Abstract
Our previous study demonstrated that mesenchymal stem cell (MSC) microvesicles (MV) reduced lung inflammation, protein permeability, and pulmonary edema in endotoxin‐induced acute lung injury in mice. However, the underlying mechanisms for restoring lung protein permeability were not fully understood. In this current study, we hypothesized that MSC MV would restore protein permeability across injured human lung microvascular endothelial cells (HLMVEC) in part through the transfer of angiopoietin‐1 (Ang1) mRNA to the injured endothelium. A transwell coculture system was used to study the effect of MSC MV on protein permeability across HLMVECs injured by cytomix, a mixture of IL‐1β, TNF‐α, and IFN‐γ (50 ng/ml). Our result showed that cytomix significantly increased permeability to FITC‐dextran (70 kDa) across HLMVECs over 24 hours. Administration of MSC MVs restored this permeability in a dose dependent manner, which was associated with an increase in Ang1 mRNA and protein secretion in the injured endothelium. This beneficial effect was diminished when MSC MV was pretreated with an anti‐CD44 antibody, suggesting that internalization of MV into the HLMVEC was required for the therapeutic effect. Fluorescent microscopy showed that MSC MV largely prevented the reorganization of cytoskeleton protein F‐actin into “actin stress fiber” and restored the location of the tight junction protein ZO‐1 and adherens junction protein VE‐cadherin in injured HLMVECs. Ang1 siRNA pretreatment of MSC MV prior to administration to injured HLMVECs eliminated the therapeutic effect of MV. In summary, MSC MVs restored protein permeability across HLMVEC in part by increasing Ang1 secretion by injured HLMVEC. Stem Cells Translational Medicine 2018;7:615–624
Keywords: Mesenchymal stem cell, Microvesicles, Human lung microvascular endothelial cell, Lung protein permeability
Significance Statement.
In this study, it was hypothesized that one of the beneficial effects of mesenchymal stem cell (MSC) microvesicle (MV) administration in lung injury was the restoration of lung protein permeability. Using a transwell coculture system with human lung microvascular endothelial cells (HLMVEC), MSC MV administration was shown to restore protein permeability to FITC‐dextran (70 kDa) across HLMVECs injured by cytomix, in part due to an increase in Ang1 mRNA and protein secretion in the injured endothelium, which prevented “actin stress fiber” formation. Ang1 siRNA pretreatment of MSC MV prior to administration to injured HLMVECs eliminated the therapeutic effect of MV. MSC MV administration restored protein permeability across HLMVEC in part by increasing Ang1 secretion.
Introduction
Acute lung injury (ALI) and its more severe clinical form, acute respiratory distress syndrome (ARDS), are life‐threatening conditions common among critically ill ventilated patients. ALI/ARDS is characterized by diffuse injury to the capillary endothelium and alveolar epithelium which leads to an increase in alveolar‐capillary permeability 1, 2, resulting in pulmonary edema formation and the accumulation of inflammatory cells in the interstitial and alveolar space. Although substantial progress have been made in the treatment of ALI/ARDS, including the application of lung‐protective ventilation 3, prone positioning 4, 5 and the use of paralytics 6, mortality remains high at approximately ∼40% 7. Moreover, no pharmacologic therapies have been developed which improve clinical outcome. Therefore, novel therapies for ALI/ARDS are needed.
Once considered cellular debris, extracellular vesicles released by human mesenchymal stem cell (MSC) have now been shown to be biologically protective in multiple preclinical models of ALI, similar to the parent stem cells 8, 9. Extracellular vesicles are anuclear membrane‐bound vesicles released constitutively from the endosomal compartment as exosomes or from the plasma membrane as microvesicles (MV), which contain numerous microRNAs, mRNAs, proteins/peptides, organelles, and bioactive lipids. More importantly, various studies have suggested that MSC MV are able to home to the injured site, participating as mediators of cell‐to‐cell communication 10, 11 and as mechanisms of organ protection via the transfer of its content 12, 13. In our previous study using both lipopolysaccharide (LPS)‐induced ALI and Escherichia coli pneumonia, we demonstrated that MSC MV reduced inflammation, lung protein permeability, and pulmonary edema in part through the transfer of keratinocyte growth factor mRNA to the injured alveolus with subsequent expression of the epithelial specific growth factor 14, 15. However, the mechanisms underlying the restoration of lung protein permeability were not fully understood. In this current study, we hypothesized that MSC MV would restore protein permeability across injured human lung microvascular endothelial cells (HLMVECs) in part by preventing “actin stress fiber” formation via the transfer of mRNA for angiopoietin1 (Ang1).
Materials and Methods
Mesenchymal Stem Cells
Human MSCs were purchased from the National Institutes of Health repository from Texas A&M Health Science Center (Temple, TX). The MSC were isolated from bone marrow of healthy donors. MSCs were cultured in α‐minimum essential medium (α‐MEM) without ribonucleosides or deoxyribonucleosides containing 2 mM L‐glutamine, 16.5% fetal bovine serum (FBS) and 1% penicillin/streptomycin, and maintained in a humidified incubator with 5% CO2 at 37°C. The culture medium was changed every 2–3 days. Cells were split when they reached 90% confluence. MSCs with the total passage number <10 were used in the experiments. Normal adult human lung fibroblast (NHLF) (Lonza, Walkersville, MD, USA, http://www.lonza.com/) were used as cellular controls.
Isolation of MVs
MVs were isolated from the conditioned medium of human bone marrow‐derived MSCs and NHLFs using ultracentrifugation as we previously described 15. Briefly, MSCs or NHLFs were serum starved in a conditioned medium (α‐MEM or fibroblast basal medium (FBM) supplemented with 0.5% Bovine Albumin Fraction [MP BioMedicals, LLC, Santa Ana, CA, http://www.mpbio.com]). After 48 hours, the conditioned medium was collected and centrifuged at 3,000 rpm for 20 minutes to remove cellular debris, then at 100,000g (Beckman Coulter Optima L‐100XP ultracentrifuge) to isolate the MVs for 1 hour at 4°C. The supernatants were aspirated and the sediments were washed in phosphate buffered saline (PBS) and centrifuged at 100,000g for 1 hour at 4°C again. The sediments containing MVs were resuspended in PBS and stored in −80°C. Ten microliter of MVs were equivalent to the MVs released by 1 million MSCs or NHLFs.
