Abstract
Neuroblastoma is one of the most common and deadly childhood cancers. Neuroblastoma arises from transformed cells of the neural crest lineage. Outcomes of the disease vary greatly, ranging from spontaneous regression to aggressive metastases. While this variability may reflect the inherent migratory capabilities and multipotency of neural crest cells, there have been few direct comparisons between neuroblastoma and embryonic neural crest cells, in part because of the limited in vivo accessibility of the mammalian neural crest lineage. Our recent studies demonstrate a novel link between anaplastic lymphoma kinase (ALK) and glycogen synthase kinase 3 (GSK3). Our work suggests that ALK-dependent regulation of GSK3 via tyrosine phosphorylation may alter the substrate specificity of GSK3, thus regulating cytoskeletal dynamics in migrating neural crest cells.
Keywords: Neuroblastoma, neural crest, mouse, human, anaplastic lymphoma kinase, ALK, glycogen synthase kinase 3, GSK3, cell migration
Comment on: Gonzalez Malagon SG, Lopez Muñoz AM, Doro D, et al. Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus. Nat Commun. 2018;9:1126. doi:10.1038/s41467-018-03512-5. PubMed PMID: 29555900; PubMed Central PMCID: PMC5859133. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5859133
Neural Crest Origins of Neuroblastoma
Neuroblastoma, one of the most common pediatric solid tumors, is thought to arise from multipotent neural crest cells (NCCs), which give rise to a variety of cell types, including the sympathetic nervous system. Indeed, simply forcing the expression of transcription factor N-Myc in NCCs is sufficient to drive a neuroblastoma-like phenotype.1 The prognosis appears linked to the differentiation status of the cells. When the tumor cells are relatively undifferentiated, the prognosis is poor. Inducing differentiation toward neuronal lineages through the therapeutic use of retinoic acid is sometimes an effective cure.2,3 This similarity to naïve, multipotent, embryonic NCCs sets up a model where neuroblastoma is the result of the delay or blockade of the developmental progression of the neural crest lineage.
We have some understanding of activation mutations in neuroblastoma.4 One of the key factors is anaplastic lymphoma kinase (ALK), a multifaceted tyrosine kinase. Prior to our work, ALK had not been functionally analyzed in NCCs. In our recent work, we directly compared embryonic NCCs with a panel of neuroblastoma cells.5 We found that ALK is indeed expressed in the embryonic neural crest and that the activity of ALK is parallel to the activation of glycogen synthase kinase 3 (GSK3), another pleiotropic kinase, which is required at multiple steps in neural crest development.6,7 Based on our data, we hypothesized that ALK may regulate GSK3 by tyrosine phosphorylation during normal neural crest development and that dysregulation of this kinase cascade might underlie the pathology of neuroblastoma.
ALK Interactions in Neuroblastoma
Activating mutations in the ALK oncogene are among the leading causes for hereditary neuroblastoma (NB). These mutations are somatically acquired in approximately 10% of sporadic cases of neuroblastoma; thus, ALK has been considered as a potential treatment target.8 The most common mutations found are F1174L and R1275Q; both lie within the ALK kinase domain. These mutations lead to ligand-independent auto-phosphorylation of ALK and increased kinase activity when compared with wild-type ALK.9,10 Activation of ALK in neuroblastoma is normally associated with poor prognosis, contributing to increased cell proliferation, survival, and migration. Interestingly, some studies have reported a close association of ALK with N-myc amplification, although the links are unclear. This association has been related to aggressive and metastatic neuroblastoma,11 presumably via a model in which the cells are prevented from differentiating. However, in other studies, this correlation was not confirmed; therefore, the possibility of synergy between these 2 oncogenes remains questionable12; thus, alternative links/effects should be explored.
