Abstract
Maternal nutritional status during pregnancy impacts fetal brain development. Vitamin B12 plays a vital role in neuronal development. However, findings from studies on the association between maternal B12 status and child cognitive functions have been inconsistent. We performed a randomized, placebo‐controlled clinical trial of oral B12 supplementation (50 µg) beginning at <14 weeks of gestation through a 6‐week post‐partum. In the present study, we report the effects of maternal B12 supplementation on cognitive development in infants at 9 months of age on Bayley Scales of Infant Development‐III (BSID‐III). One hundred eighty‐three pregnant women received vitamin B12, and 183 received placebo. Nine‐month BSID‐III development score was available in 178 infants. There were no significant differences in maternal sociodemographic characteristics and baseline biochemical measures between infants who underwent BSID‐III evaluation and infants who were not evaluated. There were no significant differences in any of the subscales of BSID‐III between infants born to mothers who received B12 supplementation (n = 78) vs. placebo (n = 100). On multiple regression analysis, elevated maternal total homocysteine (tHcy) levels adjusted for treatment group, birthweight, parity, income and home environment at second trimester of pregnancy were significantly negatively associated with expressive language (β = 3.13 points, P < 0.001), and in third trimester of pregnancy with expressive language (β = −2.29 points, P < 0.001) and fine motor (β = −1.41 points, P = 0.005) domains of BSID‐III. While no significant effects of maternal B12 supplementation were seen on cognitive development in infants at 9 months of age, elevated maternal tHcy levels were associated with poorer cognitive performance in some of the subdomains of BSID‐III. In pregnant women with elevated tHcy levels and or B12 deficiencies, it may be worthwhile to study the impact of longer term maternal supplementation on infant cognitive outcomes.
Keywords: antenatal nutrition, maternal micronutrients, maternal vitamin B12, homocysteine, infants, cognitive function
Introduction
Maternal micronutrient status is critical for optimal fetal brain development, and is likely to influence DNA methylation and cognitive performance in infancy and early childhood (Georgieff 2007; Dominguez‐Salas et al. 2012). Vitamin B12 plays an important role in brain development (Black 2008). However, findings from studies examining the association between maternal vitamin B12 status and cognitive functions in their offspring have been inconsistent. In a study from rural India with a 70% prevalence of maternal vitamin B12 deficiency, children of mothers in the lowest decile of vitamin B12 concentrations (n = 49, <77 pmol/L) performed poorly on tests of attention and short‐term memory at 9 years of age, compared with children of mothers in the highest decile of vitamin B12 concentrations (n = 59, >224 pmol/L) (Bhate et al. 2008). In contrast, a study from urban India showed no associations between maternal vitamin B12 concentration and children's cognitive functions at ages of 9–10 years (Veena et al. 2010). In well‐nourished women in Canada, maternal vitamin B12 status was not related to cognitive performance in childhood (Wu et al. 2012).
Findings from studies on the association between maternal vitamin B12 intakes and childhood cognitive functions too have been inconsistent. A large well‐nourished population from the UK but with high attrition rates (50%) did not find any associations between maternal vitamin B12 intakes in the third trimester and offspring IQ at 8 years of age (Bonilla et al. 2012). In a Mexican cohort, low maternal vitamin B12 intake (<2 µg per day) in the first trimester was associated with lower mental development index on Bayley's Scale of Infant Development [β = −1.6 points (0.3 SD); 95% CI: −2.8, −0.3] (Del Rio Garcia et al. 2009). In contrast, in a study from the United States, maternal vitamin B12 dietary intake from food and supplements during the second trimester was negatively associated with offspring receptive language (−0.4 points per 2.6 µg/day; 95% CI: −0.8, −0.1) at 3 years of age (Villamor et al. 2012).
The lack of consistent evidence between maternal vitamin B12 status and childhood cognitive functions could be a result of a variety of reasons. Studies from industrialized countries have largely been performed among well‐nourished subjects. Few reports from developing countries have noted an association between maternal vitamin B12 status or dietary intake and cognitive functions, and these are largely observational studies. In the absence of randomized controlled trials, it is difficult to draw a causal inference between maternal vitamin B12 status and its effect on child cognitive abilities. We recently completed a double‐blind, placebo‐controlled trial of oral vitamin B12 during pregnancy and early lactation in south Indian women with a high prevalence of vitamin B12 deficiency (Samuel et al. 2013). Vitamin B12‐supplemented women had significantly higher plasma B12 concentrations during pregnancy, and significantly higher B12 levels in breast milk, compared with placebo recipients (Duggan et al. 2014). In the present study, we report the effects of maternal vitamin B12 supplementation on cognitive functions in infants at 9 months of age.
Key messages.
Maternal micronutrient status is important for optimal fetal brain development, and vitamin B12 plays an important role in brain development.
Vitamin B12 deficiency is common among pregnant Indian mothers.
Effects of maternal vitamin B12 supplementation on infant cognitive outcomes have not been studied till date.
While maternal B12 supplementation did not have any effects on infant cognitive outcomes, children born to mothers with elevated homocysteine had poorer cognitive outcomes.
Longer term maternal B12 supplementation and supplementation of infants may benefit cognitive outcomes.
