Abstract
Background
Long QT syndrome (LQTS) mutation carriers have higher risk of cardiac events than unaffected family members even in the absence of QTc prolongation. Changes in T wave morphology may reflect penetrance of LQTS mutations. We aimed to assess whether T-wave morphology may improve risk stratification of LQT2 mutation carriers with normal QTc interval.
Methods
LQT2-mutation carriers with QTc<460 ms in men and <470 ms in women (n=154) were compared with unaffected family members (n=1007). Baseline ECGs recorded at age ≥18 years underwent blinded assessment. Flat, notched or negative T-waves in leads II or V5 were considered ’abnormal’. Cox regression analysis was performed to assess the association between T-wave morphology, the presence of mutations in the pore region of KCNH2 and the risk of cardiac events defined as syncope, aborted cardiac arrest, defibrillator therapy or sudden cardiac death. Gender-specific associations were estimated using interactions terms.
Results
LQT2 female carriers with abnormal T-wave morphology had significantly higher risk of cardiac events compared to LQT2 female carriers with normal T waves (HR=3.31; 95% CI: 1.68–6.52; p=0.001) whereas this association was not significant in males. LQT2 males with pore location of mutations has significantly higher risk of cardiac events than non-pore location males (HR=6.01; 95%CI: 1.50–24.08; p=0.011) whereas no such association was found in females.
Conclusion
The risk of cardiac events in LQT2 carriers with normal QTc is associated with abnormal T wave morphology in females and pore location of mutation in males. The findings further indicate sex-specific differences in phenotype and genotype relationship in LQT2 patients.
Keywords: long QT syndrome, risk stratification, gender, genotype
Introduction
Introduction of cascade genetic screening in the management of families with long QT syndrome (LQTS) has led to identification of the growing number of mutation carriers, many of whom have normal QTc. Earlier studies indicated that mutation carriers have higher risk of cardiac events than genotype-negative family members even in the absence of QTc prolongation,1 which is used as a rationale for administration of beta-blocker therapy to asymptomatic LQTS mutation carriers regardless of QTc duration.2 Mutation penetrance, however, may be manifested not only in QTc prolongation but also in aberrant T-wave morphology with most notable T-wave abnormalities associated with LQT2 genotype.
The type and location of LQT2 mutation appear to be associated with the risk of cardiac events with the highest risk confined to pore-mutations as reported in a large international LQT2 cohort.3 However, it is not known whether the risk conferred by a specific mutation type is independent of repolarization abnormalities detectable as either prolonged QTc or abnormal T-wave morphology in KCNH2 mutation carriers. In an earlier study that included patients with all three most common LQTS genotypes, the impact of the mutation type (i.e. transmembrane missense mutations) was confined only to the mutations carriers who did not exhibit QTc prolongation, while in patients with prolonged QTc the predictive effect of the genotype was attenuated.1
We hypothesized that LQTS penetrance in LQT2 mutations carriers with normal QTc interval assessed as occurrence of arrhythmic events and syncopal episodes is linked to the type of KCNH2 mutation and T wave morphology alterations that can be assessed from standard resting ECG. Our aim was to assess whether abnormal T-wave morphology and information regarding location of the genetic defect may be useful in risk stratification of adult LQT2 mutation carriers with normal QTc interval in the Rochester LQTS Registry.
Material
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study population
Patients in this study were from the Rochester-based LQTS Registry; enrollment into the registry has been previously described.4, 5 Patients were selected to the current analysis if they (1) were shown to be carriers of disease causing mutation in KCNH2 (LQT2), (2) had Bazett-corrected QT interval (QTc) < 470 ms for female and < 460 ms for male, (3) were 18 years or older in order to exclude variation of T-wave morphology that may be observed in children and adolescents. Patients were excluded from the study if they had more than one LQTS-associated mutation.
The study population selected according to these criteria comprised 154 subjects with genotype-positive LQT2 patients and normal or minimally affected QTc, and subjects belonging to families with genotype-positive probands who were genetically tested and found to be negative for LQTS-associated mutation (n=1007).