MSC MV Characterization
MSC MVs were labeled with PKH26 to separate out vesicles from debris by flow cytometry (Sigma‐Aldrich, St. Louis, MO, USA) following the manufacturer's protocol. To stain MSC MV with CD44 and CD9, MSC MVs were resuspended with antibodies for CD9‐fluorescein isothiocyanate (FITC) (eBioscience, Inc., San Diego, CA, USA), control IgG1 k‐FITC (eBioscience, Inc.), CD44‐FITC (BD Biosciences, San Jose, CA, USA), or control IgG2b k‐FITC (BD Biosciences). To detect CD44 or CD9 on MSC MV, a BD FACSAria Fusion Special Order (SORP) cell sorter (BD Biosciences) with 100 nm nozzle and ND filter 1 was used. The threshold was set on the SSC 200. Collected data were analyzed by Diva software (BD Biosciences). For fluorescence detection, we used a 586/15 band‐pass filter for PKH26 and 525/50 band‐pass filter for CD9‐FITC, CD44‐FITC, IgG2b k‐FITC, and IgG1 k‐FITC. An unstained sample was used to detect auto‐fluorescence and set the photomultiplier for all the considered channels. Standard silica beads (Apogee Mix for Flow Cytometer, Apogee Flow Systems, Ltd., Hemel Hempstead, England), with a similar refractive index of vesicles, was used to gate the MSC MVs.
MSC MVs were also characterized by scanning electron microscopy as previously described 15 and by using Nanosight NS 300 (Malvern Instruments, U.K.).
MVs with or Without Angiopoietin 1 siRNA Pretreatment
For siRNA experiments, MSCs were collected and seeded in 6‐well plates at a density of 1 × 106 cells/well, with siPORTNeoFX containing 100 nM Ang1 small interfering RNA (siRNA) (#AM16708 for Ang1 siRNA, Ambion, Waltham, MA, http://www.thermofisher.com/us/en/home/brands/invitrogen/ambion.html) for 24 hours. Pretreatment with a scrabbled siRNA (Negative Control No.1 siRNA, Ambion) was used as a siRNA control. The culture medium was then replaced by fresh MSC conditioned medium to produce MVs. Forty‐eight hours later, the conditioned medium was collected for MSC MV isolation.
Primary Cultures of HLMVECs
HLMVECs were obtained from small vessels within normal lung tissue (Lonza, Walkersville, MD, USA, http://www.lonza.com/). HLMVECs were cultured in microvascular endothelial growth medium (EBM‐2 Basal Medium supplemented with human Epidermal Growth Factor, Vascular Endothelial Growth Factor, R3‐Insulin‐like Growth factor‐1, Ascorbic Acid, Hydrocortisone, human Fibroblast Growth Factor‐Beta, 5% Fetal bovine Serum, Gentamicin/Amphotericin‐B and 1% penicillin/streptomycin [Lonza, Walkersville, MD, USA]) and incubated in a humidified incubator with 5% CO2 at 37°C. The growth medium was changed 24 hours after seeding and every other day thereafter. Cells were cultured when they were 70%–85% confluent. HLMVECs with the total passage number <9 were used in all the experiments. Although 5% FBS itself contains extracellular vesicles, we chose not to remove the serum from the culture medium due to excessive cell death of HLMVECs with serum starvation.
HLMVECs Exposed to Cytomix or Cytomix with MSC MVs (with or Without a Neutralizing Anti‐CD44 Antibody)
A transwell coculture system (0.4‐µm pore size and collagen I‐coated, Costar, Corning, Tewksbury, MA, http://www.corning.com) was used to study the effects of MSC MVs on HLMVEC monolayer injured by cytomix, a mixture of human IL‐1β, TNF‐α, and IFN‐γ (50 ng/ml) often used as a surrogate for ALI pulmonary edema fluid 16. In this system, the transwell inserts were placed in 24‐well plates. HLMVECs were seeded in the inserts at a density of 1 × 106 cells/insert and maintained in a humidified incubator with 5% CO2 at 37°C to form a monolayer. After 24 hours, we exposed the HLMVEC monolayer to cytomix or cytomix with MSC MVs at increasing doses (30 and 60 µl) with or without a neutralizing anti‐CD44 antibody. After 24 hours, the culture medium in the inserts were aspirated and replaced with 100 µl fresh culture medium contained FITC‐dextran (100 µg/ml, 70 kDa, http://www.sigmaaldrich.com). Cells were maintained in incubator for 1 hour. Then 100 µl culture mediums were obtained from the upper chamber and the lower chamber, respectively. The plate reader (Tecan, Switzerland) was used to measure the fluorescence in the medium. The unidirectional flux of FITC‐dextran from the upper chamber to the lower chamber was then calculated and express as percentage per 24 hours as protein permeability.
Microscopy with Immunofluorescence Labeling of Cytoskeleton, Tight Junction, and Adherens Junction Proteins
To observe any changes of the cytoskeletal protein F‐actin, tight junction protein zonula occludens‐1 (ZO‐1) and adherens junction protein, VE‐cadherin, HLMVECs were seeded on Lab‐Tek II chamber slides (Nalge Nunc International, Rochester, NY) at a density of 50,000 cells/chamber. When the cells reached 90% confluence, the culture was exposed to cytomix with and without MSC MVs. After 24 hours, the cell monolayer was washed twice with PBS and fixed in 4% paraformaldehyde for 10 minutes. Then the cells were washed three times with PBS for 5 minutes and permeabilized by 0.1% Triton X‐100 for 4 minutes. The slides were washed again three times with PBS for 5 minutes and then blocked with 1% BSA for 30 minutes at room temperature.
