Abstract
Angiitis‐induced critical limb ischemia (AICLI) patients constitute a remarkable proportion of no‐option critical limb ischemia (CLI) patients. Stem cell therapy has become an innovative and promising option for no‐option CLI patients. As one of these promising stem cell therapies, purified CD34+ cell transplantation (PuCeT) has shown favorable short‐term results. However, the long‐term efficacy of PuCeT has yet to be reported. This study evaluates the long‐term efficacy of PuCeT in AICLI patients. Twenty‐seven AICLI patients were enrolled from May 2009 to December 2011. Granulocyte colony‐stimulating factor (G‐CSF) and enoxaparin sodium were administered for 5 days. On day 5, CD34+ cell isolation was performed, and cells were transplanted by intramuscular injection. The primary endpoint, major‐amputation‐free survival rate (MAFS), as well as secondary endpoints, such as peak pain‐free walking time (PPFWT) and the Wong‐Baker FACES pain rating scale score (WFPRSS), were routinely evaluated during the 5‐year follow‐up period. The endpoints were as follows: the MAFS was 88.89%; PPFWT increased from 3 ± 3 to 17 ± 6 minutes; WFPRSS decreased from 7 ± 2 to 0.3 ± 1.7; the ulcer healing rate was 85.71%; the recurrence rate was 11.11%; and SF‐36v2 scores were significantly improved at 5 years after PuCeT. The rate of labor recovery 5 years after PuCeT was 65.38%, and no severe adverse effect was observed during the treatment. PuCeT demonstrated long‐term efficacy and durability as a treatment of AICLI not only in achieving limb salvage but also in recovering the labor competence and improving the quality of life of patients. Stem Cells Translational Medicine 2018;7:583–590
Keywords: Angiogenesis, Adult stem cells, CD34, Cellular therapy
Significance Statement.
Long‐term efficacy and durability of autologous transplantation of purified CD34+ cells was shown for the treatment of angiitis‐induced no‐option critical limb ischemia, including achievement of ideal limb salvage, recovery of labor competence, and improved quality of life.
Introduction
Critical limb ischemia (CLI) is a classic vascular disease caused by various etiologies and is associated with a substantial major amputation rate and mortality 1. Endovascular and surgical reconstruction are the mainstream treatments for CLI. Particularly, the rapid advancements in endovascular techniques and devices have improved the recanalization rate. Nevertheless, the classical treatments are unfeasible in approximately 15%–20% of CLI patients 1 (no‐option CLI) due to a high postoperative re‐occlusion rate or poor anatomical conditions. Among CLI patients, AICLI is defined as CLI caused by thromboangiitis obliterans (TAO) or by angiitis‐related autoimmunological diseases, such as systemic lupus erythematosus (SLE), Crohn's disease, erythema nodosum, scleroderma, and psoriasis. These diseases show a common propensity for affecting the distal small or microvessels and destroying the anatomic run‐off necessary for either the endovascular or the surgical reconstruction. Therefore, AICLI composes a remarkable proportion of the no‐option CLI population.
In the last 20 years, cell therapy has rapidly developed and has been considered a promising treatment for CLI patients. As one of the stem cell therapies, PuCeT has demonstrated encouraging outcomes 2, 3. Similarly, in our pilot study conducted from 2009 to 2011, PuCeT demonstrated favorable early outcomes in AICLI 4. The current study is an extension of our pilot study and aims to evaluate the long‐term efficacy of PuCeT in AICLI patients.
Materials and Methods
The protocol was approved by the Ethics Committee of Zhongshan Hospital affiliated to Fudan University (the number of the approval: No. 2009‐016) and was conducted according to the World Medical Association's Declaration of Helsinki. All candidates provided written informed consent before enrollment.
Patient Enrollment
From May 2009 to December 2011, 27 patients with no‐option AICLI were enrolled in the study. The inclusion and exclusion criteria have been previously described 4.
Treatment Procedures
G‐CSF and enoxaparin sodium were subcutaneously administered to the patients for 5 days. Apheresis (COM.TEC; Fresenius HemoCare, Friedberg, Germany) and subsequent cell isolation via immunomagnetic separation (CliniMACS instrument and CD34 reagent; Miltenyi Biotec, Bergisch Gladbach, Germany) was performed on day 5. Apheresis and purified products were tested for white blood cell (WBC) and CD34+ cell counts. Cell transplantation was performed by intramuscular injection under spinal anesthesia (for the lower extremities) or general anesthesia (for the upper extremities). Additional details about the procedure have been previously described 4. Patients with autoimmunological diseases were administered regular immunological treatment and follow‐up by rheumatologists.