Data collection
Standard 12-lead resting ECG was acquired at the time of enrolment in the registry. RR and QT intervals were measured on the first recorded ECG and used for calculation of the heart rate corrected QT-interval according to the Bazett’s formula (QTc). Clinical data were collected on prospectively designed forms with information on demographic characteristics, personal and family medical history, ECG findings, therapies, including QT-prolonging medications, and events during long-term follow-up. Information about beta-blocker use was also collected in order to allow time-dependent assessment of their possible impact on the incidence of cardiac events.
Endpoints
The prespecified endpoint of the study was the occurrence of a first cardiac event that included syncope (defined as transient loss of consciousness that was abrupt in onset and recovery), aborted cardiac arrest requiring defibrillation as a part of resuscitation attempts (ACA) or LQTS-related SCD (abrupt in onset without evident cause, if witnessed or death that was not explained by any other cause if it occurred in a non-witnessed setting including sleep).
ECG-phenotype characterization
All incoming ECGs were assessed by a cardiologist (WZ) blinded to subjects’ clinical characteristics in regard to the T-wave morphology in leads V5 and II, which was classified as either “normal”, “broad”, “flat”, “notched”, “negative” or “biphasic”.6, 7 For the purpose of this analysis, T-wave morphologies, which were not assessed as “normal” in either lead II or V5 were classified as “abnormal”, indicating the possible presence of ventricular repolarization abnormality/mutation penetrance.
ECGs were also assessed in regard to the conventional interval measurements such as PR, QRS, QT measured in the limb lead II and corrected using Bazett formula, and Tpeak-Tend (Tp-Te) interval measured from the absolute maximum of the T-wave to the end of the QT interval.
The study was approved by an institutional review board and study participants provided informed consent.
Genotype characterization
The presence of LQTS-causing KCNH2 mutation was verified with the use of standard genetic tests performed in academic molecular genetic laboratories reported previously.1 Genetic alterations of the amino acid sequence were characterized by location in the channel protein, which was defined as belonging to the pore-region if the coding sequence involved amino acid residues located in the S5-loop-S6 region (552 through 657). All other genetic variants were considered as non-pore mutations for the purpose of this study.
Statistical analysis
Differences in the univariate characteristics by LQT2-Normal QTc subjects versus genotype-negative control subjects were compared using the chi-square test or Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Categorical data were presented as frequency and percentage and continuous variables as mean ± SD or median and corresponding interquartile range.
The cumulative probability of the primary and secondary endpoints was assessed by the Kaplan-Meier method with significance testing by the log-rank statistic. The Cox-proportional hazard model was used to evaluate the independent contribution of clinical and genetic factors to the first occurrence of time dependent cardiac events from the age of 18 years through the end of follow-up. The Cox regression model was adjusted for the time-dependent beta-blocker use (the age at which patients were on and off beta-blocker therapy) and stratified by gender. As preselected QTc inclusion criteria overlapped with borderline QTc-prolongation, the model was also adjusted for QTc-duration with 440 ms selected as a cut-off for normal vs. borderline QTc values. In order to develop gender-specific regression parameter estimates for the clinical and genetic factors, interactions between gender and the variables of interest were utilized. The proportionality assumption was tested using time-dependent covariates created from interactions between survival time and various covariates.
All statistical tests were 2-sided, and a p value < 0.05 was considered statistically significant. Analyses were carried out with SAS software version 9.4 (SAS institute, Cary, North Carolina).
RESULTS
Study population
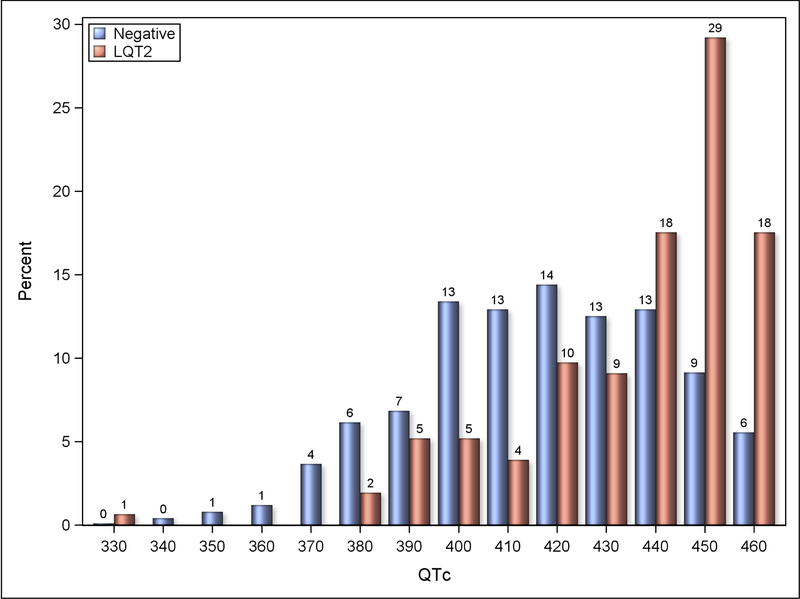
Distribution of QTc-intervals in the study group and genotype-negative control group are presented in the Figure 1. In total, 60 unique pathogenic genetic variants were reported among normal QTc mutation carriers, of whom the vast majority (n=119, 78%) carried mutations that were also reported among affected family members with prolonged QTc.
