Abstract
Among key survival circuits, defensive response circuits are one of the most intensively studied. A consensus is emerging that multiple, independent circuitries are involved in different conditioned and unconditioned defensive responses. Investigating these well-conserved defensive responses would help us to decipher the basic working mechanism of the brain at a circuitry level and thus shed light on new diagnoses and treatments for neural diseases and disorders. We showed that the visually evoked innate defensive response was modulated by a locus coeruleus-superior colliculus (LC-SC) projection. Our work demonstrates that as conserved and instinctive as the survival circuits are, they are flexible and subject to fine-tuned modulation by experience or internal states of the animals. Here, we provide more data to further discuss the possible downstream mechanisms of the LC-SC pathway for this important modulation of the defensive response, the wide range of flight latency between individual flight responses, and the interpretations of our data with additional statistical analysis.
Keywords: Locus coeruleus, superior colliculus, stress, looming, norepinephrine, defensive circuitry, modulation
Comment on: Li L, Feng X, Zhou Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol. 2018;28:859.e5-871.e5. doi:10.1016/j.cub.2018.02.005. Epub 2018 Mar 1. PubMed PMID: 29502952. https://www.ncbi.nlm.nih.gov/pubmed/29502952
Commentary
Studying the conserved neural pathways across mammals aims to decipher the basic working mechanism of the brain at a circuitry level and thus shed light on possible new diagnoses and treatments for neural diseases and disorders. Among the survival circuits, defensive responses are crucial to the survival of an individual and are intensively studied. A consensus is emerging that multiple, independent circuitries are involved in different conditioned and unconditioned defensive responses, which have been discussed in several excellent reviews.1–8
As conserved and instinctive as the defensive circuits are, they are not inflexible, but rather subjected to fine-tuned modulation. This notion is supported by our recent work published in Current Biology “Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection.”9 Looming stimulus, an expanding dark disk mimicking an aerial predator signal, provides a stable paradigm in which rodents would demonstrate innate defensive responses.10–13 We showed that a 4-day stress exposure could cause an accelerated defensive response, demonstrated as a decrease in the flight latency of the mouse to a looming stimulus in an open field with a nest apparatus. We identified that the TH::locus coeruleus-superior colliculus (LC-SC) pathway is activated by stress exposure, functions through the TH+ projection in the SC, and depends on the modulatory effect of norepinephrine (NE) locally in the SC.
The looming stimulus activates the SC, a key retino-recipient and sensory-motor integration nucleus. Several SC-related pathways have been proposed to be involved depending on the different looming stimulus parameters (upper field looming stimulus vs front-field looming stimulus) and the apparatus in which the animal received the stimulus (open field with a nest vs open field without nest).1,14–16 This includes the SC-lateral posterior nucleus (LP)-lateral amygdala pathway and SC-parabigeminal nucleus (PBGN) pathway. The SC-LP pathway mediates a long-lasting freezing behavior in an open field without nest paradigm.1 The SC-PBGN pathway mediates a flight and then freeze behavior in an open field with a nest paradigm.14 Also, a subset of retinal ganglion cells (RGCs), through their axonal collaterals to both the dorsal raphe nucleus (DRN) and SC, modulates the final looming-evoked defensive response.15 Recently, midline thalamic circuits, including the xiphoid nucleus (Xi), basal amygdala, nucleus reuniens (Re), and the medial prefrontal cortex, expanded the circuitries involved in processing looming-evoked defensive responses.17
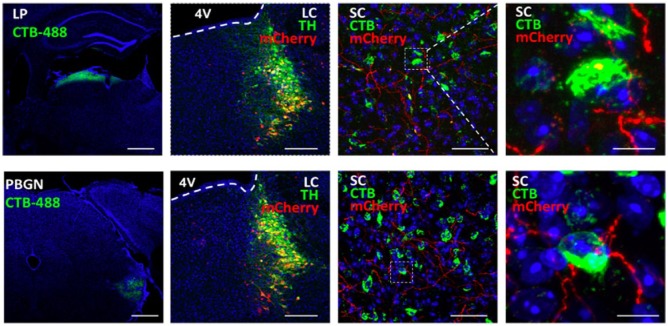
Here, we show the possible anatomical downstream projections for the LC-SC, which likely functions through the SC-LP and SC-PBGN pathways to modulate defensive responses (Figure 1). To check the possible innervation of LC-SC projections to the LP-projecting SC neurons, we first injected AAV2/9-Dio-mCherry virus (BrainVTA, China) into the LC of TH-cre mice, and 3 weeks later, Alexa Fluor 488 Conjugate Cholera toxin B subunit (CTB; C-22841; Invitrogen, Oregon, USA) was injected into the LP of the same mouse, and a week later, the animals were killed to check (1) the viral expression in the LC and its co-labeling with TH immunostaining, (2) the TH+ terminals in the SC from LC, and (3) the retrograde CTB signals from the LP. The same procedure was carried out with CTB injections in the PBGN in a second group of mice. For the detailed protocol of stereotaxic surgeries, injections, and immunostaining, refer to Li et al.9 All husbandry and experimental procedures in this study were approved by Animal Care and Use Committees at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, China.9 Our results showed that the LP-projecting SC neurons (green) or PBGN-projecting SC neurons (green) were innervated by the terminals (red) from TH+ LC neurons. These data suggest that the LC-NE system modulation might work through the reported defensive circuits from the SC, that is, SC-LP-amygdala or SC-PBGN pathways.
Figure 1.
Innervation of LP-projecting and PBGN-projecting SC neurons by terminals from TH+ LC neurons. Upper panel: LP and lower panel: PBGN; n = 3 to 4 mice per experiment (scale bars: 200, 200, 50, and 10 μm, respectively).