Data Collection and Evaluation
Endpoints
The primary endpoint was the major‐amputation‐free survival rate (MAFS). The secondary endpoints included peak pain‐free walking time (PPFWT) on a treadmill at 2.5 km/h and 10% incline, the Wong‐Baker FACES pain rating scale score (WFPRSS), the ulcer healing rate, and the recurrence rate. Data of all these parameters were collected before PuCeT and at 4, 8, 12, 24, 52 (1 year), 104 (2 years), 156 (3 years), 208 (4 years), and 260 weeks (5 years) after PuCeT.
SF‐36v2 was used to evaluate the quality of life of the patients at baseline, 1 year, and 5 years after transplantation. The questionnaires were completed either by the patients themselves or with the help of their family members or doctors. The time points at which patients returned to work were also recorded during the follow‐up evaluations.
Recurrence was defined as ischemia reoccurrence on the treated limb after its complete recovery (relief of rest pain, and healing of ulcer and gangrene), while ischemia onset was defined as new lesions on the untreated limbs.
The major adverse clinical events included all‐cause death, major amputation, peripheral blood mononuclear cell (PBMNC) therapy, repeat CD34+ cell therapy, or endovascular treatment on the ipsilateral limb after the initial transplantation. The minor adverse clinical events included any adverse events during mobilization, apheresis or injection; abnormal WBC count; and pathological angiogenesis. The incidences of all of these events were recorded at 12, 24, 52 (1 year), 104 (2 years), 156 (3 years), 208 (4 years), and 260 weeks (5 years) after transplantation. To detect potential pathological angiogenesis, the fundus oculi of the patients were examined by an ophthalmologist before transplantation and at 4, 8, 12, 24, 52 (1 year), 104 (2 years), 156 (3 years), 208 (4 years), and 260 weeks (5 years) after PuCeT.
Statistical analysis
The efficacy parameters were expressed as the mean ± standard deviation and were analyzed via t‐test. Qualitative data were expressed as percentage. A value of p < .05 was considered a statistically significant difference. The Kaplan–Meier method was used to estimate the overall survival rate (OS) and MAFS. The analysis was completed using SPSS 22 software (IBM Corporation, NY, USA).
Results
Baseline Characteristics
Detailed patients’ baseline characteristics are shown in Table 1. Among the 27 patients included from May 2009 to December 2011, 22 were from our pilot study 4, and all 27 were AICLI patients. These patients had a mean age of 40 ± 8 years (range, 23–54) and included 26 males and 1 female. Until December 2016, 26 patients underwent regular follow‐up evaluations for an average of 75 ± 8 months (range, 61–92); however, patient 22 withdrew from the study 12 months after therapy. Of the 27 patients, 23 patients were diagnosed with TAO according to the Olin criteria 5, and the remaining 4 patients were diagnosed with angiitis due to other etiologies, such as SLE, erythema nodosum, and Crohn's disease. Patients 7 and 10 were already diagnosed with erythema nodosum and Crohn's disease before ischemia. For this reason, we highly suspected that the vasculitis was secondary to their autoimmune diseases. A total of 32 limbs (4 upper limbs and 28 lower limbs) were treated. The dose of transplanted CD34+ cells was 7.02 ± 6.13 × 107 per patient, 10.20 ± 9.70 × 105 per kilogram and 9.88 ± 9.81 × 105 per kilogram per limb. Technical success was achieved in all patients.
Table 1.
Baseline characteristics
| No.a | Age/year | Sex (M/F) | Cause of CLIb | Rutherford scale |
|---|---|---|---|---|
| 1 | 42 | M | TAOc | 5 |
| 2 | 29 | M | TAO | 4 |
| 3 | 34 | M | TAO | 5 |
| 4 | 54 | M | TAO | 5 |
| 5 | 41 | M | TAO | 5 |
| 6 | 50 | M | TAO | 5 |
| 7 | 23 | M | Arteritis, erythema nodosum | 5 |
| 8 | 34 | M | TAO | 5 |
| 9 | 40 | M | TAO | 5 |
| 10 | 47 | M | Arteritis, Crohn's disease | 4 |
| 11 | 33 | M | TAO | 5 |
| 12 | 49 | M | TAO | 4 |
| 13 | 41 | M | TAO | 4 |
| 14 | 49 | M | TAO | 5 |
| 15 | 44 | M | Arteritis, SLEd | 5 |
| 16 | 45 | F | Arteritis, SLE | 5 |
| 17 | 48 | M | TAO | 5 |
| 18 | 39 | M | TAO | 5 |
| 19 | 35 | M | TAO | 4 |
| 20 | 47 | M | TAO | 5 |
| 21 | 43 | M | TAO | 5 |
| 22 | 31 | M | TAO | 5 |
| 23 | 45 | M | TAO | 5 |
| 24 | 30 | M | TAO | 5 |
| 25 | 25 | M | TAO | 5 |
| 26 | 47 | M | TAO | 5 |
| 27 | 47 | M | TAO | 4 |
No. indicates patient number.