Figure 1.
QTc distribution histogram for LQT2 patients with normal QTc and genotype-negative control subjects.
The clinical characteristics of the study population are presented in the Table 1 with comparisons between normal QTc LQT2 carriers and non-carriers. The age at enrolment in the registry and gender distribution did not differ between the groups. There were differences in QTc duration which was longer in carriers than non-carriers despite being within normal limits. Tp-Te measured in leads II and V5 was also longer in mutation carriers. Abnormal T-wave morphology was observed in 39% of LQT2 carriers with normal QTc and in 7% non-carriers. The pore mutations were identified in 15% of carriers. Syncope before the age of 18 years was observed in 23 LQT2 carriers with normal QTc, of whom five had pore mutations and 12 had abnormal T-wave morphology.
Table 1.
Clinical characteristics of the LQT2 mutation carriers with normal QTc and genotype-negative family members.
| Clinical characteristics | Number of missing values | LQT2 Normal QTc | Number of missing values | Genotype-negative controls | p-value |
|---|---|---|---|---|---|
| Number | 154 | 1007 | |||
| Male, n(%) | 0 | 67 (44) | 0 | 414 (41) | 0.574 |
| Age at ECG, years | 0 | 41±15 | 0 | 41±15 | 0.837 |
| Pore mutation | 1 | 23 (15) | 0 | 0 | - |
| Syncope <18 years, n(%) | 0 | 23 (15) | 0 | 62 (6) | <0.001 |
| Electrocardiography | |||||
| RR, ms | 0 | 959±174 | 0 | 893±166 | <0.001 |
| PR, ms | 1 | 160±23 | 32 | 161±28 | 0.320 |
| QRS, ms | 0 | 84±14 | 0 | 85±14 | 0.438 |
| QTc, ms | 0 | 436±23 | 0 | 417±26 | <0.001 |
| Tpeak-Tend lead II, ms | 4 | 94±31 | 3 | 86±21 | 0.004 |
| Abnormal T-wave in V5 or II | 12 | 55 (39) | 61 | 64 (7) | <0.001 |
| Treatment | |||||
| Beta-blockers | 0 | 81 (53) | 0 | 187 (19) | <0.001 |
| ICD | 0 | 25 (16) | 0 | 17 (2) | <0.001 |
| Cardiac events ≥18 years | |||||
| Syncope | 0 | 41 (27) | 0 | 133 (13) | <0.001 |
| ACA | 0 | 4 (3) | 0 | 3 (0) | 0.008 |
| SCD | 0 | 5 (3) | 0 | 0 (0) | <0.001 |
| Appropriate ICD shock | 0 | 1(1) | 0 | 4 (0) | 0.510 |
Data are presented as means ± standard deviations or number (%)
* T-wave characterization was missing in 12 LQT2 patients (8%) and 61 control subjects (6%)
Among the 154 LQT2 carriers there were 44 (29%) patients with cardiac events ≥ 18 years of age including 8 (5%) ACA or SCD. Out of the 1,007 non-carriers, 135 (13%) patients had at least one cardiac event including 3 (0.3%) with ACA or SCD. Due to small number of ACA/SCD in this cohort we focused our analyses on cardiac events. Of the 25 ICD recipients among LQT2 carriers none had prior history of cardiac arrest or documented torsades de pointes; however nine had a history of syncope and six had family history of sudden cardiac death. A single individual from this group who received an ICD discharge during follow-up was a carrier of non-pore mutation 209 A>G, had abnormal T-wave morphology, and a baseline QTc of 450 ms.