For F‐actin staining, the slides were incubated with a FITC‐phalloidin (Thermo Fisher, http://www.thermofisher.com) for 30 minutes at 37°C. For ZO‐1 staining, slides were incubated with ZO‐1 antibodies (10 µg/ml, Thermo Fisher) for 1 hour at room temperature. For VE‐cadherin staining, primary antibodies to VE‐cadherin (1:100, Cell signaling, Danvers, MA, http://www.cellsignal.com) were used. After washing with PBS three times, slides were incubated with secondary antibody Alexa Fluor 594 or 488 conjugated Goat Anti‐Rabbit IgG (H + L) DS Grade (1:50, Invitrogen, http://www.thermofisher.com) for 1 hour at room temperature. Then slides were washed with PBS again and dried at room temperature. Slides were then mounted with Vectashield mounting medium. Images were obtained by Leica DM 1,000 microscope.
Fluorescence Microscopy with PKH26 Stained MSC MVs
For MSC MVs staining, a PKH26 Fluorescent Cell Linker Kits (http://www.sigmaaldrich.com) was used. MVs from 1 × 107 MSCs were resuspended in 1 ml of Diluent C and then mixed with 1 ml of 2 × 106 M of PKH26 dye solution. MSC MVs were incubated at room temperature for 1–5 minutes with periodic mixing. The staining was stopped by adding an equal volume of serum and incubated for at least 1 minute to allow binding of excess dye. Then MSC MVs were centrifuged at 100,000g for 1 hour at 4°C. The sediments were resuspended in complete medium, transferred to a fresh sterile tube, centrifuged at 100,000g for 1 hour at 4°C, and washed two more times with complete medium to ensure removal of unbound dye.
To evaluate the role of CD44 in the incorporation of MSC MVs into HLMVECs, MSC MVs stained with PKH26 dye were used. Briefly, HLMVECs were seeded on Lab‐Tek II chamber slides (Nalge Nunc International) at a density of 50,000 cells/chamber. When the cells reached 90% confluence, the cultures were exposed to: (a) MSC MVs‐PKH26, (b) cytomix with MSC MVs‐PKH26, (c) cytomix with MSC MVs‐PKH26 and IgG antibody, (d) cytomix with MSC MVs‐PKH26 and a neutralizing anti‐CD44 antibody, respectively. After 24 hours, the slides were washed twice with PBS and fixed in 4% paraformaldehyde for 10 minutes. Then the slides were washed three times with PBS for 5 minutes and permeabilized by 0.1% Triton X‐100 for 4 minutes. The slides were washed again three times with PBS for 5 minutes and were mounted with Vectashield mounting medium. Images were obtained by Leica DM 1,000 microscope.
RNA Isolation and Quantitative Real‐Time Polymerase Chain Reaction
Total RNA was isolated from either MSC MVs, HLMVECs or HLMVECs treated with (a) cytomix, (b) cytomix with MSC MVs, and (c) cytomix with MSC MVs pretreated with a scrabbled siRNA or Ang1 siRNA (90 μl/ml medium) using RNeasy Mini Kit (QIAGENSciences, Redwood City, CA, http://www.qiagen.com). The quality of the RNA was assessed with the NanoDrop ND‐1000 UV‐Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA); 260/280 and 260/230 nm absorbance ratios of 1.8–2.2 suggested a pure RNA sample. Primers including the probes used for the quantitative real‐time polymerase chain reaction (qRT‐PCR) were human Ang1 and GAPDH and were purchased from Life Technologies (http://www.thermofisher.com). High‐Capacity RNA‐to‐cDNA Kit and TaqMan Fast Universal PCR Master Mix from Applied Biosystems were used for the qRT‐PCR assays. These assays were conducted following the Two‐Step qRT‐PCR protocol described by Applied Biosystems.
MSC MV Derived Secreted Soluble Factors
HLMVECs were seeded in 12‐wells plate at a density of 1 million cells per well and maintained in incubator overnight. Then the cells were exposed to cytomix with and without MSC MV or cytomix with or without MSC MV pretreated with a scrabbled siRNA or Ang1 siRNA (90 μl/ml medium). After 24 hours, the supernatants were collected and stored in −80°C. The cells were lysed, and the cell lysates were collected and stored in −80°C. The Ang1 levels in both the supernatants and cell lysates were detected using ELISA kit (R&D system, Minneapolis, MN, http://www.rndsystems.com) according to the manufacturer's instruction.
Western Blot Analyses and Rac123 Measurement
Western blot analyses were performed on lysis of HLMVEC exposed to cytomix with or without treatment with MSCs or MSC MVs as previously described 16 using antibody for phosphorylated myosin light chain 2 (1:1,000 dilution, Cell Signaling), VE‐cadherin (1 μg/ml, R&D Systems), ZO‐1 (1:1,000, Cell Signaling) and β‐actin (R&D Systems) or glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (1:1,000, Cell Signaling) as loading controls. GTP‐bound Rac1/2/3 were measured in protein lysates of HLMVECs with a commercially available activation kit (G‐LISA, kit #BK125, Cytoskeleton, Inc., Denver, CO, http://www.cytoskeleton.com/) according to the manufacturer's instructions.
Statistical Analysis
All experimental groups were repeated at least three times in triplicate for each group. Data are shown as mean ± SD. For comparisons between two groups, an unpaired two‐tailed t test was used. For comparisons between multiple groups, analysis of variance (ANOVA) with post hoc Tukey HSD test was used. A value of p < .05 was considered statistically significant. Analyses were done using GraphPad Prism software.
Results
MSC MV Characterization
MSC MV were characterized by electron microscopy, Nanosight analysis and flow cytometry. Scanning electron microscopy showed that the isolation technique yielded homogeneous population of spheroid particles (Fig. 1A). Nanosight analysis showed MSC MV mean size was 185 ± 11 nm and mode size was 115 ± 2 nm, with a concentration at 4.6 ± 0.10 × 1010 particles per ml. For flow cytometry, we labeled MSC MV with PKH26 to quantify only membrane bound vesicles and exclude debris. As a percentage of PKH26 labeled MSC MV, we found that 73% ± 10% was CD44 positive. An IgG Ab used as a control labeled only 5% ± 0.4% PKH26 MSC MVs. For CD9, only 0.3% ± 0.2% of PKH26 MSC MVs was labeled positively, suggesting that the majority of vesicles were MVs (Supporting Information Fig. S1A, S1B). All experiments were performed three times.