CLI indicates critical limb ischemia.
TAO indicates thromboangiitis obliterans.
SLE indicates systemic lupus erythematosus.
At enrollment, 26 patients (96.30%, 26/27, except patient 16) were current smokers. Although the literature and education about the harm of smoking and the importance of quitting tobacco was frequently provided, only 7 patients successfully quit smoking. The other 19 (73.08%, 19/26) relapsed within 1 year after transplantation. Eight patients (8/23) with TAO presented with superficial thrombophlebitis. None of the patients were diagnosed with hypertension, hyperlipidemia, diabetes mellitus, or cerebral or myocardial infarction. In addition, all 27 patients underwent the best medical treatment for more than 3 months prior to PuCeT. Three patients underwent toe amputations before transplantation. Three patients underwent endovascular interventions, including percutaneous transluminal angioplasty (PTA), stenting, and/or thrombolysis. Patient 10 underwent a thrombectomy twice, and patient 13 underwent the surgery once. All patients underwent antiplatelet therapy with alprostadil and/or cilostazol for more than 3 months before PuCeT.
Efficacy Evaluation
During the follow‐up period, 3 patients (patients 9, 24, and 25) underwent major amputations, 2 below and 1 above the knee. Patients 9 and 25 underwent amputations within 12 weeks of the transplantation because of the unrelieved gangrenes and rest pain, and patient 24 required an amputation below the left knee at week 120. Patient 6 had a minor amputation at week 252. The MAFS was 88.89% (24/27) at week 208 and remained the same at week 260 (Fig. 1).
Figure 1.
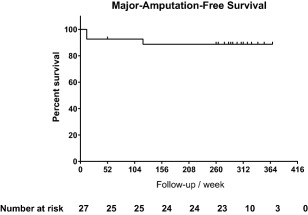
Kaplan–Meier analysis: MAFS 5 years after PuCeT.
Fifteen patients (15/27) were able to take the PPFWT test before transplantation. The PPFWT value is recorded as 0 if a patient cannot walk. The average PPFWT was 3 ± 3 minutes (n = 27, range, 0–10) at baseline and increased significantly to 9 ± 6 minutes (n = 25, range, 0–21) (p < .001) at week 12, 15 ± 7 minutes (n = 25, range, 0–21) (p < .001) at week 24, and 17 ± 6 minutes (n = 23, range, 4–21) (p < .001) at week 260. At week 260, 23 patients (23/27, 3 amputated and 1 withdrew) were able to take the PPFWT test. The serial changes of PPFWT are shown in Figure 2A. Of note, 13 of these patients completed the entire 21‐minute test: they could walk longer than 21 minutes and only needed to stop because the treadmill was set to stop at 21 minutes. However, 4 of these 13 patients were not able to walk at baseline.
Figure 2.
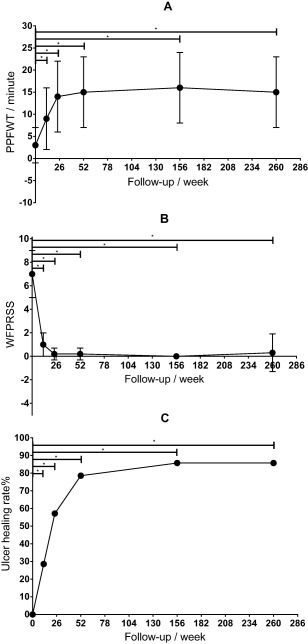
Serial changes in PPFWT (A), WFPRSS (B), and the ulcer healing rate (C). * p < .05 compared with baseline.