Clinical course in the LQT2 carriers with normal QTc
T-wave morphology
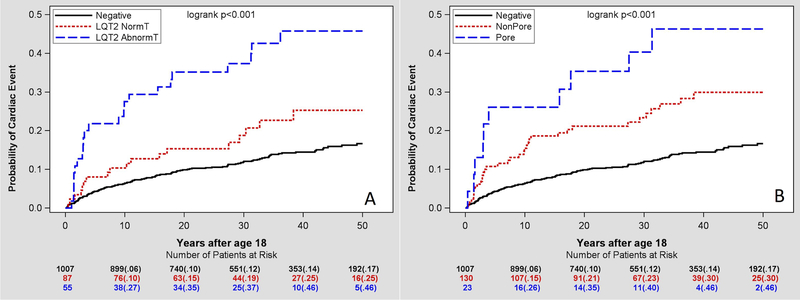
Figure 2A shows cumulative probability of cardiac events in LQT2 carriers with abnormal T wave vs. LQT2 carriers with normal T wave in comparison to non-carriers. After 10 years of follow-up from the age of 18 years old, LQT2 carriers with abnormal T wave had significantly higher rate of cardiac events than LQT2 carriers with normal T wave and non-carriers (27% vs. 10% and 6% respectively; p<0.001). After 30 years of follow-up from the age of 18 years, the rates were 37% vs. 19% (p=0.134) and 12% (p<0.001), respectively. After multivariate adjustment for sex and time-dependent beta-blocker therapy, LQT2 carriers with normal QTc and abnormal T-wave morphology had greater risk of cardiac events than LQT2 carriers with normal T-wave morphology and non-carriers (Table 2). In comparison with non-carriers, LQT2 carriers with normal T-wave morphology had a trend toward greater risk of cardiac events with hazard ratio of 1.58 (p=0.072). Tpeak-Tend did not demonstrate an independent prognostic value in the multivariable analysis and its inclusion in the model did not affect the results.
Figure 2.
Kaplan-Meier curve analysis of the risk of cardiac events in relationship to the T wave morphology (left) or LQT2 mutation type (right) in LQT2 mutation carriers with normal QTc compared with genotype-negative family members.
Table 2.
Multivariable analysis: Risk of cardiac events among LQT2 mutation carriers with normal QTc interval and genotype-negative unaffected family members (adjusted for sex, QTc and time-dependent beta blocker therapy).
| Cardiac Events |
||||
|---|---|---|---|---|
| HR | 95% CI | p-value | ||
| Lower | Upper | |||
|
T-wave
morphology | ||||
| Abnormal vs Normal T-wave LQT2 | 2.63 | 1.41 | 4.89 | 0.002 |
| Abnormal T-wave LQT2 vs Genotype-negative | 4.14 | 2.63 | 6.50 | <0.001 |
| Normal T-wave LQT2 vs Genotype-negative | 1.58 | 0.96 | 2.58 | 0.072 |
|
LQT2 mutation type | ||||
| Pore vs Non-pore | 1.93 | 0.95 | 3.92 | 0.068 |
| Pore vs Genotype-negative controls | 4.01 | 2.09 | 7.66 | <0.001 |
| Non-pore vs Genotype-negative controls | 2.07 | 1.42 | 3.03 | <0.001 |
Pore- vs. non-pore LQT2 mutation
Carrying LQT2 mutation in the pore domain appeared to be a risk indicator in the subgroup of mutation-positive patients with normal QTc (Figure 2B). After multivariate adjustment for sex and time-dependent beta-blocker therapy, normal QTc LQT2 carriers with pore-mutations had a trend toward greater risk of cardiac events than non-pore LQT2 mutations carriers (HR= 1.93; p=0.068, Table 2).
We have also performed a sensitivity analysis by excluding normal QTc LQT2-mutation carriers who had syncopal episodes prior to age of 18 years, which yielded similar Kaplan-Meier curve analysis results and did not affect risk estimates.