Figure 1.
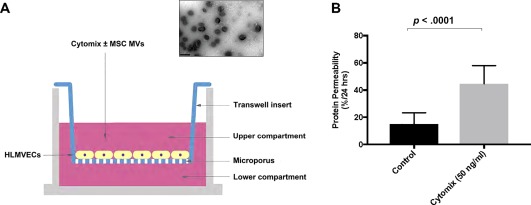
Cytomix increased protein permeability across primary cultures of human lung microvascular endothelial cells. (A): Schematic of the Transwell coculture system: HLMVEC were cultured in the Transwell insert, and culture medium containing 50 ng/ml cytomix, a mixture of IL‐1β, TNF‐α, and IFN‐γ often used as a surrogate for acute lung injury pulmonary edema fluid, was added to the upper compartment. The fluid levels between upper and lower compartments were equal, preventing any hydrostatic pressure gradient between compartments. A representative electron microscopy figure of MSC MVs demonstrating small spheroid vesicles is shown (scale bar is 0.5 μm). (B): The addition of cytomix increased permeability to FITC‐dextran (70 kDa) across HLMVECs over 24 hours. Data are presented as mean ± SD, N = 9. Abbreviations: FITC, fluorescein isothiocyanate; HLMVEC, human lung microvascular endothelial cell; MSC, mesenchymal stem cell; MVs, microvesicles.
Cytomix Increased Protein Permeability Across HLMVEC
To study the effects of MSC MVs on protein permeability across HLMVEC monolayer injured by cytomix, a transwell coculture system was used (Fig. 1A). FITC‐dextran was administered to the upper chamber as a surrogate for albumin for the measurement of protein permeability across HLMVEC from the upper to lower compartment. As shown in Figure 1B, the permeability to FITC‐dextran across normal HLMVEC monolayer was 15% ± 8%/24 hours. After exposure to cytomix, protein permeability across cytomix‐injured HLMVECs over 24 hours was increased significantly to 45% ± 13%/24 hours.
MSC MVs Restored Protein Permeability Across HLMVEC Injured by Cytomix
Prior to testing MSC MV, preliminary experiments were performed to determine whether MSCs restored protein permeability in HLMVEC exposed to an inflammatory insult. Exposure to cytomix 0.5 ng/ml increased protein permeability to 19.4% ± 6.4%/24 hours which was associated with “actin stress fiber” formation and phosphorylation of myosin light chain 2. Similar to previous studies in primary cultures of human alveolar epithelial type II cells injured with cytomix and treated with MSCs 16, the addition of MSC (only 250,000 cells in the bottom chamber) partially restored protein permeability which was associated with increased in Rac123 activation. Ang1 siRNA pretreatment of the MSC eliminated the therapeutic effect on permeability (Supporting Information Fig. S2A, S2B).
A decision was made to increase the inflammatory concentration of cytomix from 0.5 to 50 mg/ml to correlate more accurately with cytokine levels in clinical ARDS samples. Exposure to cytomix 50 ng/ml further increased protein permeability across HLMVEC by 298% of control (Fig. 2). When 1X MSC MVs (30 µl) were added to the top chamber of the transwell coculture system, protein permeability across HLMVEC exposed to cytomix was partially restored to 232% of control. Moreover, when 2X MSC MVs (60 µl) were added, protein permeability was further restored to 185% of control, suggesting that administration of MSC MVs restored protein permeability in a dose dependent manner. Administration of MVs derived from normal human lung fibroblasts (469% of control) had no effect on permeability to FITC‐dextran. Surprisingly, coculture with MSC at a concentration of 750,000 cells in the lower chamber (274% of control) had no significant effect, although there was a numerical decrease in permeability. All subsequent permeability experiments were performed with MSC MV at 60 µl.
Figure 2.
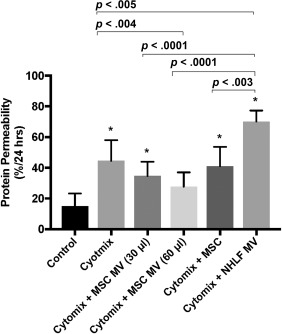
Administration of MSC microvesicles restored protein permeability across human lung microvascular endothelial cell (HLMVEC) injured by cytomix in a dose‐dependent manner. Simultaneous administration of MSC MVs (1X = 30 μl, 2X = 60 μl) restored protein permeability across HLMVEC injured by cytomix in a dose dependent manner over 24 hours. Administration of normal human lung fibroblast MVs or surprisingly MSCs (750,000 cells in the lower chamber) showed no beneficial effect on protein permeability. Data are presented as mean ± SD, N = 9, *, p is significant vs. control using ANOVA with post hoc Tukey HSD test. Individual p values are presented for comparison between groups using post hoc Tukey HSD test. Abbreviations: ANOVA, analysis of variance; MSC, mesenchymal stem cell; MVs, microvesicles; NHLF, normal human lung fibroblast.
MSC MVs Restored the Distribution of F‐Actin, ZO‐1, and VE‐Cadherin in Injured HLMVEC
Since increased protein permeability was the result of changes at the level of intercellular proteins connected to the cytoskeleton, adherens junction, and tight junction, we studied the distribution of the cytoskeletal protein F‐actin, tight junction protein zonula occludens‐1 (ZO‐1), and adherens junction protein VE‐cadherin by immunofluorescence. The result showed that MSC MV largely prevented the reorganization of cytoskeleton protein F‐actin into “actin stress fiber” in cytomix injured HLMVECs (Fig. 3A). Moreover, the loss of tight junction protein ZO‐1 in HMVEC injured by cytomix was dramatically restored by MSC MV treatment (Fig. 3C). In addition, we found that the loss of adherens junction protein VE‐cadherin in cytomix injured HLMVEC was restored as well by MSC MV treatment (Fig. 3B). By Western blot analyses, the decrease in ZO‐1 and VE‐Cadherin total protein levels in HLMVECs injured with cytomix was partially restored with MSC MVs administration (Fig. 3D).
Figure 3.