Regulation of GSK3 Activity Is Complex
Glycogen synthase kinase 3 proteins are highly abundant cellular kinases reported to have many substrates. In vertebrates, GSK3 proteins are encoded by 2 genes, GSK3α and GSK3β, which differ in the n- and c-terminal domains but are nearly identical in the kinase domain. While GSK3 is thought to be constitutively active in resting cells, it is clear that GSK3 activity levels can be dependent on phosphorylation of residues on GSK3 itself. Inactivation of GSK3 can occur via phosphorylation of N-terminal serines (S21 on GSK3α, S9 on GSK3β). This results in the N-terminus acting as a pseudosubstrate for GSK3. When phosphorylated, the N-terminus blocks access of other potential GSK3 substrates. However, as mice carrying non-phosphorylatable GSK3 variants (in which S21/S9 are mutated to alanines13) can still be inhibited in the context of Wnt signaling, there must be alternative mechanisms of GSK3 regulation.
Briefly, GSK3 is one component of the “destruction complex” of β-catenin, an effector protein of Wnt signaling. In resting cells, this complex remains in its active form in which GSK3, along with other kinases, phosphorylates β-catenin. Phosphorylated β-catenin is then ubiquitinated and targeted for proteasomal degradation. However, in response to Wnt signals, the cells respond by disassembling the destruction complex. GSK3 is no longer active and cannot phosphorylate β-catenin due to a loss of physical proximity. As β-catenin accumulates in the cytosol, it can then be translocated to the nucleus to activate transcriptional targets. One possibility is that dedicated pools of GSK3 exist within the cell, likely in complex with different partner proteins, and that these pools of GSK3 can be activated or inactivated independently. Thus, regulation of GSK3 is clearly more complicated than a simple inhibitory phosphorylation.
Positive Regulation of GSK3 via ALK Tyrosine Kinase
Because the presumption is that GSK3 proteins are “constitutively” active, positive regulatory mechanisms have been understudied. We have known for some time that GSK3 proteins can exist in a tyrosine phosphorylated form (pY-GSK3: Y216/GSK3α and Y279/GSK3β). Because it has been shown that these phosphates can be added via an auto-phosphorylation event, pY-GSK3 has generally been accepted as an indication of “active” GSK3. However, a recent study proposed that pY-GSK3 proteins are instead “hyperactive” and that phosphorylation at these residues changes the conformation of the active site in both the GSK3α and GSK3β kinase domains.14 This change in conformation would change the binding interactions with putative GSK3 substrates, raising the possibility that this is a previously unappreciated level of regulation.
While it is clear that GSK3 can autophosphorylate these tyrosine residues, GSK3 is itself a serine/threonine kinase, so it seems more likely that there is, instead, a cellular tyrosine kinase that takes on this role during normal GSK3 regulation. Anaplastic lymphoma kinase is a strong candidate for being this kinase. In a computational study for predicted substrates of ALK in neuroblastoma cell lines, GSK3α was identified as a potential target.15 Therefore, we decided to survey both neural crest and neuroblastoma lines in parallel to determine whether there was any link between ALK and GSK3.
Localization of Active ALK and Active GSK3 During a Crucial Step in NC Delamination
In our work, we found that both ALK and pY-GSK are expressed in delaminating and migrating NCCs. Anaplastic lymphoma kinase expression in the mouse embryo had not previously been studied in these cells. Interestingly, we found it expressed during the precise stages of embryonic development, from 8.5dpc, when the cranial neural crest is actively migrating. Specifically, we found that ALK co-expresses with pY-GSK3 in the right place at the right time to be controlling neural crest delamination and subsequent cell migration. More specifically, we found that in delaminating cells, pY-GSK3 is expressed at the cell side facing toward the direction of migration, and active ALK is co-expressed in these cells.
Using pharmacological inhibitors of ALK, including several used in the clinic for chemotherapy, we found that we could block neural crest delamination. Inhibition of GSK3, either genetically or pharmacologically, led to similar results. Moreover, we found that inhibition of ALK led to a loss of expression of the phospho-tyrosine form of GSK3. Thus, in mammalian NCCs, ALK is implicated in the tyrosine phosphorylation of GSK3 leading to a loss of cell migration.
Neuroblastoma Lines With High Levels of ALK Also Have High Levels of Activated GSK3
The molecular profiles of neuroblastoma are remarkably heterogeneous and there have been efforts to use these profiles to refine prognoses. As the clinical outcomes can range from spontaneous regression to a highly lethal metastatic disease, additional insights into the molecular profiles are important. Therefore, in parallel with a neural crest model, we set out to determine whether high levels of ALK in neuroblastoma correlated with active pY-GSK3. We can hypothesize that certain types of neuroblastoma reflect specific phases in the development of NCCs.