Gender-related risk of cardiac events in LQT2-mutation carriers
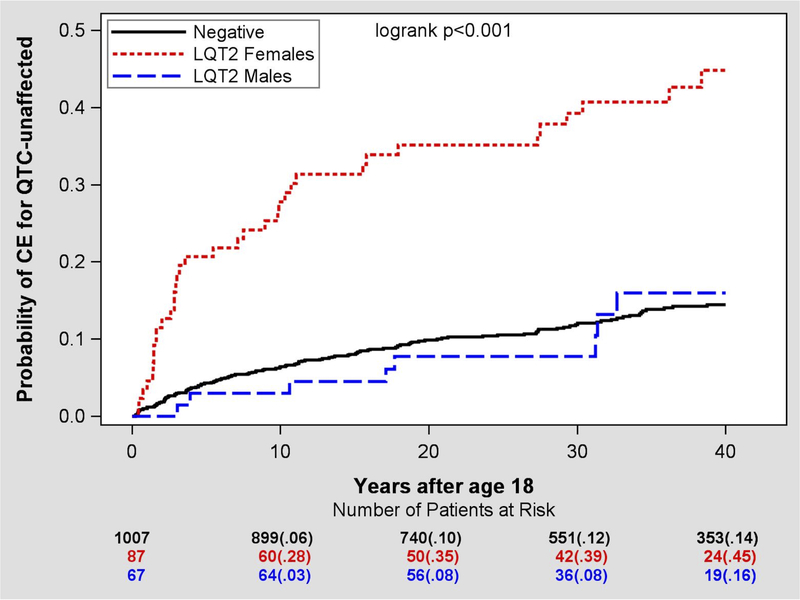
Since the risk of cardiac events is significantly different in adult LQT2 females than males we analyzed the above associations in males and females separately (Table 3). Among LQT2 carriers with normal QTc, females had greater risk of cardiac events than males (HR=4.09, 95%CI 1.89–8.81, p<0.001, Figure 3).
Table 3.
Gender-specific estimates of the risk of cardiac events among normal QTc LQT2 carriers in relationship to the T-wave morphology and mutation type. (adjusted for QTc and time-dependent beta blocker therapy).
| Women | Men | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
|
T wave
morphology | ||||||
| Abnormal T wave vs Genotype-negative | 6.01* | 3.65–9.89 | <0.001 | 1.52* | 0.54–4.27 | 0.427 |
| Normal T wave vs Genotype-negative | 1.82 | 1.05–3.14 | 0.032 | 0.94 | 0.29–3.06 | 0.918 |
| Abnormal vs Normal T wave | 3.31 | 1.68–6.52 | 0.001 | 1.58 | 0.35–7.07 | 0.549 |
|
Mutation type | ||||||
| Pore vs Genotype-negative | 3.70 | 1.62–8.47 | 0.002 | 4.39 | 1.56–12.37 | 0.005 |
| Non-Pore vs Genotype-negative | 2.71† | 1.79–4.09 | <0.001 | 0.73† | 0.26–2.05 | 0.549 |
| Pore vs Non-Pore | 1.37 | 0.57–3.29 | 0.487 | 6.01 | 1.50–24.08 | 0.011 |
* P-value for interaction 0.018; † P-value for interaction 0.020
Figure 3.
Kaplan Meier curve analysis of cardiac events risk in LQT2 male and female carriers compared with genotype-negative family members.
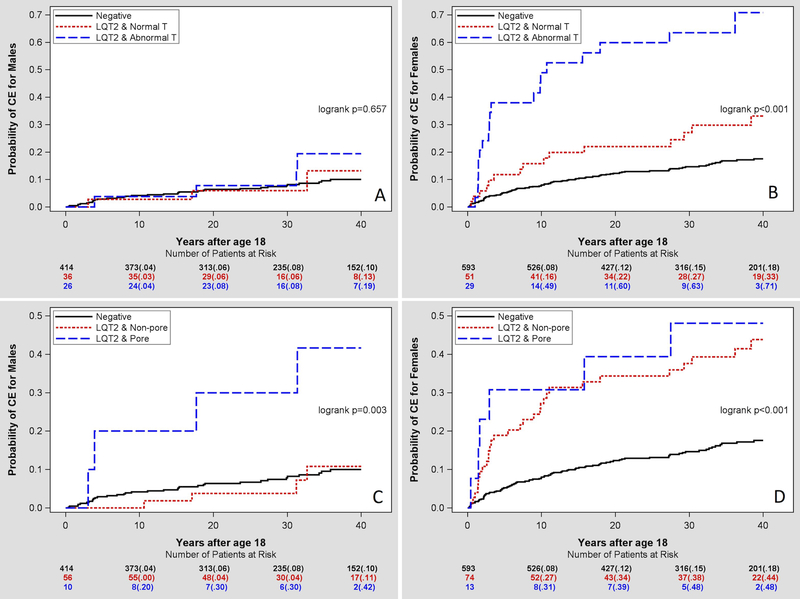
Abnormal T-wave morphology was observed in 26 men (39%) and 29 women (33%), however cardiac events were reported in only 4 (15%) men and 19 (66%) women (p-value for interaction 0.018). As shown in Table 3, female LQT2 carriers with abnormal T wave had greater risk of cardiac events than female LQT2 carriers with normal T wave morphology (HR = 3.31, p=0.001; Figure 4A and4B). There was no significant association between T wave morphology and cardiac events in males.