The severity of rest pain was evaluated using the WFPRSS, for which the average pre‐PuCeT value was 7 ± 2 (n = 27, range, 4–10). The average time to onset of rest pain relief was 3 ± 1 weeks (n = 27, range, 1–4). The WFPRSS significantly decreased to 2 ± 2 (n = 27, range, 0–6) (p < .001) and 1 ± 2 (n = 27, range, 0–6) (p < .001) at weeks 4 and 8, respectively. Except for amputees and patient 22, who was lost to follow–up, the WFPRSS was 0.2 ± 0.6 (n = 24, range, 0–2) (p < .001) at week 52 and 0.3 ± 1.7 (n = 23, range, 0–8) (p < .001) at week 260. The serial changes in the WFPRSS are shown in Figure 2B. All patients experienced rest pain before PuCeT, and 22 patients experienced complete rest pain relief by week 260. Recurrent ulcers began in patient 6 at week 252, and the WFPRSS of this patient was 8. The rate of complete pain relief at week 260 was 81.48% (22/27).
At enrollment, 7 patients had gangrenes and 14 patients had skin ulcers. And 13 of these 14 patients were completely healed 8 ± 8 months (range, 2–33 months) after therapy. By week 104, there were 2 unhealed cases (patient 6 and 15); these cases were TAO patients whose ulcers were at the bottom of their feet, and the ulcer of patient 6 finally healed at week 130. At week 108, patient 24 began experiencing recurrent ulcers and underwent amputation at week 120; however, patient 6 underwent a minor amputation at week 252. Therefore, the total healing rate of ulcers was 85.71% (12/14) at weeks 104 and 260. The serial changes in ulcer healing rates are shown in Figure 2C. Figure 3 shows the ulcer healing process for patient 18.
Figure 3.
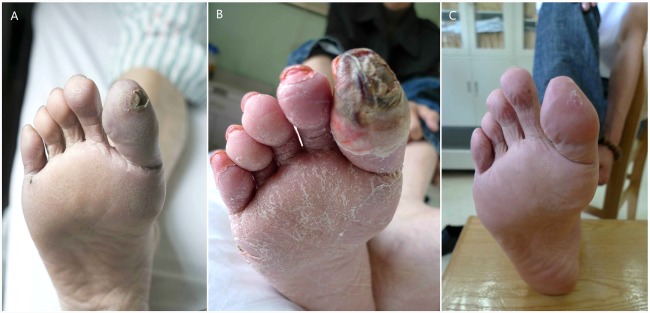
The ulcer healing process of patient 18. (A): Ulcer and cyanosis could be seen on the tip of the first toe 5 days before transplantation. (B): The ulcer was enlarged 3 months after the transplantation, but the cyanosis was improved, and scaling was observed on the bottom of the foot and toes. (C): The ulcer was completely healed 6 months after the transplantation.
Up to December 2016, 5 patients (3, 6, 19, 21, and 24) underwent a second cell transplantation. Of these 5 patients, 2 patients (19 and 21) developed a new lesion on the contralateral lower extremity. Recurrence occurred in patients 3, 6, and 24. Patient 6 developed a new ulcer at a different area of the ipsilateral foot. Ischemic symptoms reappeared at the same site in patients 3 (unhealed wound after a traffic accident) and 24 (ulcer). Ischemic symptoms appeared again on the contralateral limb of patient 25, but this patient did not receive a second transplantation. The 5‐year recurrence rate was 11.11% (3/27), and the re‐transplantation rate was 18.52% (5/27). Three patients underwent PuCeT again (3, 6, and 19), while the other 2 (21 and 24) underwent PBMNC transplantation at other hospitals. Of all 27 patients, only patient 24 received endovascular intervention on the treated limb after PuCeT. This patient was treated in the left upper limb and both lower limbs, all of which were critically ischemic. Similar symptoms reappeared 6 months later on the left lower limb, and the patient underwent PTA at another hospital. After PTA, the ischemia was relieved but reoccurred 21 months later. Patient 24 then received a PBMNC transplantation at another hospital; however, the procedure was ineffective, and at 120 weeks, the patient ultimately underwent amputation below the left knee.
Notably, 17 patients (65.38%, 17/26) not only underwent a successful limb salvage but also fully recovered their labor competence and returned to their original jobs by week 260. Results of SF‐36v2 showed a consistent conclusion. The score of all the eight sections of the questionnaire, including physiological function, role‐physical, bodily pain, general health, vitality, social function, role‐emotional, and mental health, was significantly improved at week 52 (p < .001) and week 260 (p < .001) (Fig. 4). Four patients with autoimmune diseases visited their rheumatologists regularly after PuCeT, and their diseases were well controlled according to their lab reports.