Looming-evoked defensive behaviors provide a novel paradigm for researchers to dig deep into the circuitry underlying innate defensive responses, with emerging reports in recent years.1,9,11,14–18 We believe that more details about this paradigm and its read-outs will benefit this field. We find that the flight latency of individual flight responses varies greatly. As an example, taking one group of data from the 12 stressed mice (each subjected to five trials of looming stimuli: flight latency, time to the nest, and time spent in nest were analyzed for the trials when flight to nest behavior was triggered), the range of the flight latency was 133 to 2833 ms. After calculating the mean for each mouse, the range of flight latency for this group was 493 to 1658 ms. In keeping with recommended statistical analyses for repeated measures and nested designs (CITE: Aarts et al19), we also calculated the effect of stress (or manipulation of LC-SC pathway) on defensive responses by averaging the mean for each mouse first and then performing t-tests between non-stressed control vs stressed group, mCherry control group vs opto-activated ChR2 group, and mCherry control group vs chemogenetic inhibited hM4Di group. The conclusion of these additional analyses remained the same as the published data.9 In our report, we chose to present each dot as individual trials of the mice, as it revealed more details about flight latency, the key parameter of the defensive response, which is quite dynamic between trials but is also sensitive enough to show the effect of stress on the magnitude of the defensive response. Interestingly, another form of stress, “prolonged social isolation stress,” was reported to cause an increase in the freezing time at post-stimulus period to upper field looming stimulus in an open field without nest apparatus, not during the 10 second looming presentation.18 In Li et al,9 parameters including flight latency, time to the nest, and time spent in nest were analyzed for the trials with flight to nest behavior. There were also a very low percentage of trials that the animal did not flee to nest, and additional parameter like percentage of flight might serve the purpose of describing the defensive behaviors well. All these indicate that detailed readouts of the defensive behaviors are crucial to employ the looming test in answering specific scientific questions.
Also, we re-analyzed the results of our c-fos expression experiments. In our study, mice of the stressed group underwent a 4-day repeated stress protocol that included (1) being held in a restraint, (2) forced swim, and (3) foot shock. The stressors were given to the mice every day in a randomized order and repeated for 4 days, and then on day 5, the mice were euthanatized and brains were harvested for c-fos staining. Importantly, post-stress behavioral tests/post-stress chemogenetic inhibition of LC were also performed on day 5, for consistency between different experiments (for chemogenetic inhibition, clozapine N-oxide (CNO) was injected on day 5, 1 hour before looming test).9 To obtain cluster-based analysis of the results,19 we averaged the cell counting of all the sections from one mouse first and used the mean for subsequent t-tests. The conclusions for c-fos experiments remained the same as published.9
A significant number of studies focus on the neuronal circuitries of looming-evoked defensive responses,1,2,9,14,15,17 and yet, vital questions still remain unanswered, such as (1) what is and how to detect “key trigger stimulus” of looming-evoked defensive behaviors,4 (2) how many “multiple, independent circuits that process different types of fear”3 exist, (3) where does the behavioral decision happen concerning the “hierarchical taxonomy of defensive behaviors,”7 and (4) the possible interaction between adaptive behaviors, innate circuitry, and external/internal challenges. Efforts in answering these questions might enlighten us on the physiological and pathological properties of the brain at circuitry levels.
Acknowledgments
We would like to acknowledge Drs Johannes Bohacek, Sophie van der Sluis, Pierre-Luc Germain, and Kirsten Porter-Stransky for their thoughtful comments on the statistical analyses and results of our original publication.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by National Natural Science Foundation of China (NSFC 31630031 and NSFC 31471109) and Shenzhen Governmental Grant JCYJ20150529143500959, KQJSCX20160301144002, JCYJ20140417113430710, and JCYJ20151030140325151.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LL wrote the manuscript; LW reviewed and approved the final manuscript.
ORCID iD: Liping Wang  https://orcid.org/0000-0001-6893-3809
https://orcid.org/0000-0001-6893-3809
References
- 1. Wei P, Liu N, Zhang Z, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. 2015;6:6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang H, Yang J, Xi W, et al. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat Neurosci. 2016;19:283–289. [DOI] [PubMed] [Google Scholar]
- 3. Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. [DOI] [PubMed] [Google Scholar]
- 4. LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adolphs R. The biology of fear. Curr Biol. 2013;23: R79–R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LeDoux J, Daw ND. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat Rev Neurosci. 2018;19:269–282. [DOI] [PubMed] [Google Scholar]
- 8. Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. [DOI] [PubMed] [Google Scholar]
- 9. Li L, Feng X, Zhou Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol. 2018;28:859.e5–871.e5. [DOI] [PubMed] [Google Scholar]
- 10. Yilmaz M, Meister M. Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol. 2013;23:2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao X, Liu M, Cang J. Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron. 2014;84:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG. Vision guides selection of freeze or flight defense strategies in mice. Curr Biol. 2016;26:2150–2154. [DOI] [PubMed] [Google Scholar]
- 13. Vale R, Evans DA, Branco T. Rapid spatial learning controls instinctive defensive behavior in mice. Curr Biol. 2017;27:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shang C, Liu Z, Chen Z, et al. BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348:1472–1477. [DOI] [PubMed] [Google Scholar]
- 15. Huang L, Yuan T, Tan M, et al. A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behaviour. Nat Commun. 2017;8:14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shang C, Chen Z, Liu A, et al. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat Commun. 2018;9:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salay LD, Ishiko N, Huberman AD. A midline thalamic circuit determines reactions to visual threat. Nature. 2018;557:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zelikowsky M, Hui M, Karigo T, et al. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell. 2018;173:1265.e19–1279.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aarts E, Verhage M, Veenvliet JV, Dolan CV, Van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 2014;17:491–496. [DOI] [PubMed] [Google Scholar]