Materials and methods
The parent randomized controlled trial was registered at clinicaltrials.gov as NCT00641862. Mother and infant follow‐up was conducted at Hosahalli Hospital, a government maternity health centre catering predominantly to the needs of pregnant women from lower socioeconomic strata in urban Bangalore. The children of the mothers who participated in the vitamin B12 supplementation trial were the subjects of the present study. Details of the trial have been previously published (Duggan et al. 2014). Briefly, healthy mothers ≥18 years of age were recruited and supplemented with either oral vitamin B12 (50 µg) or placebo beginning at or before 14 weeks of gestational age throughout pregnancy and early lactation (6 weeks post‐partum). Women with multiple gestations, those diagnosed with chronic medical conditions (diabetes mellitus, hypertension, heart disease or thyroid disease), those who tested positive for hepatitis B, HIV or syphilis, those who anticipated moving out of the city before delivery, those who were already consuming vitamin B12 supplements or those who were treated for infertility were excluded. All mothers received iron and folic acid supplements per standard of care.
Trained research assistants obtained sociodemographic information, and measured maternal weight, height and infant weight, length and head circumference. The weights of the mothers were recorded using a digital balance (Salter's 9016; Tonbridge) to the nearest 100 g, and the heights were measured using a stadiometer to the nearest 0.1 cm. BMI was calculated as weight in kilograms divided by the height in metres squared. Gestational age (weeks) was calculated from reported first day of the last menstrual period. Low birthweight was defined as birthweight < 2500 g, and small for gestational age was defined as birthweight less than the 10th percentile of norms for gestational age (Villar et al. 2014).
Biochemical assessments
Approximately 10 mL of blood was obtained from the mothers by venipuncture, and collected in both EDTA and plain vacutainers (BD Franklin Lakes, NJ, USA) that were kept on ice until separation in a refrigerated centrifuge, usually within 4 h. Details of biochemical assessments have been previously described (Duggan et al. 2014). Haemoglobin and complete blood count were analysed on whole‐blood samples in an automated Coulter counter (ABX Pentra C+; Horiba Medicals, CA, USA). The plasma and RBCs were separated and stored at −80 °C until analysis for vitamin B12, total homocysteine (tHcy), methylmalonic acid (MMA) and erythrocyte folate concentrations. The plasma vitamin B12 was measured by the electrochemiluminescence method (Roche Diagnostics Mannheim, USA). The measurement of tHcy and MMA was performed by GC‐MS (model 3800; Varian, Palo Alto, CA, USA). The intraday and interday assay CVs for vitamin B12 were 0.54% and 2.44%, respectively. The interday assay CVs for MMA and tHcy were 5.57% and 5.04%, respectively, and the intraday assay CVs were 6.92% and 5.60%, respectively. Erythrocyte folate was measured by a competitive immunoassay with direct chemiluminescence detection on an automated immunoanalyzer (ADVIA Centaurs; Bayer Health Care Diagnostics, Tarrytown, New York, USA), with intra‐assay and inter‐assay variabilities of 1.9% and 5.2%, respectively. The folate concentration in the hemolysate was converted to values for whole blood by adjusting for the hematocrit. The following cut‐off values were used for the biochemical measures: anaemia was defined as Hb < 11.0 g/dl, low vitamin B12 as <150 (pmol/L), elevated MMA (high MMA) as >0.26 (µmol/L) and elevated Hcy (high tHcy) as >15.0 (µmol/L). Blood samples were collected at baseline (12 weeks) and at 24 weeks and 33 weeks of pregnancy.
Neurocognitive assessment of the infants
The Bayley Scales of Infant Development 3rd edition (BSID‐III) was used to assess infant neurodevelopment status at 9 months of age within a window period of 2 weeks. Early post‐natal period is characterized by dramatic and rapid changes in neuronal development (Rosales et al. 2009). Furthermore, brain development during these periods is sensitive to various environmental insults, including nutritional deficiencies (Thompson & Nelson 2001). We chose to assess the children at 9 months, as at this age, language functions begin to appear, children develop greater motor coordination and are able to engage in tasks for longer period of time. In addition, the risk of confounding increases as the time between maternal supplementation and early childhood neurocognitive assessment increases. The BSID‐III has been used in earlier studies that examined the effects of maternal nutrient status on infant and early childhood cognitive outcomes (Del Rio Garcia et al. 2009; Morales et al. 2012; Wu et al. 2012). The BSID‐III assesses the developmental status of infants from 1 to 42 months of age. The scales assess five domains: cognitive, language (receptive and expressive), motor (fine and gross), social–emotional and adaptive (conceptual, social, practical). The first three domains are assessed by means of items administered to the infant by an examiner. We did not administer the latter two domains, as several of the items were not culturally appropriate. The test instructions were translated into the local language (Kannada), and back‐translated to ensure that it conformed to the original test instructions. A standard script was used while administering the tests. We used the raw scores as age‐specific norms were not available for our population. A similar approach was used in an earlier study on the effect of stunting and wasting on the neurodevelopment scores in Tanzanian children (McDonald et al. 2013). Testing was carried out in a quiet room in a central location, with a parent or guardian present. Testing was performed by two master's level psychologists experienced in child developmental testing who were blind to treatment group assignment. The two psychologists were trained in the administration and scoring of BSID‐III by an international expert. During the course of the study, some of the BSID‐III assessment sessions were videotaped (n = 5) and reviewed by the expert for correctness in administration and scoring. Feedback was given to the tester if any discrepancies in test administration or scoring were identified. The assessment was rescheduled if a child was obviously ill or febrile.
Assessment of home environment
We captured aspects of home environment using Bradley's home inventory for infants/toddlers (Bradley et al. 1989). This scale captures information on parenting behaviour under six domains (responsivity, acceptance, organization, learning materials, parental involvement and variety of stimulation at home), and has been used extensively in research on the influence of home environment on child health outcomes and cognitive abilities (Bradley et al. 1996; Bacharach & Baumeister 1998). Trained study personnel administered this questionnaire to participating families at 1 year post‐partum.