Figure 4.
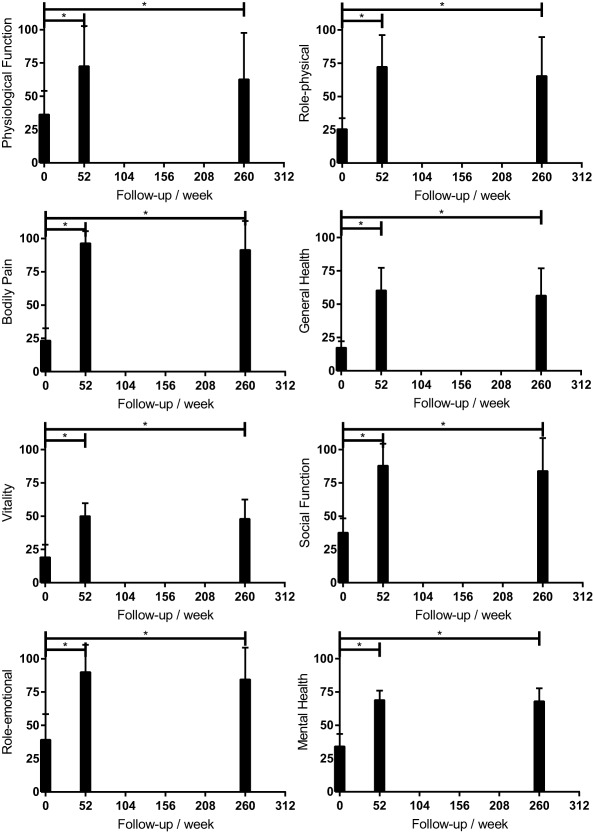
Results of the SF‐36v2 questionnaire at baseline, 1 year, and 5 years after the PuCeT. *p < .05 compared with baseline.
Details regarding the outcomes of the patients with and without total smoking cessation are shown in Table 2. There was no significant difference between patients with and without smoking cessation in terms of the major amputation rate, the PPFWT, the WFPRSS, the ulcer healing rate, or the recurrence rate. In addition, a subanalysis (Table 3) was performed to focus on the TAO patients given that these individuals were the primary focus of our study (88.89%, 24/27). The results of this analysis showed similar outcomes in TAO patients compared to the entire cohort.
Table 2.
Outcomes of patients with and without total smoking cessation
| Outcome | Time | Cessation | Relapse | p value |
|---|---|---|---|---|
| Major amputation | 260 weeks | 14.29% (1/7) | 11.11% (2/18) | .645 |
| PPFWTa/min | Baseline | 4 ± 4 (n = 7) | 2 ± 3 (n = 18) | .388 |
| 260 weeks | 18 ± 6 (n = 7) | 17 ± 14 (n = 18) | .84 | |
| WFPRSSb | Baseline | 6 ± 2 (n = 7) | 7 ± 2 (n = 18) | .655 |
| 260 weeks | 0 ± 0 (n = 7) | 0 ± 2 (n = 18) | .204 | |
| Ulcer healing | 260 weeks | 100% (4/4) | 80% (8/10) | .714 |
| Recurrence | 260 weeks | 0% (0/7) | 16.67% (3/18) | .355 |
PPFWT indicates peak pain‐free walking time.
WFPRSS indicates the Wong‐Baker FACES pain rating scale score.
Table 3.
Subanalysis of TAO patients
| Baseline | 12 weeks | 24 weeks | 52 weeks | 156 weeks | 260 weeks | |
|---|---|---|---|---|---|---|
| Major amputation | 0% (0/22) | 9.09% (2/22) | 9.09% (2/22) | 9.09% (2/22) | 13.64% (3/22) | 13.64% (3/22) |
| PPFWTa/min | 3 ± 4 | 10 ± 7*b | 15 ± 8* | 15 ± 9* | 16 ± 8* | 15 ± 8* |
| WFPRSSb | 7 ± 1 | 1 ± 2* | 0 ± 1* | 0 ± 1* | 0 ± 0* | 0.4 ± 17* |
| Ulcer healing | 0% (0/12) | 25% (3/12) | 58.33% (7/12) | 83.33% (10/12) | 91.67% (11/12) | 91.67% (11/12) |
| Recurrence | 0% (0/22) | 0% (0/22) | 0% (0/22) | 4.55% (1/22) | 9.09% (2/22) | 13.64% (3/22) |
PPFWT indicates peak pain‐free walking time.