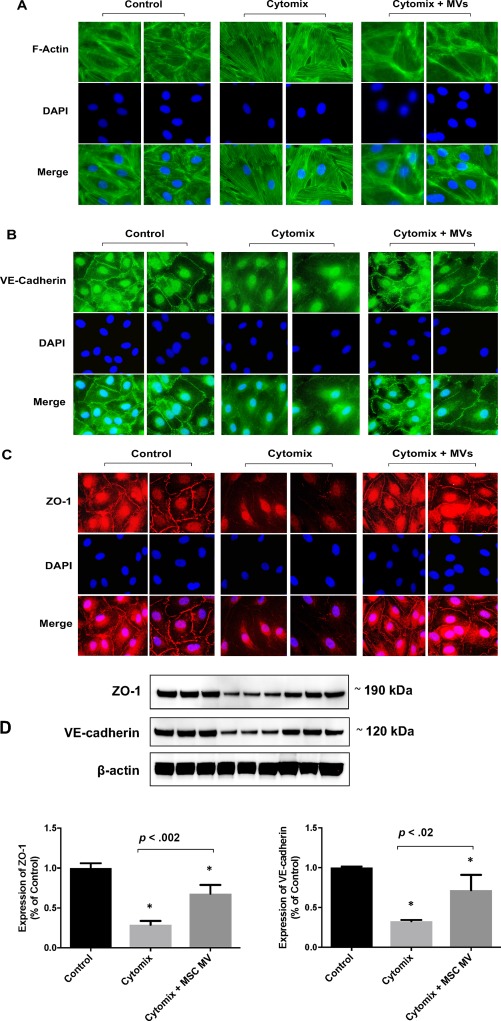
Administration of MSC microvesicles restored the distribution of the cytoskeleton protein, F‐action, the tight junction protein, ZO‐1, and adherens junction protein, VE‐cadherin, in injured human lung microvascular endothelial cell (HLMVEC). HLMVECs were grown on glass slide and stained with phalloidin (green) for F‐actin (A) and VE‐cadherin (B), and rhodamine (red) for ZO‐1 (C). Baseline control staining for F‐actin in HLMVEC showed a typical peripheral distribution as well as intense staining of VE‐cadherin and ZO‐1 at the junctions between cells. After exposure to cytomix for 24 hours, total cellular F‐action was reorganized toward the center of the cells to form “actin stress fibers” with a redistribution of VE‐cadherin and ZO‐1 staining away from cell contact. There was also an increase in pores between cells, potentially the cause of increase in protein permeability. Administration of MSC MV largely prevented the reorganization of cytoskeleton protein F‐action into “actin stress fiber” and restored the staining of VE‐cadherin and ZO‐1 between cells in cytomix injured HLMVECs. Images are representative for each condition run in triplicates. DAPI (blue) was used to stain the cell nuclei. (D): By Western blot analyses, the decrease in ZO‐1 and VE‐Cadherin total protein levels with cytomix injury was partially restored by MSC MV treatment. Data are presented as mean ± SD, N = 3, *, p is significant vs. control using ANOVA with post hoc Tukey HSD test. Individual p values are presented for comparison between groups using post hoc Tukey HSD test. Abbreviations: ANOVA, analysis of variance; DAPI, 4′,6‐diamidino‐2‐phenylindole; MSC, mesenchymal stem cell; MV, microvesicle.
MSC MV CD44 Was Required for Restoration of Protein Permeability
Our previous study showed that CD44 played a role in the therapeutic effect of MSC MV on E. coli induced severe pneumonia 15. However, the underlying mechanism remained unclear. Here, we stained MSC MV with PKH26 dye and used the anti‐CD44 antibody to block the CD44 receptor on PKH26 stained MSC MVs. We found that the uptake of MSC MV by HLMVEC was decreased significantly compared to the IgG control group using fluorescence microscopy (Fig. 4A, 4B). Moreover, the beneficial effect of MSC MVs on protein permeability across HLMVECs was diminished when MSC MVs were pretreated with an anti‐CD44 antibody in a transwell coculture system (Fig. 4C), suggesting that internalization of MVs into the HLMVECs was required for the therapeutic effect.
Figure 4.
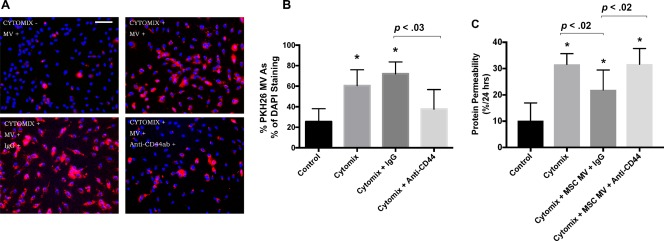
MSC MV surface receptor, CD44, was required for therapeutic effect of MSC MVs on the restoration of protein permeability across injured human lung microvascular endothelial cell (HLMVEC). MSC MV uptake by HLMVECs was dependent on the cell surface receptor for hyaluronic acid, CD44. (A): Images from fluorescent microscopy (scale bar = 20 μm) and (B) the result from quantitative analysis of these images showed that exposure to cytomix for 24 hours increased the uptake of PKH26 labeled MSC MV into HLMVECs. The percentage of PKH26 labeled MSC MV as a percentage of DAPI staining was obtained from each slide and expressed as mean (%) ± SD for each condition, N = 9. Addition of anti‐CD44 antibody decreased the uptake of MSC MV. (C): In a transwell coculture system, the therapeutic effect of MSC MVs on protein permeability was diminished when MSC MVs were pretreated with an anti‐CD44 antibody compared with MSC MVs pretreated with IgG control. Data is presented as mean ± SD, N = 9, *, p is significant vs. control using ANOVA with post hoc Tukey HSD test. Individual p values are presented for comparison between groups using post hoc Tukey HSD test. Blue, DAPI; Red, MV stained with PHK26 dye. Abbreviations: ANOVA, analysis of variance; DAPI, 4′,6‐diamidino‐2‐phenylindole; MSC, mesenchymal stem cell; MV, microvesicle.
