Abstract
Breast cancer is one of the most common cancer types in humans. Magnetic resonance imaging (MRI) is an efficient method for the detection of human breast cancer. However, the efficacy of MRI in detecting breast cancer in the early stage requires to be improved. The present study investigated the diagnostic efficacy of a combination of MRI and detection of gene expression in patients with breast cancer in the early stage. The gene expression levels of Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2 were used as an auxiliary diagnostic index for patients with breast cancer in the early stage. Higher expression levels of TPA and C2erbB22 were observed in tumor tissue obtained from diagnostic biopsy and determined by immunohistochemistry, which indicated a higher risk of breast cancer in a total of 84 participants. Diagnostic data revealed that combination MRI and detection of gene expression had a significantly higher diagnostic rate (66/84) in diagnosing breast cancer in an early stage compared with either MRI (78/360) or detection of gene expression (72/84; P<0.01). It was indicated that the combination of MRI and detection of gene expression had a higher diagnostic rate (94.5%) than either MRI (81.4%) or detection of gene expression (75.5%). Histological analysis confirmed the diagnosis determined by MRI and detection of gene expression. These results suggest that the combination of MRI and detection of gene expression may be a potential diagnostic method for assessing patients with early-stage breast cancer.
Keywords: breast cancer, gene expression, detection, magnetic resonance imaging, early diagnosis
Introduction
Breast cancer is one of the most common gynecological tumor types worldwide (1). The majority of breast cancer-associated mortalities is caused by local migration and distant metastases of tumor cells (2). While the current therapeutic schedules, including surgical techniques, as well as radiation, chemotherapy and gene targeted therapy, have improved the overall survival (OS) of breast cancer patients, the outcome remains generally poor (3,4). Of note, previous studies have indicated that numerous genes are involved in epigenetic modifications among patients with breast cancer (5–7). Genetic analyses and testing for inherited gene mutations in patients with breast cancer have been reported in clinical studies (8,9). Therefore, it is essential to explore potential genes for diagnosing patients with breast cancer in the early stage.
At present, ultrasound, fluorodeoxyglucose-positron emission tomography (PET)/computed tomography (CT) and magnetic resonance imaging (MRI) are widely used for diagnosing and staging of human breast cancer (10–12). Eminently, MRI provides a higher sensitivity and accuracy in the detection of breast cancer than CT and ultrasound (13). Application of dynamic contrast enhancement MRI and post-processing techniques are helpful for making the correct diagnosis for patients with suspected breast cancer (14). Youk et al (15) have suggested that MRI may be used to identify malignant breast lesions by analyzing their morphological and kinetic features. In addition, MRI is more efficient than CT in assessing the response to neo-adjuvant chemotherapy and is beneficial for identifying the primary tumor in breast cancer patients (16). Furthermore, comparison between PET/CT and MRI in the diagnosis, staging and follow-up of breast cancer patients revealed that MRI is useful for distinguishing between benign and malignant pulmonary nodules, has a high sensitivity and specificity for nodal staging, and is helpful for evaluating the early response to systemic chemotherapy (17). However, the diagnostic accuracy of single MRI for breast cancer is insufficient (18). In recent years, genetic diagnosis of breast cancer has been applied, which may be an accurate and novel diagnostic method for the evaluation of sentinel lymph node metastasis in breast cancer patients (19–21).
In the present study, the diagnostic efficacy of MRI in combination with detection of gene expression in breast cancer patients was evaluated. It was reported that MRI combined with detection of gene expression not only improves the accuracy, but also contributes to the selection of efficient treatments for patients with breast cancer.
Materials and methods
Patients and selection
A total of 84 female patients (median age, 46.2 years; range, 28.4–65.5 years) with suspected breast cancer who were assessed at the Radiology Department of Beijing Tiantan Hospital (Beijing, China) between May 2015 and October 2016 were recruited for the present study. All patients underwent pre-operative MRI and/or detection of gene expression (Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2). All patients were finally diagnosed by histopathology in diagnostic biopsy samples. Patients with a family history of cancer, chronic renal failure and heart disease, as well as those with a history of ipsilateral breast surgery, chest radiotherapy and oncoplastic breast cancer were excluded from the study. Patients who received neoadjuvant chemotherapy or radiotherapy were also excluded from the recruitment. The present study was approved by the Ethics Committee of Capital Medical University (Beijing, China). All patients provided written informed consent.