*p < .05 compared with baseline.
WFPRSS indicates the Wong‐Baker FACES pain rating scale score.
Safety Evaluation
As previously described, five patients underwent a second cell transplantation. However, only three of these patients (patients 3, 6, and 24) underwent transplantation on the ipsilateral limb. Of these individuals, patient 24 also received endovascular intervention on the ipsilateral limb. Three patients underwent major amputations during the follow‐up period. At week 260, the OS was 100%, and the occurrence rate of major adverse clinical events was 22.22% (6/27). During the mobilization, apheresis, and injection components of treatment, no serious adverse events were observed, and the WBC counts decreased to normal within 1 week after transplantation in all patients. No pathological angiogenesis was observed on the fundus oculi.
Discussion
Therapeutic angiogenesis has been considered an innovative and promising treatment for no‐option CLI. Many studies using different methods, such as vascular endothelial growth factor (VEGF)‐related gene transfer 6, 7, bone‐marrow mononuclear cell (BMMNC) 8, 9, 10, 11, 12, PBMNC 13, 14, 15, 16, 17, 18, or CD34+ cell 2, 3, 4 transplantation, have been completed and have proven their safety and efficacy. The long‐term outcome after CD34+ cell therapy in CLI patients was already reported by Kinoshita et al. in 2012 19. However, there were only 17 patients enrolled (5 ASO patients and 12 TAO patients) in Kinoshita's study 19 and the long‐term results elucidating the efficacy and durability of PuCeT for AICLI remain rare. Our previous study launched in 2009 assessed the feasibility and short‐term safety and efficacy of PuCeT in treating CLI patients 4. At the beginning of the study, ASO‐induced CLI patients were also included. However, as the study progressed, we decided to select AICLI patients, instead of ASO patients, as the priority targets for the following reasons. First, particularly with the rapid development of devices and skills for endovascular intervention, surgical and endovascular revascularization demonstrated higher success rates and better early results in ASO patients than in AICLI patients. Second, the outcomes in the three ASO patients we reported on in our pilot study appeared to be poor 4. All of the ASO patients underwent major amputations within 3 months after PuCeT, similar to the disappointing results from ASO patients in other studies 2, 8. In contrast, the outcomes of AICLI patients showed extreme promise. The short‐ and mid‐term results of our study revealed a favorable limb salvage rate after PuCeT and suggested that a satisfying long‐term outcome could be expected in certain patients. In addition, given that most of the AICLI patients were significantly younger and had longer life expectancies, the ideal results of treatment for these patients were not only limb salvage but also recovery of labor competence and improvement on the quality of life.
There are numerous revascularization options for AICLI patients, but the effects are far from satisfactory. Taking TAO cases as an example, these patients represent the vast majority of AICLI patients and can be treated by many methods, including surgical bypass, endarterectomy, embolectomy, sympathectomy, superficial vein arterialization, PTA, thrombolysis, and stenting; however, none of these methods provide ideal mid‐ to long‐term efficacy. Without cell therapy, the major amputation rate of TAO patients, including CLI and claudicants, has been reported to be as high as 12%–31% 20, 21, 22. Ohta et al. observed that 118 TAO patients presented a total major amputation rate of 19%; moreover, this study revealed that the primary patency rates of the surgical bypass subgroup were 41% (1 year), 32% (5 years), and 30% (10 years), respectively, and the secondary patency rates of this group were 54% (1 year), 47% (5 years), and 39% (10 years), respectively. Without smoking cessation, the patency rate decreased by another 50%, and the major amputation rate after bypass occlusion was 14% 22. Another study examining 69 TAO cases showed that the patients suffered from acute ischemia 5.4 times after the initial attack. The 5‐year major amputation rate was 26%, and 34.8% of the patients lost their ability to work before the age of 42 years old 20.