Figure 4.
Kaplan-Meier curve analysis of cardiac events in relationship to the presence of the abnormal T-wave morphology (A and B) or transmembrane pore KCNH2 mutation (C and D) in normal QTc LQT2 mutation carriers compared with genotype-negative family members presented separately for men and women.
Pore location of LQT2 mutations indicated significantly increased risk of cardiac events than in non-pore LQT2 mutation males (HR=3.70; p=0.002). However, there was no such association in females (HR=1.37, p=0.487; Figure 4C and4D). LQT2 pore-mutation carriers constituted the only male subgroup that demonstrated significant hazard compared to non-carriers (Figure 4C).
Discussion
We have shown that among carriers of disease-causing mutations in the KCNH2 gene with normal QTc interval further risk stratification can be achieved by combining information regarding patient gender, the affected KCNH2 region, and T-wave morphology. Our findings extend earlier reported hazard associated with female gender to the subgroup of LQT2-mutation carriers with normal QTc interval. Furthermore, patients with normal T-wave morphology and non-pore KCNH2 mutations generally have low risk of cardiac events, however, important differences in risk stratification value of these characteristics exist between men and women. Women carrying LQT2 mutations demonstrate significantly increased risk of cardiac events compared to genotype-negative controls, which is linked to the presence of abnormal T waves and not related to the type of mutation. On the contrary, carrying pore mutation was the only risk factor that identified a subgroup with elevated risk of CE among men.
Carriers of LQTS-causing mutations with normal QTc represent a challenging patient group. Though the risk of ACA/SCD is believed to be low, as shown by our group previously,1 it still accounts for about 4% cumulative risk by the age of 40 years and is 10-fold increased compared to unaffected family members. This observation has led to recommendation of beta-blocker therapy in all LQTS mutation carriers regardless of QTc duration.2 Even though beta blockers are known for their excellent safety profile, family members diagnosed as a result of widely implemented cascade genetic family screening are often young and healthy individuals, who are being exposed to a long-term beta blocker therapy and their associated side effects, which may significantly affect their quality of life. Further attempts to risk stratify LQTS mutations carriers with normal QTc are therefore needed.
We hypothesized that mutation penetrance and associated arrhythmic risk may not only be assessed by the degree of QTc prolongation but also by the alterations in T-wave shape visible also in the normal QTc range. Among the two most common LQTS genetic variants, the type 2 is known for its characteristic T-wave distortion first described in 19956 and could be reliably distinguished by computer-based methods.8–10 Our findings of strong association between the cardiologist-adjudicated abnormal T-wave morphology and the risk of arrhythmic events among patients with type 2 LQTS syndrome indicate that previously used terminology of “phenotype-negative” LQTS based exclusively on QTc assessment should be redefined and, at least in the context of type 2 LQTS, be based on assessment of both QTc and the shape of T wave.
Despite demonstrating lower risk of arrhythmic events compared to patients with abnormal T-wave morphologies, LQT2 females with normal T waves still had increased risk of cardiac events compared with genotype-negative family members, which was not observed among men. Significant residual risk observed among women with normal T waves may be due to the well-recognized impact of sex hormones on arrhythmogenesis reviewed recently,11 which may not be directly translated in the abnormalities on surface ECG. However, it is also possible that the link between individual genetic variants and their expression on surface ECG is mutation-specific and the arrhythmic risk may not be bound to the ECG expression of ion channel defect.