MRI analysis
The MRI protocol was in accordance with that described in a previous study (22). In brief, a Siemens Verio 3.0 T magnet MRI machine (Siemens AG, Munich, Germany) was used to analyze breast tumor lesions and determine their volume. All patients with suspected breast cancer were subjected to MRI screening. MR images were analyzed using a SUN T2000 workstation (Sun Microsystems Inc., Mountain View, CA, USA) with the Eigentool image analysis software 3.0 (Image Analysis Lab, Henry Ford Hospital, Detroit, MI, USA). Threshold ranges were determined using histogram analysis from regions of interest placed around the identified lesion using 95% confidence intervals determined from signal intensity.
Detection of gene expression
Total RNA was extracted from tumor cells (1.0 µg) in biopsy samples using an RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Total RNA (1 µg) was reverse transcribed into complementary (c)DNA using a QuantiTect Reverse Transcription kit (cat. no. 205310; Qiagen) according to the manufacturer's protocol. The cDNA (10 ng) was subjected to quantitative polymerase chain reaction analysis using SYBR Green Master mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All primers were synthesized by Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA) as described previously (23). The reaction conditions were accordance with those of a previous study (24). Relative mRNA expression changes were calculated using the 2−ΔΔCq method (25). The results are expressed as a fold of the control.
Histopathology
Breast tumor staging was performed based on the American Joint Committee on Cancer Staging manual, sixth edition (26). Histological analysis was performed using the modified Bloom-Richardson classification (27). Primary breast cancer tissues were evaluated from formalin-fixed, paraffin-embedded tumor sections using immunohistochemistry. Tumor sections were incubated with primary antibodies rabbit anti-human against estrogen receptor (ER; 1:1,000; cat. no. clone SP1; Neomarkers for Lab Vision, Fremont, CA, USA) and progesterone receptor (1:1,000, PR; cat. no. clone PgR 636; Dako, Glostrup, Denmark) for 12 h at 4°C. Tumor tissues were then incubated with using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G monoclonal antibody (1:1,000, cat. no. PV-6001; Zhongshan Goldenbridge-BIO, Beijing, China) for 2 h at 37°C. A Ventana Benchmark automated staining system (Olympus BX51, Olympus; Tokyo, Japan) was used to assess protein expression in tumor tissues. The staining results were semi-quantitatively evaluated by multiplying the staining intensity and the percentage of positively stained cells (magnification, ×400). The cutoff value for ER and PR positivity was >10% staining on immunohistochemistry (27).
Treatment
Standard treatments for patients with breast cancer in the present study were in accordance with those described in a previous study (28). In brief, breast cancer patients received breast surgery and breast radiotherapy.
Statistical analysis
Values are expressed as the mean ± standard deviation of triplicate experiments. Pearson's correlation analysis was performed to assess correlations. All data were analyzed with SPSS version 20 (IBM Corp., Armonk, NY, USA) using one-way analysis of variance followed by Tukey's multiple comparison post-hoc test. The OS rates were calculated using the Kaplan-Meier method and the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
MRI diagnosis of patients with breast cancer
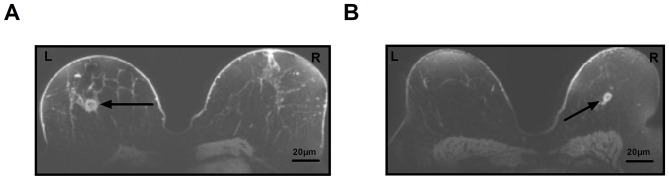
MRI was used to diagnose patients with suspected breast cancer in a total of 84 cases. In a total of 78 patients, a breast lump was detected, which required further confirmation by pathological analysis. Representative MRI images of breast lumps in patients with suspected breast cancer are displayed in Fig. 1.
Figure 1.
Diagnosis of breast cancer by magnetic resonance imaging. Representative images of breast cancer tissue. (A) Tumor lesion (arrow) in the left breast. (B) Tumor lesion (arrow) in the right breast (magnification, ×0.25). Scale bar, 20 µm. L, left breast; R, right breast.