From the neuroblastoma lines that we analyzed, we found a clear association between the expression of ALK and tyrosine phosphorylation of GSK3. These cell lines included ones that were genetically mutated such as the Kelly line that carries the F1174L activating mutation, as well as lines with elevated levels of ALK due to non-genetic causes. We found that the subset of NB cell lines expressing high levels of ALK also exhibited high levels of pY-GSK3. Conversely, the NB cell lines that showed minimal ALK expression (eg, LS line) did not express pY-GSK3.
GSK3 Inhibition Can Block Cell Migration in “High ALK” Neuroblastoma Lines
To test whether GSK3 phosphorylation was downstream of ALK activity in the neuroblastoma lines, we used scratch assays where we block GSK3 activity using pharmacological inhibitors. We first assessed the Kelly line. As noted, the Kelly NB cell line is well characterized and is known to carry an activating ALK mutation (F1174L) as well as amplification of N-myc. Based on our knowledge of the developmental program of NCCs, this combination of factors (ALK and MYC) would suggest an analogy between Kelly cells and NCCs just at the cusp of migration. Indeed, inhibition of GSK3 led to a block in neural crest migration and Kelly NB line.
In contrast, the LS NB cell line does not carry an activating mutation of ALK. Application of GSK3 inhibitors led to a very surprising response in LS cells, which formed aggregates and showed an increase in apoptosis. Closer examination of the LS cells suggested that, when cultured, these cells behaved more like pseudoepithelial cells rather than fully mesenchymal cells. Taken together, we propose that LS and Kelly lines, if superimposed on the neural crest developmental clock, are not at the same developmental phase. It seems likely that the LS cells are more analogous to premigratory NCCs while the Kelly cells represent a more migratory mesenchymal population. It would be interesting to study more NB cells with different molecular backgrounds to understand better if the behavioral outcome of NB represents a specific stage of neural crest development where aggressive, metastatic tumors behave similarly to the migratory neural crest population (see Figure 1).
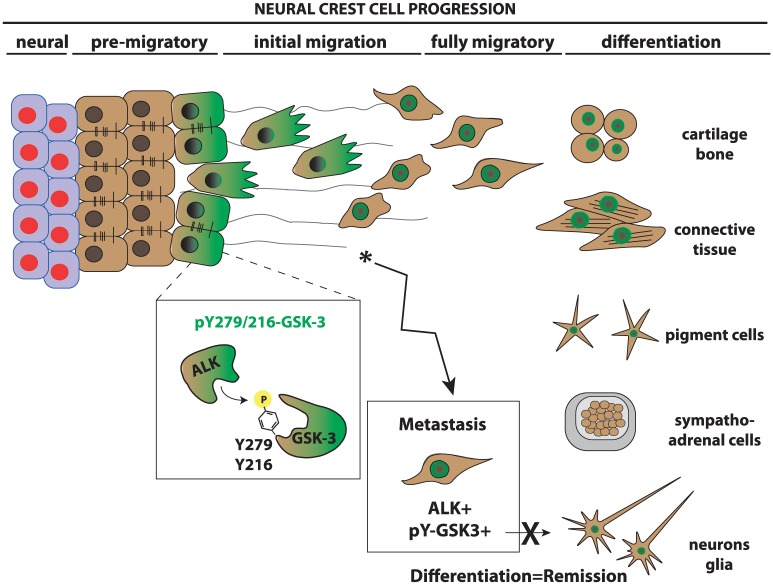
Figure 1.