Data analysis
Examination of normality was conducted for all the variables. The sociodemographic, psychosocial, biochemical and anthropometric characteristics of the participants were analysed using descriptive statistics. Each subscale of the BSID‐III was compared between the vitamin B12 and placebo groups using the Mann–Whitney U test. The BSID‐III raw scores were adjusted for the infant's gestational age at birth. The association of maternal sociodemographic characteristics such as age, education, income, parity, vitamin B12 biomarker status in pregnancy, birthweight of the infant and home environment with each subscale of the BSID‐III was examined using simple linear regression analyses. Collinearity between the independent variables was examined using variance inflation factor, and multicollinearity was confirmed if variance inflation factor was >5 (Hair et al. 2009). Separate multiple linear regression analyses for each BSID‐III subscale with trimester‐specific maternal biomarker status, anthropometry, birthweight, treatment group and home environment were performed. Group assignment was included in all regression analyses as the data emerged from a randomized control trial. The assumptions of the linear regression were examined using residual plots. The interaction effects of group assignment and all predictor variables were examined separately. Further, all BSID‐III subscales were considered together in multivariate analysis of variance with covariates (MANCOVA). This analysis examined the effect of maternal nutritional biomarker status in each trimester on BSID‐III subscales while adjusting for covariates such as birthweight, treatment group and home environment score. The level of significance used for interpreting the data was P < 0.05. All data were analysed using STATA for Windows, Version 12.0. As suggested in an earlier study of multivitamin supplementation on neurocognitive outcomes in infants, with 100 children in each group and an alpha of 0.05, we would have 80% power to detect a difference of 1.2 in the mean raw cognitive score between the two groups (Manji et al., 2014).
Results
From the 366 women who were recruited in the intervention study, birth details were available for 256 deliveries. The 9‐month BSID‐III development score was obtained in 178 infants (Fig. 1). The infants who underwent BSID‐III evaluation showed no significant differences compared with the infants who were not evaluated based on sociodemographic and baseline biochemical measures.
Figure 1.
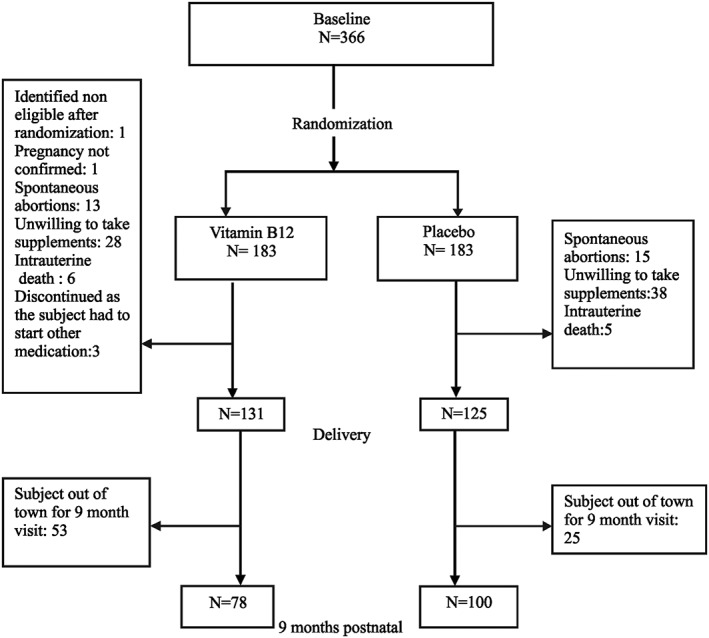
Summary of recruitment and randomization.
The mean (SD) age of the women was 23 years (3.6). The majority of women had at least a high school education (75%), had household income in the range of Rs 1500–5000 (39.9%), and were primiparous (61.8%) (Table 1). In addition, nearly one‐third of the women (30%) were anaemic at baseline, 50–60% of the women had low concentrations of B12 (<150 pmol/l), and nearly three fourths of the women had elevated MMA across the three trimesters. One‐fourth of the women had elevated tHcy at baseline, and later in the second and the third trimesters, the prevalence of elevated tHcy was 12% (Table 2). The mean (SD) birthweight of the infants was 2.9 (0.5) kilograms, and 42 (24%) were small for gestational age (Villar et al. 2014). The composite score on the infant toddler version of the home environment inventory was 37 ± 2.3. While comparative scores from other populations in India are not available, the scores obtained in the present study are similar to earlier reports (Bacharach & Baumeister 1998).
Table 1.
Sociodemographic and anthropometric characteristics of 178 pregnant women whose infants underwent BSID‐III testing at 9 months of age
| Variable | Mean +/− SD, or n (%) |
|---|---|
| Age, years | 23.1 ± 3.6 |
| Education | |
| None/Primary | 44 (25) |
| High school | 79 (45) |
| >High school | 53 (30) |
| Monthly household income, INR | |
| 1500–5000 | 71 (40) |
| 5001–8000 | 44 (25) |
| 8001–30000 | 63 (35) |
| Parity | |
| Primiparous | 110 (62) |
| Multiparous | 68 (38) |
| Trimester 1 weight, kg | 47.8 ± 8.0 |
| Trimester 1 height, cm | 152.8 ± 5.7 |
| BMI, kg/m2 | 20.4 ± 3.3 |
100 INR was equivalent to approximately US $2 at the time the study was conducted.
Table 2.