Analysis of breast cancer-associated gene expression in biopsy samples of patients with breast cancer
To identify differences in gene expression in patients with breast cancer, the gene expression levels of Ki-67, B-cell CLL/lymphoma 11a (BCL11A), forkhead box (FOX)C1, homeobox (HOX)D13; protocadherin γ subfamily B, 7 (PCDHGB7) and her-2 were detected in patients with breast cancer. The analysis identified 72 patients with elevated gene expression of Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2, while 32 patients had lower expression (Table I). Of the 78 patients in which a breast lump was detected on MRI, 72 had higher gene expression. The present study demonstrated that there were a total of 66 patients with and 12 patients without breast tumors (Table I).
Table I.
Gene expression in patients with suspected breast cancer.
| Gene | Patients with breast tumors (n=66; %) | Individuals without tumors (n=12; %) |
|---|---|---|
| Ki-67 | 9.74±2.47 | 2.2±1.2 |
| BCL11A | 16.2±4.2 | 1.8±0.7 |
| FOXC1 | 8.4±3.2 | 2.3±1.0 |
| HOXD13 | 7.5±3.0 | 2.6±1.5 |
| PCDHGB7 | 6.8±2.6 | 1.7±0.7 |
| Her-2 | 9.3±3.5 | 2.5±1.5 |
BCL11A, B-cell CLL/lymphoma 11a; FOX, forkhead box; HOX, homeobox; PCDHGB7, protocadherin γ subfamily B, 7. Values are expressed as the mean ± SD.
Pathological analysis of breast cancer tissue
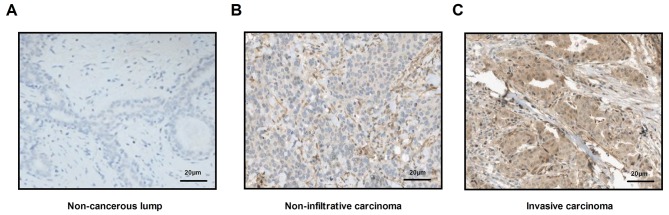
Histopathological analysis was used to confirm whether patients had breast cancer. Analyses demonstrated that all cancer tissues were either non-infiltrative carcinoma or early invasive carcinoma. Representative images of non-cancerous lump, as well as non-infiltrative carcinoma and invasive carcinoma tissue sections are displayed in Fig. 2. A total of 66 patients were finally diagnosed with breast cancer, including 45 cases of non-infiltrative carcinomas and 21 cases of invasive carcinoma (Table II).
Figure 2.
Pathological analysis of breast cancer tissue. Immunohistochemistry was performed to analyze normal breast and breast cancer tissue (magnification, ×40). (A) Non-cancerous lump. (B) Non-infiltrative breast cancer tissue. (C) Invasive breast carcinoma tissue. Scale bar, 20 µm.
Table II.
Characteristics of the groups, divided by pathological analysis.
| Type | n | Tumor size (cm) |
|---|---|---|
| Non-infiltrative carcinoma | 45 | <2 |
| Invasive carcinoma | 21 | >2 |
| Healthy individuals | 16 | 0 |
Efficacy of combined diagnosis of MRI and detection of gene expression for patients with breast cancer
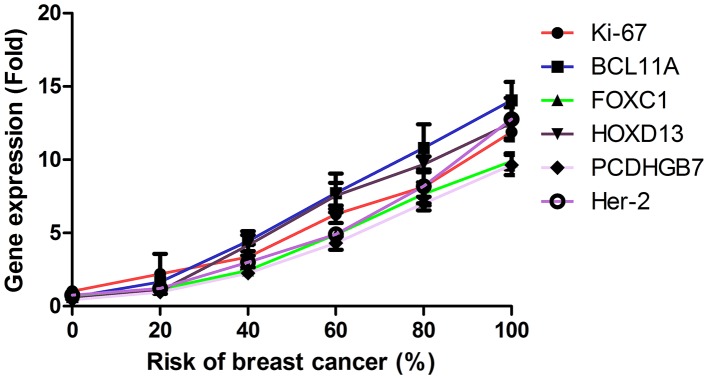
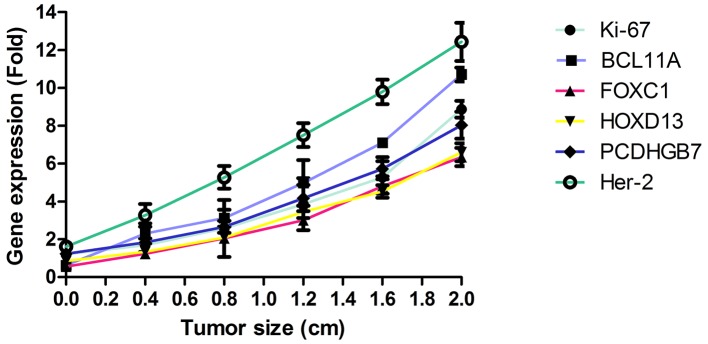
The efficacy of combined diagnosis of MRI and detection of gene expression was evaluated in patients with breast cancer. Pearson's R test was used to assess the correlation between gene expression and the risk of breast cancer. A significant positive correlation was observed between expression levels of the genes Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2, and the risk of breast cancer (Pearson's R=0.516; P<0.01; Fig. 3). In addition, a significant positive correlation was observed between gene expression levels and tumor size in patients with breast cancer (Pearson's R=0.410; P<0.01; Fig. 4). It was demonstrated that the efficacy of combined diagnosis by MRI and detection of gene expression improved the diagnostic rate compared with that achieved by either MRI or detection of gene expression alone (Fig. 5).