Altered Ang1 and S1PK mRNA Expression in HLMVEC Injured by Cytomix Exposed to MSC MV
Recently, MSC MV was shown to be biologically active due to the presence of microRNAs, mRNAs, proteins/peptides, organelles, and lipids. To understand the therapeutic effect of MSC MV, we measured several MSC MV associated soluble factors potentially involved in restoring endothelial permeability in HLMVEC injured by cytomix. We found that Ang1 mRNA levels in injured HMVECs were significantly increased after MSC MVs treatment, especially at 6 and 12 hours (Fig. 5A). In addition, we also found that sphingosine 1 phosphate (S1P) kinase1 mRNA levels were significantly elevated, indicating that S1P secretion may also play a role in the restoration of protein permeability across HLMVECs by MSC MV (Fig. 5B).
Figure 5.
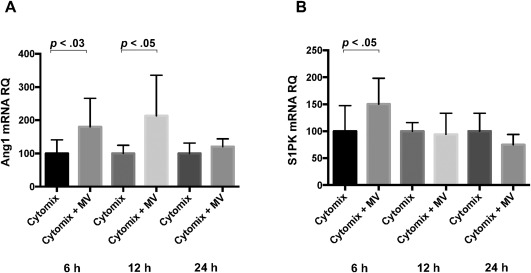
Administration of mesenchymal stem cell (MSC) MVs altered gene transcription in human lung microvascular endothelial cell (HLMVEC) injured by cytomix. Ang1 is an important endothelial survival and vascular stabilization factor. It can reduce endothelial permeability and protect intercellular junctions. MSC MVs increased (A) Ang1 mRNA levels in HLMVECs exposed to cytomix over 24 hours compared to control. Sphingosine‐1‐phosphate (S1P) is a bioactive lipid second messenger which plays a significant role in inflammation, the differentiation of lymphocytes, and endothelial permeability. It is generated intracellularly from the phosphorylation of sphingosine by sphingosine kinases (S1PK). Interestingly, MSC MVs also increased (B) S1PK1 mRNA levels in HLMVEC injured by cytomix at 6 hours, indicating S1P signaling may potentially also play a role in the restoration of endothelial permeability by MSC MV. Data are presented as mean ± SD, N = 9. Abbreviations: MV, microvesicle; RQ, relative quantification.
MSC MVs Increased Ang1 Secretion in Cytomix‐Injured HLMVEC
We also detected Ang1 levels in the culture medium of HLMVEC injured by cytomix at 6, 12, and 24 hours. The result showed that Ang1 levels in the culture medium of HMVECs exposed to cytomix were increased dramatically compared with the control group, especially at 6 hours (Fig. 6). The total Ang1 protein level found in 60 µl of MSC MVs was only 36 ± 19 pg, which suggested that the increase in Ang1 levels could not be accounted for by Ang1 protein in the MVs alone. Based on the Ang1 PCR results and protein levels in the culture medium, the beneficial effect of MSC MV on HLMVEC permeability may possibly be through increased expression of Ang1 by cytomix injured HLMVECs treated with MSC MV.
Figure 6.
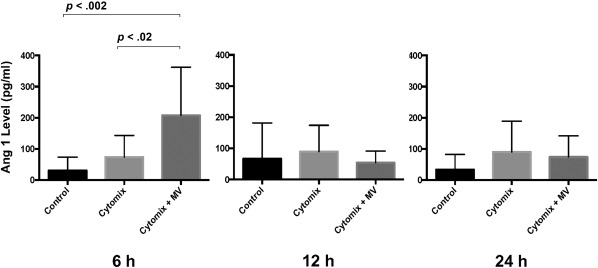
Administration of mesenchymal stem cell (MSC) MVs increased Ang1 protein secretion by human lung microvascular endothelial cell (HLMVEC) injured by cytomix. Ang1 levels in the culture medium of HLMVEC injured with or without cytomix at 6, 12, and 24 hours were detected by ELISA. MSC MVs treatment significantly increased Ang1 secretion at 6 hours. There were no significant differences in Ang1 levels at 12 or 24 hours. Data are presented as mean ± SD, N = 9, Individual p values are presented for comparison between groups using ANOVA post hoc Tukey HSD test. Abbreviation: ANOVA, analysis of variance; MV, microvesicle.
Effect of Ang1 siRNA Pretreatment of MSCs on the Therapeutic Effect of MSC MV on Protein Permeability of Cytomix Injured HLMVEC
To confirm the role of MSC MV derived Ang1 in the restoration of protein permeability across HLMVEC monolayer, we pretreated MSCs with Ang1 siRNA prior to the isolation of the MV. We previously found that pretreatment of MSCs with Ang1 siRNA significantly reduced Ang1 mRNA in the cells. Administration of MVs derived from MSCs pretreated with Ang1 siRNA for 24 hours significantly reduced Ang1 mRNA levels in cytomix injured HLMVECs at 6 hours by RT‐PCR compared with Neg siRNA MSC MV treatment group. In addition, Ang1 levels in the culture medium of cytomix injured HLMVECs were significantly reduced at 6 hours (Supporting Information Fig. S3 and Fig. 7A, 7B). More importantly, the therapeutic effect of MSC MV pretreated with Ang1 siRNA on protein permeability across cytomix injured HLMVEC were largely eliminated (Fig. 7C). Ang1 siRNA pretreatment of MSC eliminated much of beneficial effects of MSC MVs, suggesting that the therapeutic effect of MSC MV was related to its ability to increase Ang1 expression by HLMVECs injured by cytomix.
Figure 7.
