450. doi: 10.1371/journal.pone.0128450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders RD, Giombini M, Ma D, Ohashi Y, Hossain M, Fujinaga M, Maze M. Dexmedetomidine exerts dose-dependent age-independent antinociception but age-dependent hypnosis in Fischer rats. Anesth Analg. 2005;100:1295–1302. doi: 10.1213/01.ANE.0000149595.41576.B3. [DOI] [PubMed] [Google Scholar]
- 9.Zhu M, Wang H, Zhu A, Niu K, Wang G. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: Different administration and different dosage. PLoS One. 2015;10:e0123728. doi: 10.1371/journal.pone.0123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Hu J, Liu X, Yan J. Effects of intravenous dexmedetomidine on emergence agitation in children under sevoflurane anesthesia: A meta-analysis of randomized controlled trials. PLoS One. 2014;9:e99718. doi: 10.1371/journal.pone.0099718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Xu J, Wang H, Xu C, Ji C, Wang Y, Feng C, Zhang X, Xu Z, Wu A, et al. Isoflurane-induced spatial memory impairment by a mechanism independent of amyloid-beta levels and tau protein phosphorylation changes in aged rats. Neurol Res. 2012;34:3–10. doi: 10.1179/1743132811Y.0000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 13.Zhou YF, Wang QX, Zhou HY, Chen G. Autophagy activation prevents sevoflurane-induced neurotoxicity in H4 human neuroglioma cells. Acta Pharmacol Sin. 2016;37:580–588. doi: 10.1038/aps.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao M, Liu D, Qiao L, Li N, Zheng J, et al. CHOP mediates ASPP2-induced autophagic apoptosis in hepatoma cells by releasing Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2. Cell Death Dis. 2014;5:e1323. doi: 10.1038/cddis.2014.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trisciuoglio D, de Luca T, Desideri M, Passeri D, Gabellini C, Scarpino S, Liang C, Orlandi A, Del Bufalo D. Removal of the BH4 domain from Bcl-2 protein triggers an autophagic process that impairs tumor growth. Neoplasia. 2013;15:315–327. doi: 10.1593/neo.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.