Association between mutations in the transmembrane pore region of KCNH2 and increased arrhythmia risk was first reported 200212 and later expanded in a large international cohort of LQT2 patients.3 In line with earlier observations,2 the prevalence of the pore mutations in our study was relatively low in our group of patients with normal QTc. In a previous analysis of the impact of mutation-specific characteristics on the outcome in patients with different variants of LQTS,3 it was suggested that mutation characteristics had prognostic impact only among those with normal QTc interval while in patients with prolonged QTc it was the degree of QTc prolongation rather than the mutation type that affected the risk of arrhythmias. It is remarkable, however, that even low-risk non-pore mutations in our study were associated with three-fold increased risk of cardiac events in LQT2-mutation male carriers with normal QTc compared with genotype-negative family members.
Our findings further illustrate the importance of gender in the risk assessment of LQT2 mutation carriers. While female gender association with elevated risk of CE in LQT2 has been repeatedly reported earlier13, 14, our findings extend this knowledge to the growing group of phenotype-negative mutation-carrying family members resulting from implementation of genetic cascade screening in the clinical routine. Even though T-wave morphology assessment could be useful in identifying the highest risk group in females, even LQT2-mutation carrying women with ostensibly normal ECG, i.e. normal T waves and normal QTc, appear to be at significantly elevated risk compared to non-carriers. Furthermore, the risk of CE associated with LQTS genotype persists through the life-time as our group has shown previously15 and our current findings confirm the validity of this observation in women with normal QTc, who remain at risk through the late postmenopausal period. On the contrary, men carrying LQT2 mutations appear to be at a very low risk of events if they have normal QTc independently from T-wave morphology so that their risk of events remains undistinguishable from the genotype-negative controls with exception for the small minority of male subjects carrying pore mutations who had four-fold risk increase compared to non-carriers.
Finally, our findings raise questions whether primary preventive beta-blocker therapy, which is currently advocated in mutation carriers with normal QTc,2 should be applied indiscriminately. Our findings of gender-related differences regarding the risk of cardiac events among LQT2 mutation carriers with normal QTc suggest that men carrying a non-pore LQT2 mutation, which stands for the vast majority of male mutation carriers, are at the risk of arrhythmic events that is not distinguishable from the one observed in genotype-negative family members and thus may not have sufficient risk-benefit ratio for justifying life-long beta-blocker therapy.
Study limitations
Our findings are only applicable to adult LQT2 mutation carriers since we have intentionally excluded individuals under 18 years of age due to a greater variation of age-related normal variants of T-wave morphology. It remains therefore unproven whether similar risk stratification scheme could be developed for children and infants born with disease-causing KCNH2 variant and should be treated with beta blockade according to the current recommendations.2
T-wave morphology adjudication was performed without use of automatic computer-based algorithms and therefore might have underestimated ECG manifestations of potassium-channel malfunction possibly attributable to the arrhythmic risk in patients with normal T-wave morphologies.
Finally, even though the model was adjusted for time-dependent beta-blocker use, we have not been able to access the information concerning circumstances surrounding reported CE. Therefore we cannot account for possible triggers, exposure to potential QT-prolonging drugs or predisposing factors, which may have contributed to syncopal episodes in LQT2-mutation carriers.
Conclusion
Among electrocardiographically unaffected LQT2 patients, female gender, the T-wave morphology, and the type of LQT2 mutations are independently associated with the risk of cardiac events and should be considered in weighing risks and benefits of primary preventive therapies. Genotype-positive female LQT2 patients with normal QTc are at higher risk for cardiac events than control population, although the presence of T-wave abnormality is associated with a higher risk than normal T-wave morphology. Mutation type is useful in risk stratifying cardiac events in male LQT2 carriers with normal QTc: non-pore LQT2 mutations are not at significantly different risk of cardiac events than unaffected family members. The findings indicate that risk stratification within the unaffected LQT2 mutation carriers is possible and advocate patient-tailored use of prophylactic beta-blocker therapy.
WHAT IS KNOWN.
Genotype-positive patients with long QT syndrome (LQTS) are at risk for cardiac events even if QTc durations does not exceed normal limits and beta-blocker therapy is advocated for asymptomatic genotype-positive LQTS patients regardless of their QTc duration.