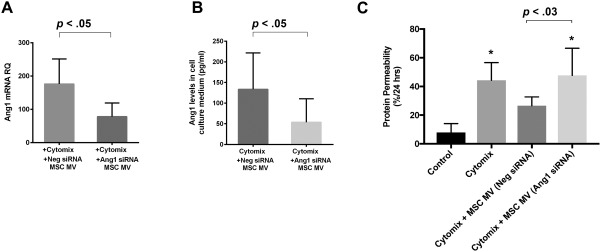
Effect of Ang1 siRNA pretreatment of MSCs on the therapeutic effect of MSC MV on protein permeability across cytomix injured human lung microvascular endothelial cell (HLMVEC). Pretreatment of MSCs with Ang1 siRNA reduced Ang1 mRNA in the MSC MVs. (A): Administration of MSC MVs pretreated with Ang1 siRNA decreased Ang1 mRNA levels in cytomix injured HLMVECs at 6 hours by real‐time polymerase chain reaction compared to MSC MV pretreated with a Neg siRNA treatment group. Moreover, (B): Ang1 levels in the cell culture medium of HLMVECs injured by cytomix were significantly reduced at 6 hours to control levels following administration of MSC MV pretreated with Ang1 siRNA compared to MSC MV pretreated with a Neg siRNA treatment group. (C): In a transwell coculture system, the therapeutic effect of MSC MV pretreated with Ang1 siRNA on protein permeability across cytomix injured HLMVEC were largely eliminated. Data are presented as mean ± SD, N = 9, *, p is significant vs. control using ANOVA with post hoc Tukey HSD test. Individual p values are presented for comparison between groups using post hoc Tukey HSD test. Abbreviations: ANOVA, analysis of variance; MSCs, mesenchymal stem cells; MV, microvesicle; siRNA, small interfering RNA.
Discussion
The major findings of our current study are (a) MSC MVs restored protein permeability across injured HLMVECs in a dose dependent manner; (b) the restoration of protein permeability by MSC MV was associated with the prevention of “actin stress fibers” and the restoration of tight and adherens junction proteins following inflammatory injury; (c) internalization of MSC MV via surface receptors such as CD44 was critical for its therapeutic effect; (d) transfer of mRNA for Ang1 from the MSC MV to the injured endothelium with subsequent expression of the soluble factor was one of the mechanism underlying the restoration of protein permeability; and (e) Ang1 siRNA pretreatment of MSCs prior to the isolation of the MVs eliminated the therapeutic effect of MSC MVs.
It is now accepted that most cell types including stem, hematopoietic and myeloid, cancer, epithelial, endothelial, mesenchymal, etc. release MVs 17. More importantly, these MVs are biologically active in both physiological and pathophysiological processes as mediators of cell‐to‐cell communication or through direct effects on target cells 18, 19, 20. Recently, MVs derived from MSC have been shown to be biologically active and able to exert therapeutic effects on various organ injury models 12, 13, 21. Our previous studies demonstrated that MSC MV significantly reduced pulmonary edema in models of both lipopolysaccharide or the E. coli pneumonia in mice 14, 15 and an ex vivo perfused human lung model of ischemia/reperfusion 22. However, the underlying mechanisms were not fully known.
In this present study, we used an in vitro model of protein permeability across HLMVEC injured by cytomix using FITC‐dextran (70 KDa) as a surrogate of albumin in a transwell coculture system in order to study the effects of MSC MV (Fig. 1A) on endothelial permeability as previously described 16, 23. Administration of MSC MVs restored protein permeability across HLMVEC exposed to cytomix in a dose dependent manner to near control values, whereas administration of MV from normal human lung fibroblasts had no beneficial effect (Fig. 2). Surprisingly, coculture with MSCs in the lower chamber of the Transwell plate had minimal beneficial effects as well. They are several potential explanations for this finding: (a) the dose of MSCs used (750,000 cells) may not be equivalent in terms of potency to MSC MV used to restore protein permeability (60 µl MV is the MVs isolated from 6 million MSCs); (b) the degree of injury, IL‐1β, TNF‐α, and IFN‐γ at 50 ng/ml, or the inflammatory milieu may itself modify MSC, diminishing its therapeutic potency. Further studies are needed.
Cytoskeleton and intercellular junctions among endothelial cells including adherens and tight junctions are essential structures for the regulation of endothelial permeability. F‐actin, a critical cytoskeleton protein, is typically distributed in the periphery of cells, forming a tight monolayer, by immunofluorescent staining. After exposure to cytomix for 24 hours, total cellular F‐actin was reorganized toward the center of the cells to form “actin stress fibers,” creating pores between cells and leading to increased protein permeability. Exposure to cytomix for 24 hours also decreased the total amount of VE‐cadherin and ZO‐1 dramatically as seen between cells. Administration of MSC MV largely prevented the reorganization of cytoskeleton protein F‐actin into “actin stress fiber” as well as the loss of VE‐cadherin and ZO‐1 in cytomix injured HLMVECs (Fig. 3). In addition, by Western blot analyses, MSC MV restored total protein levels of ZO‐1 and VE‐cadherin in injured HLMVECs. In previous studies 15, 21, 22, 24, CD44 receptor played an important role in the uptake of MSC MV in the target tissue. In the current study, CD44 was also required for MSC MV to incorporate into HLMVEC injured by cytomix, which was essential for restoring protein permeability (Fig. 4).
MSC MV is enriched with microRNAs, mRNAs, proteins/peptides, organelles, bioactive lipids, etc. To understand the potential mechanisms underlying the therapeutic effect of MSC MVs, we studied several mRNAs contained in the MV which may be translated into a soluble factor by the target cells, such as Ang1. Ang1, as a ligand for the receptor‐tyrosine kinase Tie2, is responsible for a quiescent vascular phenotype and is known as an endothelial survival 25 and vascular stabilization factor that reduces endothelial permeability and inhibits leukocyte‐endothelium interactions by modifying endothelial cell adhesion molecules and cell junctions 26, 27. Multiple studies have investigated its anti‐inflammatory, antipermeability, and endothelial protective characteristics. In LPS induced ALI in mice, MSC or MSC transfected with the human Ang1 gene reduced pulmonary vascular injury and the recruitment of inflammatory cells into the lung 28, 29. We also previously found that the secretion of Ang1 by MSCs was critical in restoring fluid transport across primary culture of human alveolar epithelial type II cells 16. More recently, Tang et al. 30 found that Ang1 in MSC MV was important in reducing inflammation and pulmonary edema in LPS induced ALI in mice. In the current study, we found that Ang1 gene expression in cytomix‐injured HLMVEC was significantly increased at 6 and 12 hours and Ang1 protein secretion was significantly increased at 6 hours following MSC MV treatment. More importantly, the therapeutic effect of MSC MV on restoring protein permeability was eliminated when MSC was pretreated with Ang1 siRNA (Figs. 5, 6, 7).