Figure 3.
Correlation between gene expression levels of Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2 and the risk of breast cancer. The mean gene expression levels were determined by reverse transcription-quantitative polymerase chain reaction analysis. BCL11A, B-cell CLL/lymphoma 11a; FOX, forkhead box; HOX, homeobox; PCDHGB7, protocadherin γ subfamily B7.
Figure 4.
Correlation between gene expression levels and the tumor size of breast cancer. BCL11A, B-cell CLL/lymphoma 11a; FOX, forkhead box; HOX, homeobox; PCDHGB7, protocadherin γ subfamily B7.
Figure 5.
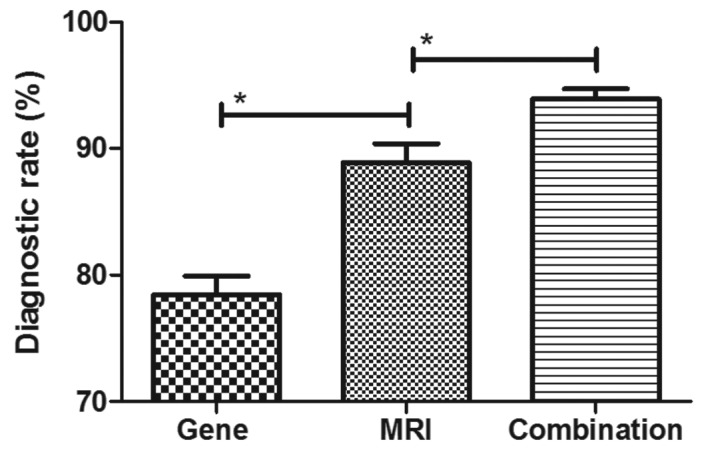
Diagnostic efficacy of a combination of MRI and gene detection for breast cancer patients. *P<0.01. MRI, magnetic resonance imaging.
Association between gene expression levels and breast cancer subtypes
The present study then analyzed the association between gene expression levels and the breast cancer subtype, including non-infiltrative carcinoma and early invasive carcinoma. As presented in Fig. 6, breast tumor-associated genes were lower expressed in non-infiltrative carcinoma compared with early invasive carcinoma.
Figure 6.
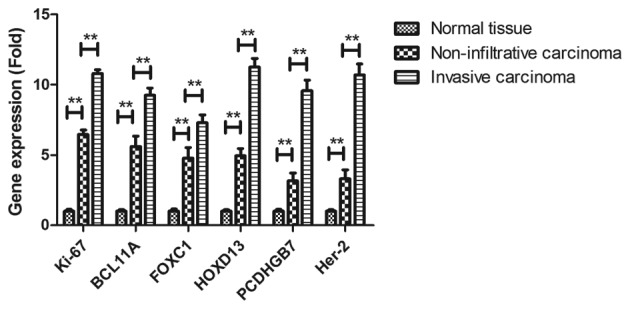
Breast cancer-associated mean gene expression of Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2 levels in non-infiltrative carcinoma and early invasive carcinoma (n=8). **P<0.01.