Biochemical characteristics of 178 pregnant women whose infants underwent BSID‐III testing at 9 months of age. All data are N (%) or *median (Q1, Q3)
| Variables | Trimester 1 | Trimester 2 | Trimester 3 |
|---|---|---|---|
| Vitamin B12, <150 pmol/L | 85 (49.4) | 72 (49.3) | 80 (60.6) |
| MMA, >0.26 µmol/L | 136 (77.3) | 101(68.7) | 79 (59.9) |
| tHcy, >15.0 µmol/L | 44 (25.0) | 17 (11.6) | 17 (13.0) |
| Erythrocyte folic acid, nmol/L* | 385 (292, 497) | 606 (497, 750) | 514 (421, 632) |
| Anemia, Hb < 11.0 g/dL | 54 (30.3) | 83 (55.3) | 67 (48.6) |
*All data are N (%) or median (Q1, Q3)
The distribution of BSID‐III subscales for infants at 9 months by groups is presented in Fig. 2. There were no significant differences in any of the subscales of BSID‐III between the infants born to mothers who received vitamin B12 supplementation (n = 78) vs. those who received placebo (n = 100). Hence, both the intervention and the control group were combined for the remaining analyses.
Figure 2.
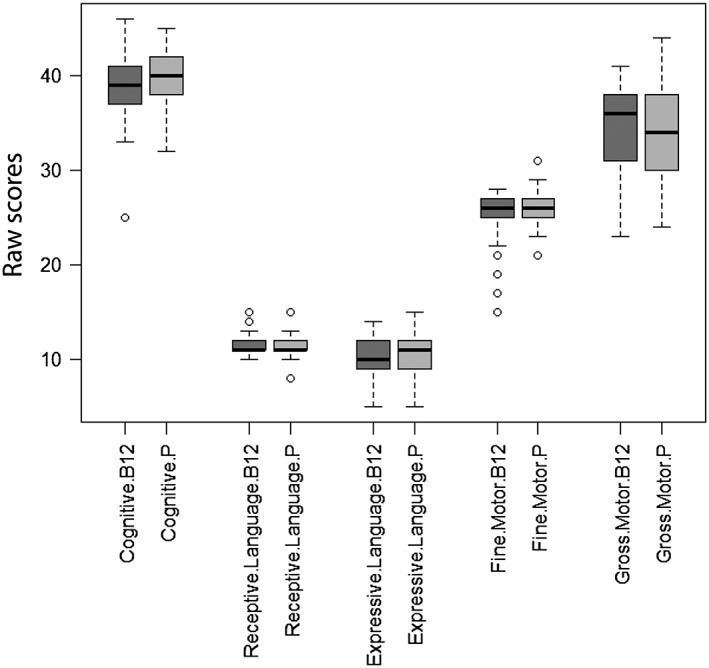
Distribution of Bayley developmental scales of infants 9 months of age (n = 178). The figure represents the distribution of data for the various domains of Bayley Scales of Infant Development‐III by intervention group: minimum, first quartile, median, third quartile and maximum data are presented.
Linear regression analyses were performed to identify factors associated with BSID‐III subscores (Table 3). Home environment score was positively associated with receptive language, and birthweight was positively associated with motor skills. Interestingly, family income was negatively associated with receptive language skills. The level of maternal education was not associated with BSID‐III scores. The elevated tHcy concentration in all three trimesters was associated with lower expressive language score, and the elevated tHcy in trimesters 1 and 3 was associated with lower fine motor scores.
Table 3.
Bivariate and multivariate linear regression of Bayley developmental scales in infants 9 months of age
| Bayley subscale | Receptive language | Expressive language | Fine motor | Gross motor | ||||
|---|---|---|---|---|---|---|---|---|
| Bivariate analyses | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI |
| Income (Indian Rupees) | −0.22 | (−0.38, −0.06) | −0.25 | (−0.61, 0.11) | −0.27 | (−0.62, 0.07) | −0.32 | (−1.08, 0.44) |
| Parity | −0.10 | (−0.40, 0.19) | −0.31 | (−0.96, 0.34) | 0.35 | (−0.27, 0.98) | 0.01 | (−1.37, 1.35) |
| Home environment (total score) | 0.09 | (0.03, 0.15) | 0.09 | (−0.05, 0.23) | 0.12 | (−0.01, 0.25) | 0.03 | (−0.27, 0.32) |
|
Elevated tHcy (>15.0 µmol/L) |
||||||||
| Trimester 1 | −0.14 | (−0.47, 0.19) | −1.10 | (−1.81, −0.39) | −1.04 | (−1.72, −0.35) | 0.42 | (−1.10, 1.95) |
| Trimester 2 | −0.03 | (−0.53, 0.47) | −2.87 | (−3.83, −1.91) | −0.56 | (−1.60, 0.48) | 0.68 | (−1.58, 2.94) |
| Trimester 3 | −0.06 | (−0.53, 0.40) | −2.25 | (−3.27, −1.23) | −1.27 | (−2.26, −0.27) | 0.77 | (−1.44, 2.98) |
| Birthweight (gms) | 0.13 | (−0.18, 0.44) | 0.29 | (−0.41, 1.0) | 1.03 | (0.37, 1.69) | 2.12 | (0.70, 3.55) |
| Multivariate analysis* | ||||||||
|
Elevated tHcy (>15.0 µmol/L) |
||||||||
| Trimester 1 | −0.31 | (−0.63, 0.02) | −1.30 | (−2.05, −0.55) | −1.06 | (−1.76, −0.37) | 0.38 | (−1.20, 1.96) |
| Trimester 2 | −0.23 | (−0.72, 0.26) | −3.13 | (−4.13, −2.14) | −0.96 | (−1.98, 0.05) | 0.19 | (−2.15, 2.52) |
| Trimester 3 | −0.12 | (−0.55, 0.31) | −2.29 | (−3.35, −1.23) | −1.41 | (−2.40, −0.41) | 0.42 | (−1.81, 2.66) |
Values in bold are statistically significant predictors; the inclusion of tester code in multivariable model did not modify the results. The interactions of group assignment and predictors were evaluated separately. The interactions being not statistically significant are not included in the model.