The pathological features of AICLI were considered to be the cause of the poor effect of the conventional revascularization methods. First, AICLI frequently involves distal middle and small vessels and thus destroys the outflow necessary for successful endovascular intervention and surgical bypass. Second, as the predominant form of AICLI, TAO is a type of vasculitis that is characterized by a highly inflammatory thrombus with relative sparing of the vessel wall and that is apparently different from ASO due to the preservation of the internal elastic lamina 21. As a result of these characterizations of TAO, during conventional interventions, the guidewire could easily pass through the lesion, and the balloon could inflate without much resistance; however, the involved vessel frequently recoils shortly after surgical intervention. This is unlike ASO lesions, which show a characteristic loss of the wall elasticity (damaged elastic lamina) and plaque‐induced occlusion of the lumen; as such, these lesions tend to stay open after ballooning and stenting 23. Third, superficial phlebitis, which was observed in 40%–60% of TAO cases 24, often damaged the great saphenous vein that was considered an ideal vascular graft for the bypass. Finally, the inflammatory nature of AICLI probably precipitates the intimal hyperplasia after either endovascular or surgical intervention and results in re‐occlusion. Although there are many treatment options available, tobacco cessation seemed to be the only successful treatment for AICLI. Despite this, tobacco cessation failed to reduce the risk of amputation.
AICLI caused by other etiologies, such as SLE and giant cell arteritis (GCA), were not as common as TAO and were rarely reported by other studies focusing on the long‐term outcomes of stem cell therapy treating CLI 8, 13, 19. However, the prevalence of CLI in these patients is not something to be ignored. Lie reported that the prevalence of GCA‐induced upper and lower extremity ischemia was 26% and 18%, respectively 25. The CLI prevalence in SLE patients was reported to be 1.4% in a retrospective study of 485 patients, with 71.43% of patients (5/7) having suffered from digit loss 26.
Thus, it would be meaningful to seek a new, effective and durable method of revascularization for AICLI patients. According to the 5‐year results of this study, the PPFWT was significantly increased, the total pain relief rate was 81.48% (22/27), the complete healing rate of ulcers reached 85.71% (12/14), and the MAFS was 88.89% (24/27), which were similar to the results reported by Kinoshita et al. 19. Importantly, the quality of life of the patients was noticeably improved according to the results of the SF‐36v2 questionnaire. Moreover, 65.38% (17/26) of the patients fully recovered their labor competence and returned to their previous employment; however, the remaining nine patients did not return to work but could live a physically independent life. The long‐term improvement of the quality of life is quite important for AICLI patients since they are significantly younger, but the evaluation of the quality of life was absent in previous studies 8, 13, 19.
The limitation of the current study was the absence of a placebo control group, which would have reinforced the results. A cross‐over design could have provided a suitable option for a true placebo group. However, the unsatisfactory outcomes of medical, surgical, and/or endovascular management before PuCeT in all patients could be regarded, to some extent, as control data. Second, the number of angiitis patients except those with Buerger's disease was very small in this study and the sample size was relatively small because it was based upon the previous pilot study.
Conclusion
PuCeT demonstrated long‐term efficacy and durability in treating AICLI, not only in achieving limb salvage but also in recovering the labor competence and improving the quality of life of AICLI patients. Confirmation of the conclusion of the current study is expected in the coming years as additional evidence is obtained from the long‐term outcomes of a larger number of patients.
Author Contributions
Y.F.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Z.W.: conception and design, collection and/or assembly of data; B.C.: administrative support, provision of study material or patients; T.P.: collection and/or assembly of data; S.G., Z.S., T.Z.: provision of study material or patients, collection and/or assembly of data; P.L.: administrative support; D.G., X.X., J.J., and J.Y.: provision of study material or patients; Y.S. and Y.L.: collection and/or assembly of data; Z.D. and W.F.: conception and design, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Funds (Grant No. 30801122), Shanghai Talent Development Funds (Grant No. 2010017), and Excellent Core Member Training Program of Zhongshan Hospital, Fudan University (Grant No. 2015ZSYXGG02).
Contributor Information
Zhihui Dong, Email: dzh926@126.com.
Weiguo Fu, Email: fuweiguo6288@126.com.