Survival of breast cancer patients diagnosed by MRI and detection of gene expression
The survival of breast cancer patients diagnosed by MRI and detection of gene expression enrolled in the present study was then investigated. A significant improvement of patients' survival time was observed after diagnosis by MRI and detection of gene expression compared with the mean survival of breast cancer patients diagnosed by MRI and detection of gene expression (Fig. 7). These outcomes suggest that diagnosis with a combination of MRI and detection of gene expression contributes to the early implementation of treatment and therefore patient survival.
Figure 7.
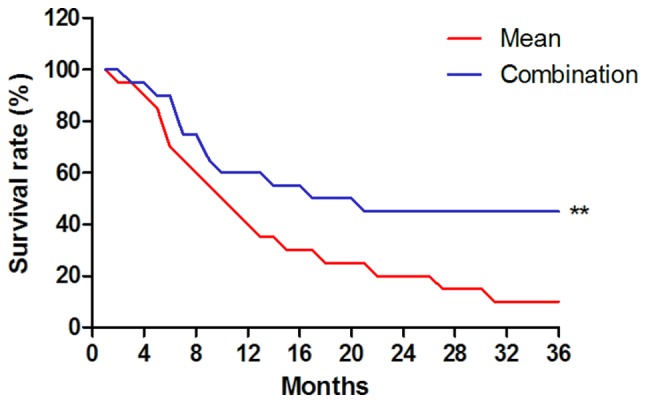
Survival of breast cancer patients diagnosed by magnetic resonance imaging and gene detection. **P<0.01 vs. mean survival of breast cancer patients.
Discussion
The efficacy of contrast-enhanced MRI in the diagnosis of breast cancer has been previously proven (29). In addition, diagnosis of breast cancer using malignancy-associated biomarkers has been widely accepted (30). The present study analyzed the efficacy of the combination of MRI and detection of gene expression in diagnosing patients with breast cancer in the early stage. The results indicated that combination of MRI and detection of gene expression not only markedly improved the diagnostic accuracy, but also contributed to early tumor treatments, which further resulted in a long survival time of breast cancer patients.
Although various methods for the early-stage diagnosis of breast cancer have been introduced, including contrast-enhanced MRI, it is difficult to differentiate between breast tumors and normal lumps in women with suspected breast cancer (31–33). Molecular diagnosis comprising the detection of high-frequency mutations in breast cancer patients has provided a novel strategy for the early diagnosis of human breast cancer (34). In the present study, the expression levels of six genes, namely Ki-67, BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2, was determined to analyze the risk of breast cancer in a total of 84 patients with suspected breast cancer. The results indicated a significant positive correlation between gene expression levels and the risk of breast cancer, as well as between gene expression levels and tumor size in patients with breast cancer. In the present study, gene expression analysis identified 72 patients with an elevated risk of breast cancer among 84 suspicious patients.
Ki-67 has been regarded as a prognostic marker based on a molecular subtype of breast cancer (35). The present study reported that Ki-67 expression was higher in invasive carcinoma compared with that in non-infiltrative carcinoma. BCL11A is overexpressed in triple-negative breast cancer compared with that in normal mammary epithelial cells (36). In the present study, BCL11A was 12–20-fold increased in tumor biopsy samples in patients with breast cancer compared with that non-malignant breast lumps in healthy individuals. A previous study has indicated that FOXC1 overexpression is a marker of poor response to anthracycline-based adjuvant chemotherapy in triple-negative breast cancer (37). In addition, the prognostic significance of HOXD13 expression in human breast cancer has been elucidated and a previous study indicated that HOXD13 is a potential prognostic marker for patients with breast cancer (38). Furthermore, quantification of her-2 expression in immunohistochemically-identified biopsies can be used to diagnose breast cancer (39). The present study indicated that the expression levels of BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2 were upregulated in patients with breast cancer. The present study analyzed the diagnostic efficacy of the combination of MRI and detection of gene expression for patients with breast cancer in the early stage.