β‐regression coefficient, 95% CI.
Variables included in the multivariable model were income, parity, home environment, treatment group and birthweight.
The multiple linear regression analyses of BSID‐III subscales were then performed, using tHcy as the main exposure variable because it was collinear with MMA. We examined the independent effect of elevated maternal tHcy concentrations at each time point during pregnancy while adjusting for significant covariates (treatment group, birthweight, parity, income and home environment) (Table 3). Separate analyses for predictor variables specific to Trimester 1, 2 and 3, respectively, are presented. In trimester 1, elevated tHcy concentrations continued to have a significant negative association with the expressive language and fine motor subscales of BSID‐III. In the second trimester, elevated tHcy concentration was negatively associated with the expressive language (β = −3.13 points; P < 0.001) subscale of the BSID‐III. In the third trimester, elevated tHcy concentration was negatively associated with expressive language (β = −2.29 points; P < 0.001) and fine motor (β = −1.41 points; P = 0.005) subscales of BSID‐III. In multiple regression analyses, birthweight and home environment were positively associated with fine motor score. Lower income (β = −0.39, 95% CI: −0.58, −0.21) and higher home environment scores were associated (β = 0.13, 95% CI: 0.06, 0.21) with higher receptive language score.
Further, the BSID‐III subscale scores were considered together as the dependent variable in MANCOVA to examine the effect of elevated tHcy in the three trimesters (Table 4). The MANCOVA analysis showed that elevated tHcy in all three trimesters were separately associated with lower BSID‐III score after adjusting for home environment and birthweight as covariates. The P values corresponding to the Wilk's Lambda test for elevated tHcy in trimesters 1, 2 and 3 were 0.004, <0.001 and 0.0004, respectively. The test satisfied the null hypothesis assumption for MANCOVA in that the coefficients of the covariates (treatment group, birthweight and home environment) were similar between the elevated and normal tHcy groups.
Table 4.
Multivariate analysis of covariance of Bayley subscales with maternal homocysteine status in three trimesters of pregnancy
|
Elevated tHcy (>15.0 µmol/L) |
Wilk's Lambda | P value |
|---|---|---|
| Trimester 1 | 0.86 | 0.0004 |
| Trimester 2 | 0.75 | <0.001 |
| Trimester 3 | 0.80 | 0.0002 |
Additional covariates adjusted for in the analysis were home environment and birthweight.
Discussion
In this randomized, double‐blind trial of vitamin B12 supplementation during pregnancy and early lactation, we observed no effect of maternal vitamin B12 supplementation on early neurocognitive outcomes in infants at 9 months of age using the BSID‐III. However, in the group taken together as a whole, higher maternal plasma concentrations of tHcy across all the three trimesters were associated with poorer performance on language expression, and a higher concentration of tHcy in the first trimester was associated with poorer fine motor function, adjusted for a variety of confounders. To our knowledge, this study is the first randomized controlled trial of vitamin B12 supplementation in pregnant Indian women that examined cognitive outcomes in early childhood.
Two studies from low‐resource countries observed an association between maternal B12 concentrations and B12 intakes with cognitive outcomes in children. A study from rural India (n = 108) found that the children of the mothers with lowest vitamin B12 concentration, compared with the children of the mothers with the highest concentration of vitamin B12, performed less well on tests of sustained attention (colour trail test: 182 vs. 159 s) and short‐term memory (digit span backward test: 4.3 vs. 4.4 digits) at the age of 9 years (Bhate et al. 2008). In a study from Mexico, the children of the mothers with low B12 intake (<2 µg/day) compared with the children of the mothers with adequate dietary B12 intake scored 0.3 SD lower in mental development index computed from BSID‐II (Del Rio Garcia et al. 2009). However, others have failed to find any association between maternal vitamin B12 status and cognitive function in children across ages using a variety of exposures such as maternal plasma B12 and holotranscobalamin concentrations (Wu et al. 2012), maternal B12 concentrations (Veena et al. 2010) and an average B12 intake at first and second trimesters (Boeke et al. 2013).
There could be several reasons for the lack of effect of maternal vitamin B12 supplementation on infant cognitive outcomes in the present study. Maternal B12 supplementation was stopped at 6 weeks post‐partum and thus, the effects of supplementation on both maternal and infant B12 status may have waned. We have indirect support for this observation with higher levels of B12 in breast milk in the supplemented group at 6 weeks, but not at later time points (Duggan et al. 2014). A recent study linked higher infant B12 status, as well as lower tHcy and MMA concentrations, with better cognitive outcomes at 12–18 months (Strand et al. 2013). Thus, infant B12 supplementation may have resulted in better cognitive outcomes as shown in a recent study, with B12 supplemented infants having improved gross motor function (Torsvik et al. 2013). Another possibility is the modest sample size of the infants on whom cognitive outcomes were available. Finally, the inability to demonstrate the effects of maternal B12 supplementation on infant cognitive outcomes could be a result of difficulties in reliably capturing cognitive measures in infants and young children (Marks et al. 2008).