There are several limitations which require further study: (a) the transfer of Ang1 mRNA from the MVs to the injured endothelium is not the only mechanism for restoring protein permeability. We also found that the mRNA for S1P kinase, responsible for the phosphorylation of sphingosine to S1P, was also elevated following MSC MV treatment. S1P is a potent angiogenic factor that enhances lung endothelial cell integrity and prevents vascular permeability and alveolar flooding in various preclinical animal models of ALI 31. Clearly, other mRNA, microRNA, and even organelles (i.e., mitochondria 32) may be involved and should be studied; (b) Figure 2 seems to suggest that the biological effect of MSC MV may be stronger than that of MSCs. Whether the results are due to limitations of the Transwell system (i.e., number of MSCs that can be plated in the bottom chamber) or whether MSC has a therapeutic ceiling, whereas MV do not, needs to be studied further.
Conclusion
MSC MVs restored protein permeability across HLMVECs injured by an inflammatory insult in part by maintaining intercellular junctions and preventing “actin stress fiber” formation. Incorporation of MSC MVs into HLMVECs through the surface receptor CD44 was required for restoration of protein permeability. The therapeutic effect of MSC MV was associated with the transfer of Ang1 from the MV to the injured HLMVECs with subsequent secretion of the antipermeability factor.
Author Contributions
S.H.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; J.P., A.L., J.L., X.Z., and Q.H.: collection and assembly of data; J.‐W.L.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowlegments
We thank Seonguk Kim, M.D., Ph.D., for assistance with the experiments and help in the interpretation of the data. This research was supported by Grant HL‐113022 from NHLBI (Dr. Jae‐Woo Lee).
References
- 1. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122:2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acute Respiratory Distress Syndrome Network , Brower RG, Matthay MA et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 4. Guerin C, Reignier J, Richard JC et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159–2168. [DOI] [PubMed] [Google Scholar]
- 5. Hu SL, He HL, Pan C et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: A meta‐analysis of randomized controlled trials. Crit Care 2014;18:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papazian L, Forel JM, Gacouin A et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107–1116. [DOI] [PubMed] [Google Scholar]
- 7. Bellani G, Laffey JG, Pham T et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- 8. Bruno S, Collino F, Tetta C et al. Dissecting paracrine effectors for mesenchymal stem cells. Adv Biochem Eng Biotechnol 2013;129:137–152. [DOI] [PubMed] [Google Scholar]
- 9. Rani S, Ryan AE, Griffin MD et al. Mesenchymal stem cell‐derived extracellular vesicles: Toward cell‐free therapeutic applications. Mol Ther 2015;23:812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turturici G, Tinnirello R, Sconzo G et al. Extracellular membrane vesicles as a mechanism of cell‐to‐cell communication: Advantages and disadvantages. Am J Physiol Cell Physiol 2014;306:C621–C633. [DOI] [PubMed] [Google Scholar]
- 11. Ratajczak J, Wysoczynski M, Hayek F et al. Membrane‐derived microvesicles: Important and underappreciated mediators of cell‐to‐cell communication. Leukemia 2006;20:1487–1495. [DOI] [PubMed] [Google Scholar]
- 12. Gatti S, Bruno S, Deregibus MC et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia‐reperfusion‐induced acute and chronic kidney injury. Nephrol Dial Transplant 2011;26:1474–1483. [DOI] [PubMed] [Google Scholar]
- 13. Lai RC, Arslan F, Lee MM et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 14. Zhu YG, Feng XM, Abbott J et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells 2014;32:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monsel A, Zhu YG, Gennai S et al. Therapeutic effects of human mesenchymal stem cell‐derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med 2015;192:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang X, Neyrinck AP, Matthay MA et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin‐1. J Biol Chem 2010;285:26211–26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirsova P, Ibrahim SH, Verma VK et al. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016;64:2219–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schorey JS, Harding CV. Extracellular vesicles and infectious diseases: New complexity to an old story. J Clin Invest 2016;126:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J Clin Invest 2016;126:1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zappulli V, Friis KP, Fitzpatrick Z et al. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest 2016;126:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindoso RS, Collino F, Bruno S et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev 2014;23:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gennai S, Monsel A, Hao Q et al. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant 2015;15:2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu J, Li X, Huang L et al. HSPA12B inhibits lipopolysaccharide‐induced inflammatory response in human umbilical vein endothelial cells. J Cell Mol Med 2015;19:544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bruno S, Grange C, Deregibus MC et al. Mesenchymal stem cell‐derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20:1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwak HJ, So JN, Lee SJ et al. Angiopoietin‐1 is an apoptosis survival factor for endothelial cells. FEBS Lett 1999;448:249–253. [DOI] [PubMed] [Google Scholar]
- 26. Kim I, Moon SO, Park SK et al. Angiopoietin‐1 reduces VEGF‐stimulated leukocyte adhesion to endothelial cells by reducing ICAM‐1, VCAM‐1, and E‐selectin expression. Circ Res 2001;89:477–479. [DOI] [PubMed] [Google Scholar]
- 27. Gamble JR, Drew J, Trezise L et al. Angiopoietin‐1 is an antipermeability and anti‐inflammatory agent in vitro and targets cell junctions. Circ Res 2000;87:603–607. [DOI] [PubMed] [Google Scholar]
- 28. Mei SH, McCarter SD, Deng Y et al. Prevention of LPS‐induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007;4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCarter SD, Mei SH, Lai PF et al. Cell‐based angiopoietin‐1 gene therapy for acute lung injury. Am J Respir Crit Care Med 2007;175:1014–1026. [DOI] [PubMed] [Google Scholar]
- 30. Tang XD, Shi L, Monsel A et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang‐1 mRNA. Stem Cells 2017;35:1849–1859. [DOI] [PubMed] [Google Scholar]
- 31. Natarajan V, Dudek SM, Jacobson JR et al. Sphingosine‐1‐phosphate, FTY720, and sphingosine‐1‐phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol 2013;49:6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrison TJ, Jackson MV, Cunningham EK et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med 2017;196:1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3