References
- 1. Norgren L, Hiatt WR, Dormandy JA et al. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45: S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Losordo DW, Kibbe MR, Mendelsohn F et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv 2012;5:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawamoto A, Katayama M, Handa N et al. Intramuscular transplantation of G‐CSF‐mobilized CD34(+) cells in patients with critical limb ischemia: A phase I/IIa, multicenter, single‐blinded, dose‐escalation clinical trial. Stem Cells 2009;27:2857–2864. [DOI] [PubMed] [Google Scholar]
- 4. Dong Z, Chen B, Fu W et al. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia. J Vasc Surg 2013;58:404–411. [DOI] [PubMed] [Google Scholar]
- 5. Olin JW. Thromboangiitis obliterans (Buerger's disease). N Engl J Med 2000;343:864–869. [DOI] [PubMed] [Google Scholar]
- 6. Makinen K, Manninen H, Hedman M et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: A randomized, placebo‐controlled, double‐blinded phase II study. Mol Ther 2002;6:127–133. [DOI] [PubMed] [Google Scholar]
- 7. Mohler ER, Rajagopalan S, Olin JW et al. Adenoviral‐mediated gene transfer of vascular endothelial growth factor in critical limb ischemia: Safety results from a phase I trial. Vasc Med 2003;8:9–13. [DOI] [PubMed] [Google Scholar]
- 8. Idei N, Soga J, Hata T et al. Autologous bone‐marrow mononuclear cell implantation reduces long‐term major amputation risk in patients with critical limb ischemia: A comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circ Cardiovasc Interv 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 9. Matoba S, Tatsumi T, Murohara T et al. Long‐term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (therapeutic angiogenesis by cell transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J 2008;156:1010–1018. [DOI] [PubMed] [Google Scholar]
- 10. Miyamoto K, Nishigami K, Nagaya N et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation 2006;114:2679–2684. [DOI] [PubMed] [Google Scholar]
- 11. Tateishi‐Yuyama E, Matsubara H, Murohara T et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone‐marrow cells: A pilot study and a randomised controlled trial. Lancet 2002;360:427–435. [DOI] [PubMed] [Google Scholar]
- 12. Walter DH, Krankenberg H, Balzer JO et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized‐start, placebo‐controlled pilot trial (PROVASA). Circ Cardiovasc Interv 2011;4:26–37. [DOI] [PubMed] [Google Scholar]
- 13. Horie T, Onodera R, Akamastu M et al. Long‐term clinical outcomes for patients with lower limb ischemia implanted with G‐CSF‐mobilized autologous peripheral blood mononuclear cells. Atherosclerosis 2010;208:461–466. [DOI] [PubMed] [Google Scholar]
- 14. Hoshino J, Ubara Y, Hara S et al. Quality of life improvement and long‐term effects of peripheral blood mononuclear cell transplantation for severe arteriosclerosis obliterans in diabetic patients on dialysis. Circ J 2007;71:1193–1198. [DOI] [PubMed] [Google Scholar]
- 15. Kawamura A, Horie T, Tsuda I et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J Artif Organs 2006;9:226–233. [DOI] [PubMed] [Google Scholar]
- 16. Kawamura A, Horie T, Tsuda I et al. Prevention of limb amputation in patients with limbs ulcers by autologous peripheral blood mononuclear cell implantation. Ther Apher Dial 2005;9:59–63. [DOI] [PubMed] [Google Scholar]
- 17. Moriya J, Minamino T, Tateno K et al. Long‐term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv 2009;2:245–254. [DOI] [PubMed] [Google Scholar]
- 18. Ozturk A, Kucukardali Y, Tangi F et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J Diabetes Complications 2012;26:29–33. [DOI] [PubMed] [Google Scholar]
- 19. Kinoshita M, Fujita Y, Katayama M et al. Long‐term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor‐mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis 2012;224:440–445. [DOI] [PubMed] [Google Scholar]
- 20. Borner C, Heidrich H. Long‐term follow‐up of thromboangiitis obliterans. Vasa 1998;27:80–86. [PubMed] [Google Scholar]
- 21. Piazza G, Creager MA. Thromboangiitis obliterans. Circulation 2010;121:1858–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohta T, Ishioashi H, Hosaka M et al. Clinical and social consequences of Buerger disease. J Vasc Surg 2004;39:176–180. [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi M, Sugimoto M, Komori K. Endarteritis obliterans in the pathogenesis of Buerger's disease from the pathological and immunohistochemical points of view. Circ J 2014;78:2819–2826. [DOI] [PubMed] [Google Scholar]
- 24. Juergens L, Spittel A, Fairbairn F. Thromboangiitis obliterans, (Buerger's Disease, TAO), in Peripheral Vascular Diseases. Philadelphia, PA: WB Saunders, 1980. [Google Scholar]
- 25. Lie JT. Aortic and extracranial large vessel giant cell arteritis: A review of 72 cases with histopathologic documentation. Semin Arthritis Rheum 1995;24:422–231. [DOI] [PubMed] [Google Scholar]
- 26. Jeffery RC, Narshi CB Isenberg DA. Prevalence, serological features, response to treatment and outcome of critical peripheral ischaemia in a cohort of lupus patients. Rheumatology (Oxford) 2008;47:1379–1383. [DOI] [PubMed] [Google Scholar]