Our finding that elevated maternal tHcy levels adjusted for a variety of confounders across all three trimesters were associated with poorer cognitive functions in infants is in agreement with earlier observations that hyperhomocysteinemia is associated with a variety of adverse health outcomes in children (Bjoorke Monsen & Ueland 2003; Obeid & Herrmann 2005; Acilmis et al. 2011; Hogveen et al. 2012; Puri et al. 2013). In relation to cognitive outcomes in children, several studies did not find an association between maternal tHcy levels and cognitive measures (Tamura et al. 2005; Bhate et al. 2008; Veena et al. 2010; Wu et al. 2012). However, there were methodological limitations with some studies not reporting details of elevated tHcy levels (Bhate et al. 2008; Wu et al. 2012), and in another study, there was a low prevalence of elevated tHcy levels (3%) among mothers during pregnancy (Veena et al. 2010). Our finding of poorer cognitive function in children of mothers with elevated tHcy levels is in agreement with a more recent study that reported better cognitive outcomes in children with higher B12 concentrations as well as lower tHcy and MMA concentrations (Strand et al. 2013). It is relevant to note here that in our parent trial, maternal B12 supplementation did not significantly lower tHcy blood concentrations in pregnant mothers (Duggan et al. 2014). This was also true for the subsample of mothers whose children participated in the present study. Thus, it is possible that the growing fetus was exposed to high concentrations of maternal tHcy levels regardless of treatment group through the pregnancy, impacting fetal brain development, and resulting in poorer cognitive outcomes.
Elevated levels of tHcy serve as a biomarker of functional deficiency of different B vitamins such as B12, B6 and riboflavin, but they are most closely linked to folate deficiency (Torbjorn et al. 2011). Several studies especially in the elderly have reported an association between hyperhomocysteinemia and poorer cognitive abilities independent of serum B12 and folate levels (Miller et al. 2003; West et al. 2011), suggestive of a direct neurotoxic effect. In contrast, a recent study among Swedish adolescents noted a positive association between tertiles of folate intake and academic achievement, but not tertiles of tHcy, adjusted for various confounders (Torbjorn et al. 2011). It is possible that the direct neurotoxic effects of tHcy may be more apparent during critical periods of neural development such as in growing fetus. Our findings of an association between hyperhomocysteinemia and poorer cognitive performance add to the growing literature on the effects of antenatal maternal nutrient status on neural development and cognitive performance in children.
The association between lower monthly household income and higher receptive language score is in contrast to findings from earlier studies that report adverse effects of low family income and poverty on cognitive outcomes in children (Santos et al. 2008; Berger et al. 2009). However, robust associations between low income and adverse childhood cognitive development are noted among children who are persistently poor (Aber et al. 1997). Income is more accurately estimated over a longer time period, and permanent income is more closely related to child outcomes (Korenman et al. 1995). However, in the present study, we captured details about family income at the baseline and not during follow‐up when the income status of the family could have changed. In addition, in the Indian context, capturing all sources of family income is challenging as households engage in a variety of economic activities, and most instruments that measure socioeconomic status in India typically include physical assets and material possessions in addition to family income to categorize the socioeconomic status of an individual or a family (Tiwari et al. 2005). Finally, studies have shown that the effects of income on cognitive outcomes in pre‐school children are largely mediated through home environment and cognitive stimulation (Brooks‐Gunn & Markman 2005). In the present study, home environment scores were positively associated with receptive language and fine motor score.
There is a paucity of studies that examine the effects of maternal micronutrient supplementation on cognitive development in children from developing countries where pregnant women are at risk of multiple micronutrient deficiencies, and thus the present report fills an important gap in literature. There was a high prevalence of B12 deficiency in our cohort of mothers prior to randomization (Samuel et al. 2013). In addition, we captured several possible confounders that may impact child cognitive development both during prenatal and post‐natal periods. However, a number of limitations deserve mention. The sample size may have been inadequate to detect significant differences in infant cognitive performance between groups. Although the cognitive assessments were carried out by master's level psychologist experienced in child development testing, as only five sessions were reviewed for correctness of administration, a possible drift in administration and scoring of BSID‐III during the course of the study cannot be ruled out. In addition, we could not study the association between the current child micronutrient status and cognitive performance as a result of unavailability of infant bloods at the time of cognitive testing. Finally, we did not measure maternal intelligence, which is known to influence child cognitive abilities.
In summary, maternal supplementation with 50 µg of oral vitamin B12 during pregnancy and early lactation did not influence cognitive outcomes in children at 9 months of age. However, in the entire sample of children, the elevated maternal tHcy levels across the three trimesters were significantly and negatively related with several of the subscales of BSID III. In pregnant women with elevated tHcy levels and or B12 deficiencies, it may be worthwhile to study the impact of longer term maternal supplementation on infant cognitive outcomes. In addition, in future studies, the benefits of supplementation of infants directly on cognitive outcomes can be examined.
Source of funding
Supported by the Indian Council of Medical Research grant 5/7/192/06‐RHN and the US National Institutes of Health grants R03 HD054123 and K24 DK104676 (CD).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
KS, CD and AVK developed the protocol; DCB provided the training and supervised the administration of Bayley Scales of Infant Development‐III; KS, AVK, AR, ARMK and CD were involved in the conduct of the study and assisted in manuscript preparation; TT and RJB analysed the data and assisted in manuscript preparation; KS wrote the initial draft of the manuscript, and all the coauthors reviewed all sections of the text and approved the final version of the manuscript.