Neural crest development. Neural crest cells are induced at the border of the neural plate. On induction, premigratory neural crest must delaminate and undergo an EMT from the neuroepithelium becoming fully mesenchymal. Migratory neural crest cells then move to their final destinations where they differentiate into a variety of cell types. Our work suggests that ALK acts in the premigratory neural crest to phosphorylate GSK3 at tyrosine 279 on GSK3α and tyrosine 216 on GSK3β. This serves to activate GSK3 in the cells undergoing EMT. GSK3 is proposed to regulate a signaling cascade controlling lamellipodial dynamics at the leading edge of the migratory front. In neuroblastoma cells, ALK activation correlates with high levels of phospho-tyrosine GSK3; this may maintain the migratory and proliferative capacity of the neuroblastoma cells while preventing differentiation. ALK indicates anaplastic lymphoma kinase; EMT, epithelial-mesenchymal transition; GSK3, glycogen synthase kinase 3.
ALK Regulates GSK3 in Migratory Neural Crest, but Not During Neural Crest Induction
By using primary neural crest explants, we were able to assess the effect of ALK and GSK3 inhibition on the following populations: neural plate cells, newly induced premigratory (epithelial) cells, and migratory (mesenchymal) cells. In these assays, we found that inhibition of GSK3 led to an increase in the area occupied by the premigratory population and a decrease in the migratory populations. However, ALK inhibition only significantly affected the migratory population. This suggests that while ALK regulates GSK3 activity in the migratory population, GSK3 has an independent function in the premigratory neural crest. This premigratory function is most likely related to the role of GSK3 in Wnt signaling, as others have shown that GSK3 inhibition upregulates β-catenin activity leading to an expansion of neural crest induction,16,17 while subsequent down-regulation of Wnt is necessary for neural crest migration.18,19
All together, our work identifies a new role for ALK-dependent regulation of GSK3 during normal and pathological development of the neural crest. Further studies should focus on the contextual regulation of these 2 important kinases and the potential for GSK3 inhibition in the treatment of neuroblastoma.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SGGM and KJL wrote the manuscript.
ORCID iDs: Sandra G Gonzalez Malagon  https://orcid.org/0000-0003-4479-4001
https://orcid.org/0000-0003-4479-4001
Karen J Liu  https://orcid.org/0000-0002-2483-2165
https://orcid.org/0000-0002-2483-2165
References
- 1. Schulte JH, Lindner S, Bohrer A, et al. MYCN and ALKF1174L are sufficient to drive neuroblastoma development from neural crest progenitor cells. Oncogene. 2013;32:1059–1065. [DOI] [PubMed] [Google Scholar]
- 2. Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barone G, Anderson J, Pearson AD, et al. New strategies in neuroblastoma: therapeutic targeting of MYCN and ALK. Clin Cancer Res. 2013;19:5814–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chesler L, Weiss WA. Genetically engineered murine models—contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin Cancer Biol. 2011;21:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez Malagon SG, Lopez Munoz AM, Doro D, et al. Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus. Nat Commun. 2018;9:1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu KJ, Arron JR, Stankunas K, et al. Chemical rescue of cleft palate and midline defects in conditional GSK-3beta mice. Nature. 2007;446:79–82. [DOI] [PubMed] [Google Scholar]
- 7. Szabo-Rogers H, Yakob W, Liu KJ. Frontal bone insufficiency in Gsk3β mutant mice. PLoS ONE. 2016;11:e0149604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palmer RH, Vernersson E, Grabbe C, et al. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108:1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. [DOI] [PubMed] [Google Scholar]
- 11. Hasan MK, Nafady A, Takatori A, et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci Rep. 2013;3:3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McManus EJ, Sakamoto K, Armit LJ, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stamos JL, Chu ML, Enos MD, et al. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. Elife. 2014;3:e01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rush J, Moritz A, Lee KA, et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotech. 2005;23:94–101. [DOI] [PubMed] [Google Scholar]
- 16. Leung AW, Murdoch B, Salem AF, et al. WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development. 2016;143:398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saint-Jeannet JP, He X, Varmus HE, et al. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci U S A. 1997;94:13713–13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rabadan MA, Herrera A, Fanlo L, et al. Delamination of neural crest cells requires transient and reversible Wnt inhibition mediated by Dact1/2. Development. 2016;143:2194–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maj E, Kunneke L, Loresch E, et al. Controlled levels of canonical Wnt signaling are required for neural crest migration. Dev Biol. 2016;417:77–90. [DOI] [PubMed] [Google Scholar]