Female gender is associated with higher risk of cardiac events in patients with LQTS.
Disease-causing mutation penetrance may be manifested in both QTc prolongation and alterations of T-wave morphology
WHAT THE STUDY ADDS.
Among adult LQT2 mutation carriers with normal QTc, further risk stratification is possible using gender, T-wave morphology and mutation localization within the KCNH2 gene.
Female patients with LQT2 remain to be at higher risk of cardiac events than men even in the normal range QTc while male patients do not demonstrate risk increase compared to control population.
Performance of electrocardiographic and genetic risk indicators is gender-specific and can be used for patient-tailored risk stratification and beta-blocker use.
Sources of Funding
The study was performed with support from NIH grant HL123483. Pyotr Platonov was supported by the research grant from The Swedish Heart-Lung Foundation (grant #20150574) and scholarship grants from the Fulbright Commission, Maggie Stephens Foundation, Swedish Society of Medicine and donation funds at Skåne University Hospital (Lund, Sweden).
Footnotes
Disclosures:None.
References
- 1.Goldenberg I, Horr S, Moss AJ, Lopes CM, Barsheshet A, McNitt S, Zareba W, Andrews ML, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Napolitano C, Platonov PG, Priori SG, Qi M, Schwartz PJ, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Zhang L. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol. 2011;57:51-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793-867. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu W, Moss AJ, Wilde AA, Towbin JA, Ackerman MJ, January CT, Tester DJ, Zareba W, Robinson JL, Qi M, Vincent GM, Kaufman ES, Hofman N, Noda T, Kamakura S, Miyamoto Y, Shah S, Amin V, Goldenberg I, Andrews ML, McNitt S. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg I, Mathew J, Moss AJ, McNitt S, Peterson DR, Zareba W, Benhorin J, Zhang L, Vincent GM, Andrews ML, Robinson JL, Morray B. Corrected QT variability in serial electrocardiograms in long QT syndrome: the importance of the maximum corrected QT for risk stratification. J Am Coll Cardiol. 2006;48:1047-52. [DOI] [PubMed] [Google Scholar]
- 5.Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm. 2011;8:1537-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JL, Schwartz PJ, Towbin JA, Vincent GM, Lehmann MH. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation. 1995;92:2929-34. [DOI] [PubMed] [Google Scholar]
- 7.Zareba W, Moss AJ, Rosero SZ, Hajj-Ali R, Konecki J, Andrews M. Electrocardiographic findings in patients with diphenhydramine overdose. Am J Cardiol. 1997;80:1168-73. [DOI] [PubMed] [Google Scholar]
- 8.Vaglio M, Couderc JP, McNitt S, Xia X, Moss AJ, Zareba W. A quantitative assessment of T-wave morphology in LQT1, LQT2, and healthy individuals based on Holter recording technology. Heart Rhythm. 2008;5:11-8. [DOI] [PubMed] [Google Scholar]
- 9.Kanters JK, Fanoe S, Larsen LA, Bloch Thomsen PE, Toft E, Christiansen M. T wave morphology analysis distinguishes between KvLQT1 and HERG mutations in long QT syndrome. Heart Rhythm. 2004;1:285-92. [DOI] [PubMed] [Google Scholar]
- 10.Cortez D, Bos JM, Ackerman MJ. Vectorcardiography identifies patients with electrocardiographically concealed long QT syndrome. Heart Rhythm. 2017;14:894-899. [DOI] [PubMed] [Google Scholar]
- 11.Odening KE, Koren G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm. 2014;11:2107-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss AJ, Zareba W, Kaufman ES, Gartman E, Peterson DR, Benhorin J, Towbin JA, Keating MT, Priori SG, Schwartz PJ, Vincent GM, Robinson JL, Andrews ML, Feng C, Hall WJ, Medina A, Zhang L, Wang Z. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105:794-9. [DOI] [PubMed] [Google Scholar]
- 13.Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm. 2011;8:1537-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ, Benhorin J, Hall WJ, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329-37. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Moss AJ, Bradley J, Polonsky S, Peterson DR, McNitt S, Zareba W, Andrews ML, Robinson JL, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Qi M, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long-QT syndrome after age 40. Circulation. 2008;117:2192-201.</References> [DOI] [PubMed] [Google Scholar]