Acknowledgements
We are grateful to Dr B Nirmala for helping us obtain all the clearances to conduct the study at Hosahalli Hospital. We thank Ms P. Vijaya, Ms S. Surekha and Ms C. Darshini for technical support. The authors thank Ms Sarita for conducting the analyses of total homocysteine and methylmalonic acid, and Ms Shanti and Ms Beena for conducting the analyses of vitamin B12.
Srinivasan, K. , Thomas, T. , Kapanee, A. R. M. , Ramthal, A. , Bellinger, D. C. , Bosch, R. J. , Kurpad, A. V. , and Duggan, C. (2017) Effects of maternal vitamin B12 supplementation on early infant neurocognitive outcomes: a randomized controlled clinical trial. Maternal & Child Nutrition, 13: e12325. doi: 10.1111/mcn.12325.
References
- Aber J.L., Bennett N.G., Conley D.C. & Li J. (1997) The effects of poverty on child health and development. Annual Reviews of Public Health 18, 463–483. [DOI] [PubMed] [Google Scholar]
- Acilmis Y.G., Dikensoy E., Kutlar A.I., Balat O., Cebesoy F.B., Ozturk E. et al. (2011) Homocysteine, folic acid and vitamin B12 levels in maternal and umbilical cord plasma and homocysteine levels in placenta in pregnant women with preeclampsia. The Journal of Obstetrics and Gynaecology Research 37, 45–50. [DOI] [PubMed] [Google Scholar]
- Bacharach V.R. & Baumeister A.A. (1998) Direct and indirect effects of maternal intelligence, maternal age, income, and home environment on intelligence of preterm, low‐birth‐weight children. Journal of Applied Developmental Psychology 19, 361–375. [Google Scholar]
- Berger L.M., Paxson C. & Waldfogel J. (2009) Income and child development. Child Youth Services Review 31, 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhate V., Deshpande S., Bhat D., Joshi N., Ladkat R., Watve S. et al. (2008) Vitamin B12 status of pregnant women and cognitive function in their 9‐yr‐old children. Food and Nutrition Bulletin 29, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjoorke Monsen A.L. & Ueland P.M. (2003) Homocysteine and methymalonic acid in diagnosis and risk assessment from infancy to adolescence. The American Journal of Clinical Nutrition 78, 7–21. [DOI] [PubMed] [Google Scholar]
- Black M.M. (2008) Effects of vitamin B12 and folate deficiency on brain development in children. Food and Nutrition Bulletin 29 (2 Suppl), S126–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke C.E., Gillman M.W., Hughes M.D., Rifas‐Shiman S.L., Villamor E. & Oken E. (2013) Choline intake during pregnancy and child cognition at age 7 years. American Journal of Epidemiology 177, 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla C., Lawlor D.A., Taylor A.E., Gunnell D.J., Ben‐Shlomo Y., Ness A.R. et al. (2012) Vitamin B12 status during pregnancy and child's IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PLoS One 7, e51084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.H., Caldwell B.M., Rock S.L., Ramey C.T., Barnard K.E., Gray C. et al. (1989) Home environment and cognitive development in the first 3 years of life: a collaborative study involving six sites and three ethnic groups in North America. Developmental Psychology 25 (2), 217. [Google Scholar]
- Bradley R.H., Corwyn R.F. & Whiteside‐Mansell L. (1996) Life at home: same time, different places—an examination of the HOME inventory in different cultures. Early Development and Parenting 5 (4), 251–269. [Google Scholar]
- Brooks‐Gunn J. & Markman L. (2005) The contribution of parenting to ethnic and racial gaps in school readiness. The Future of Children 15, 139–168. [DOI] [PubMed] [Google Scholar]
- Del Rio Garcia C., Torres‐Sanchez L., Chen J., Schnaas L., Hernandez C., Osorio E. et al. (2009) Maternal MTHFR 677C > T genotype and dietary intake of folate and vitamin (B12): their impact on child neurodevelopment. Nutritional Neuroscience 12, 13–20. [DOI] [PubMed] [Google Scholar]
- Dominguez‐Salas P., Cox S.E., Prentice A.M., Hennig B.J. & Moore S.E. (2012) Maternal nutritional status, C (1) metabolism and offspring DNA methylation: a review of current evidence in human subjects. Proceedings of Nutrition Society 71 (1), 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan C., Srinivasan K., Thomas T., Samuel T., Rajendran R., Muthayya S. et al. (2014) Vitamin B12 supplementation during pregnancy and early lactation increases maternal, breast milk and infant measures of vitamin B12 status. Journal of Nutrition. DOI: 10.3945/jn.113.187278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff M.K. (2007) Nutrition and the developing brain: nutrient priorities and measurements. The American Journal of Clinical Nutrition 85, 614S–620S. [DOI] [PubMed] [Google Scholar]
- Hair J.F., Black W.C., Babin B.J., Anderson R.E. & Tatham R.L. (2009) Multivariate data analysis. 3rd edition. Multiple Regression Analysis 4, 193–292. [Google Scholar]
- Hogveen M., Blom H.J. & den Heijer M. (2012) Maternal homocysteine and small‐for‐gestational age offspring: systematic review and meta‐analysis. The American Journal of Clinical Nutrition 95, 130–136. [DOI] [PubMed] [Google Scholar]
- Korenman S., Miller J. & Sjaastad J. (1995) Long‐term poverty and child development in the United States: results from the NLSY. Children and Youth Services Review 17, 127–155. [Google Scholar]