Studies have indicated that MRI is an accurate diagnostic method for patients with breast cancer (40,41). The present study reported that out of 78 patients in whom a breast lump was detected on MRI, 72 were indicated to have breast cancer according to detection of gene expression. In fact, only 66 patients were confirmed to have breast cancer by pathological analysis. The results of the present study also identified that MRI and detection of gene expression alone are not sufficient for breast cancer diagnosis. The results of the present study also indicated that breast tumor-associated genes were lower expressed in non-infiltrative carcinoma compared with those in in early invasive carcinoma. Of note, the survival time of patients diagnosed by MRI and detection of gene expression was longer than the mean survival of breast cancer patients reported previously (42).
In conclusion, the present study analyzed the efficacy of MRI and detection of gene expression in diagnosing breast cancer patients. It was demonstrated that the detection of the gene expression levels of BCL11A, FOXC1, HOXD13, PCDHGB7 and her-2 may be regarded as an auxiliary method for MRI in diagnosing human breast cancer. However, further studies in large populations of patients with suspected breast cancer are required to determine the combined diagnostic efficacy of MRI and detection of gene expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DK, RY, LJ analyzed and interpreted the patient data regarding the hematological disease and the transplant. DK performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Capital Medical University (Beijing, China). All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Novik AV, Protsenko SA, Baldueva IA, Ivantsov AO, Nekhaeva TL, Akhaeva ZY, Yanus GA, Iyevleva AG, Imyanitov EN. Vemurafenib-induced progression of breast cancer: A case report and review of the literature. Target Oncol. 2016;11:235–238. doi: 10.1007/s11523-015-0384-7. [DOI] [PubMed] [Google Scholar]
- 2.Araki K, Ito Y. A review multigene assays for Clinical Utility in breast cancer. Gan to kagaku ryoho. Gan To Kagaku Ryoho. 2016;43:1332–1340. (In Japanese) [PubMed] [Google Scholar]
- 3.Plourde N, Brown HK, Vigod S, Cobigo V. Contextual factors associated with uptake of breast and cervical cancer screening: A systematic review of the literature. Women Health. 2016;56:906–925. doi: 10.1080/03630242.2016.1145169. [DOI] [PubMed] [Google Scholar]
- 4.Post KE, Flanagan J. Web based survivorship interventions for women with breast cancer: An integrative review. Eur J Oncol Nurs. 2016;25:90–99. doi: 10.1016/j.ejon.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Thivya KS, Sakthivel P, Venkata Sai PM. Analysis of framelets for breast cancer diagnosis. Technol Health Care. 2016;24:21–29. doi: 10.3233/THC-151042. [DOI] [PubMed] [Google Scholar]
- 6.Korkmaz SA, Korkmaz MF, Poyraz M. Diagnosis of breast cancer in light microscopic and mammographic images textures using relative entropy via kernel estimation. Med Biol Eng Comput. 2016;54:561–573. doi: 10.1007/s11517-015-1361-0. [DOI] [PubMed] [Google Scholar]
- 7.Zuo X, Chen L, Liu L, Zhang Z, Zhang X, Yu Q, Feng L, Zhao X, Qin T. Identification of a panel of complex autoantigens (LGALS3, PHB2, MUC1, and GK2) in combination with CA15-3 for the diagnosis of early-stage breast cancer. Tumour Biol. 2016;37:1309–1317. doi: 10.1007/s13277-015-3932-y. [DOI] [PubMed] [Google Scholar]
- 8.Wang DY, Done SJ, McCready DR, Boerner S, Kulkarni S, Leong WL. A new gene expression signature, the ClinicoMolecular Triad Classification, may improve prediction and prognostication of breast cancer at the time of diagnosis. Breast Cancer Res. 2011;13:R92. doi: 10.1186/bcr3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miki Y. Gene expression-based diagnosis of efficacy of chemotherapy for breast cancer. Breast Cancer. 2010;17:97–102. doi: 10.1007/s12282-009-0180-2. [DOI] [PubMed] [Google Scholar]
- 10.Richman I, Asch SM, Bendavid E, Bhattacharya J, Owens DK. Breast density notification legislation and breast cancer stage at diagnosis: Early evidence from the SEER registry. J Gen Intern Med. 2017;32:603–609. doi: 10.1007/s11606-016-3904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdoli G, Bottai M, Sandelin K, Moradi T. Breast cancer diagnosis and mortality by tumor stage and migration background in a nationwide cohort study in Sweden. Breast. 2017;31:57–65. doi: 10.1016/j.breast.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Tang S, Zhou F, Sun Y, Wei L, Zhu S, Yang R, Huang Y, Yang J. CEA in breast ductal secretions as a promising biomarker for the diagnosis of breast cancer: A systematic review and meta-analysis. Breast Cancer. 2016;23:813–819. doi: 10.1007/s12282-016-0680-9. [DOI] [PubMed] [Google Scholar]
- 13.Kruger S, Mottaghy FM, Buck AK, Maschke S, Kley H, Frechen D, Wibmer T, Reske SN, Pauls S. Brain metastasis in lung cancer. Comparison of cerebral MRI and 18F-FDG-PET/CT for diagnosis in the initial staging. Nuklearmedizin. 2011;50:101–106. doi: 10.3413/Nukmed-0338-10-07. [DOI] [PubMed] [Google Scholar]
- 14.Peng KQ, Huang ZL, Xie CM, Chen L, Ouyang Y, Zheng QS, Zhang Y, He HQ, Wu PH. Application of dynamic contrast enhancement MRI and post-processing technique for diagnosis of breast cancer. Ai Zheng. 2009;28:549–554. (In Chinese) [PubMed] [Google Scholar]
- 15.Youk JH, Son EJ, Kim EK, Kim JA, Kim MJ, Kwak JY, Lee SM. Diagnosis of breast cancer at dynamic MRI in patients with breast augmentation by paraffin or silicone injection. Clin Radiol. 2009;64:1175–1180. doi: 10.1016/j.crad.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011;378:1804–1811. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Cheng T, Xu W, Yan WL, Liu J, Yang HL. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40:523–531. doi: 10.1007/s00256-010-0963-8. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima H, Inokuchi M, Furukawa H, Kitamura S. Accuracy for a diagnosis of breast cancer spread using 3.0T MRI. Nihon rinsho. Nihon Rinsho. 2012;70(Suppl 7):S5306–S5308. (In Japanese) [PubMed] [Google Scholar]
- 19.Kwong A, Chen J, Shin VY, Ho JC, Law FB, Au CH, Chan TL, Ma ES, Ford JM. The importance of analysis of long-range rearrangement of BRCA1 and BRCA2 in genetic diagnosis of familial breast cancer. Cancer Genet. 2015;208:448–454. doi: 10.1016/j.cancergen.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Woodson AH, Muse KI, Lin H, Jackson M, Mattair DN, Schover L, Woodard T, McKenzie L, Theriault RL, Hortobágyi GN, et al. Breast cancer, BRCA mutations, and attitudes regarding pregnancy and preimplantation genetic diagnosis. Oncologist. 2014;19:797–804. doi: 10.1634/theoncologist.2014-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derks-Smeets IA, de Die-Smulders CE, Mackens S, van Golde R, Paulussen AD, Dreesen J, Tournaye H, Verdyck P, Tjan-Heijnen VC, Meijer-Hoogeveen M, et al. Hereditary breast and ovarian cancer and reproduction: an observational study on the suitability of preimplantation genetic diagnosis for both asymptomatic carriers and breast cancer survivors. Breast Cancer Res Treat. 2014;145:673–681. doi: 10.1007/s10549-014-2951-5. [DOI] [PubMed] [Google Scholar]
- 22.Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, Kim SG, Moy L. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: Correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol. 2016;85:815–823. doi: 10.1016/j.ejrad.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Ethier JL, Amir E. The Role of the 21-gene recurrence score in breast cancer treatment. Mol Diagn Ther. 2016;20:307–313. doi: 10.1007/s40291-016-0209-0. [DOI] [PubMed] [Google Scholar]
- 24.Hoogland AM, Bottcher R, Verhoef E, Jenster G, van Leenders GJ. Gene-expression analysis of gleason grade 3 tumor glands embedded in low- and high-risk prostate cancer. Oncotarget. 2016;7:37846–37856. doi: 10.18632/oncotarget.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Printz C. New AJCC cancer staging manual reflects changes in cancer knowledge. Cancer. 2010;116:2–3. doi: 10.1002/cncr.24848. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, Park S, Kim JH, Lee JH, Choi SY, Park BW, Lee KS. Clinicopathologic features and outcomes of metaplastic breast carcinoma: Comparison with invasive ductal carcinoma of the breast. Yonsei Med J. 2010;51:864–869. doi: 10.3349/ymj.2010.51.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JH, Park JM, Park HS, Park S, Kim SI, Park BW. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J Surg Oncol. 2013;108:531–536. doi: 10.1002/jso.23439. [DOI] [PubMed] [Google Scholar]