- Manji K.P., McDonald C.M., Kupka R., Bosch R.J., Kisenge R., Aboud S. et al. (2014) Effect of multivitamin supplementation on the neurodevelopment of HIV-exposed infants : A Randomized double‐blind, placebo‐controlled clinical trial. Journal of Tropical Pediatrics 60, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks K., Glascoe F.P., Aylward G.P., Shevell M.I., Lipkin P.H. & Squires J.K. (2008) The thorny nature of predictive validity studies on screening tests for developmental–behavioral problems. Pediatrics 122, 866–868. [DOI] [PubMed] [Google Scholar]
- McDonald C.M., Manji K.P., Kupka R., Bellinger D.C., Spiegelman D., Kisenge R. et al. (2013) Stunting and wasting are associated with poorer psychomotor and mental development in HIV‐exposed Tanzanian infants. Journal of Nutrition 143, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.W., Green R., Ramos M.I., Allen L.H., Mungas D.M., Jagust W.J. et al. (2003) Homocysteine and cognitive function in the Sacramento area Latino study on aging. The American Journal of Clinical Nutrition 78, 441–447. [DOI] [PubMed] [Google Scholar]
- Morales E., Guxens M., Llop S., Rodriguez‐Bernal C.L., Tardon A., Riano I. et al. INMA Project (2012) Circulating 25‐hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics 130, e913–920. [DOI] [PubMed] [Google Scholar]
- Obeid R. & Herrmann W. (2005) Homocysteine, folic acid and vitamin B12 in relation to pre‐ and post‐natal health aspects. Clinical Chemistry and Laboratory Medicine 43, 1052–1057. [DOI] [PubMed] [Google Scholar]
- Puri M., Kaur L., Walia G.K., Mukhopadhyay R., Sachdeva M.P., Trivedi S.S. et al. (2013) MTFHR C677T polymorphism, folate, vitamin B12 and homocysteine in recurrent pregnancy losses: a case control study among north Indian women. Journal of Perinatal Medicine 41, 549–554. [DOI] [PubMed] [Google Scholar]
- Rosales F.J., Reznick J.S. & Zeisel S.H. (2009) Understanding the role of nutrition in the brain and behavioural development of toddlers and preschool children: identifying and overcoming methodological barriers. Nutritional Neuroscience 12, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel T.M., Duggan C., Thomas T., Bosch R., Rajendran R., Virtanen S.M. et al. (2013) Vitamin B12 intake and status in early pregnancy among urban South Indian women. Annals of Nutrition and Metabolism 62, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D.N., Assis A.M.O., Bastos A.C.S., Santos L.M., Santos C.A., Strina A. et al. (2008) Determinants of cognitive function in childhood: a cohort study in a middle income context. BMC Public Health 8, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand T.A., Taneja S., Veland P.M., Refsum H., Bahl R., Schneede J. et al. (2013) Cobalamin and folate status predicts mental development scores in North Indian children 12–18 months of age. The American Journal of Clinical Nutrition 97, 310–317. [DOI] [PubMed] [Google Scholar]
- Tamura T., Goldenberg R.L., Chapman V.R., Johnston K.E., Ramey S.L. & Nelson K.G. (2005) Folate status of mothers during pregnancy and mental and psychomotor development of their children during pregnancy and mental and psychomotor development of their children at five years of age. Pediatrics 116, 703–708. [DOI] [PubMed] [Google Scholar]
- Thompson R.A. & Nelson C.A. (2001) Developmental science and the media. Early brain development. American Psychologist 56, 5–15. [DOI] [PubMed] [Google Scholar]
- Tiwari S.C., Kumar A. & Kumar A. (2005) Development and standardization of a scale to measure socio‐economic status in urban and rural communities in India. Indian Journal of Medical Research 122, 309–314. [PubMed] [Google Scholar]
- Torbjorn K.N., Yngve A., Bottiger A.K., Hurtig‐Wennlof A. & Sjostrom M. (2011) High folate intake is related to better academic achievement in Swedish adolescents. Pediatrics 128, e358–e365. [DOI] [PubMed] [Google Scholar]
- Torsvik I., Ueland P.M., Markestad T. & Bjorke‐Monsen A.L. (2013) Cobalamin supplementation improves motor development and regurgitations in infants: results from a randomized intervention study. The American Journal of Clinical Nutrition 98, 1233–1240. [DOI] [PubMed] [Google Scholar]
- Veena S.R., Krishnaveni G.V., Srinivasan K., Wills A.K., Muthayya S., Kurpad A.V. et al. (2010) Higher maternal plasma folate but not vitamin‐B12 concentrations during pregnancy are associated with better cognitive function scores in 9–10 year old children in South India. Journal of Nutrition 140, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E., Rifas‐Shiman S.L., Gillman M.W. & Oken E. (2012) Maternal intake of methyl donor nutrients and child cognition at 3 years of age. Paediatric and Perinatal Epidemiology 26, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J., Ismail L.C., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. et al. (2014) International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross‐Sectional Study of the INTERGROWTH‐21st Project. Lancet 384, 857–68. [DOI] [PubMed] [Google Scholar]
- West R.K., Beeri M.S., Schmeidler J., Mitchell D.B., Carlisle K.R., Angelo G. et al. (2011) Homocysteine and cognitive function in very elderly nondemented subjects. The American Journal of Geriatric Psychiatry 19, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B.T., Dyer R.A., King D.J., Richardson K.J. & Innis S.M. (2012) Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One 7, e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]