- 29.Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G, Moschetta M. Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn Reson Imaging. 2016;34:173–176. doi: 10.1016/j.mri.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Masood S, El-Gabry E, Zhang C, Wang Z. The potential of identification of a malignancy-associated biomarker in breast cancer diagnosis and research: hTERT gene DNA methylation. Diagn Cytopathol. 2016;44:670–675. doi: 10.1002/dc.23505. [DOI] [PubMed] [Google Scholar]
- 31.Yoon H, Yoon D, Yun M, Choi JS, Park VY, Kim EK, Jeong J, Koo JS, Yoon JH, Moon HJ, et al. Metabolomics of Breast Cancer Using High-Resolution Magic Angle Spinning Magnetic Resonance Spectroscopy: Correlations with 18F-FDG Positron Emission Tomography-Computed Tomography, Dynamic Contrast-Enhanced and Diffusion-Weighted Imaging MRI. PLoS One. 2016;11:e0159949. doi: 10.1371/journal.pone.0159949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, Berg WA, Zuley ML, Kurland BF, Jankowitz RC, Nishikawa R, Gur D, Sumkin JH. Breast MRI contrast enhancement kinetics of normal parenchyma correlate with presence of breast cancer. Breast Cancer Res. 2016;18:76. doi: 10.1186/s13058-016-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung MT, Lourenco AP, Mainiero MB. Screening Breast MRI in Women with a Personal History of Breast Cancer. Breast J. 2016;22:252–253. doi: 10.1111/tbj.12563. [DOI] [PubMed] [Google Scholar]
- 34.Mannan AU, Singh J, Lakshmikeshava R, Thota N, Singh S, Sowmya TS, Mishra A, Sinha A, Deshwal S, Soni MR, et al. Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: Implications of embracing a multi-gene panel in molecular diagnosis in India. J Hum Genet. 2016;61:515–522. doi: 10.1038/jhg.2016.4. [DOI] [PubMed] [Google Scholar]
- 35.Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. 2016;13:496–504. doi: 10.20892/j.issn.2095-3941.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaled WT, Choon Lee S, Stingl J, Chen X, RazaAli H, Rueda OM, Hadi F, Wang J, Yu Y, Chin SF, et al. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun. 2015;6:5987. doi: 10.1038/ncomms6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu YL, Yao R, Li J, Zhou YD, Mao F, Pan B, Sun Q. FOXC1 overexpression is a marker of poor response to anthracycline-based adjuvant chemotherapy in sporadic triple-negative breast cancer. Cancer Chemother Pharmacol. 2017;79:1205–1213. doi: 10.1007/s00280-017-3319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong ZB, Shan M, Qian C, Liu T, Shi QY, Wang J, Liu Y, Liu Y, Huang YX, Pang D. Prognostic significance of HOXD13 expression in human breast cancer. Int J Clin Exp Pathol. 2015;8:11407–11413. [PMC free article] [PubMed] [Google Scholar]
- 39.Mendoza G, Portillo A, Olmos-Soto J. Accurate breast cancer diagnosis through real-time PCR her-2 gene quantification using immunohistochemically-identified biopsies. Oncol Lett. 2013;5:295–298. doi: 10.3892/ol.2012.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swayampakula AK, Dillis C, Abraham J. Role of MRI in screening, diagnosis and management of breast cancer. Expert Rev Anticancer Ther. 2008;8:811–817. doi: 10.1586/14737140.8.5.811. [DOI] [PubMed] [Google Scholar]
- 41.Tozaki M. Diagnosis of breast cancer: MDCT versus MRI. Breast Cancer. 2008;15:205–211. doi: 10.1007/s12282-008-0049-9. [DOI] [PubMed] [Google Scholar]
- 42.Schwentner L, Wolters R, Wischnewsky M, Kreienberg R, Wockel A. Survival of patients with bilateral versus unilateral breast cancer and impact of guideline adherent adjuvant treatment: A multi-centre cohort study of 5292 patients. Breast. 2012;21:171–177. doi: 10.1016/j.breast.2011.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.