Abstract
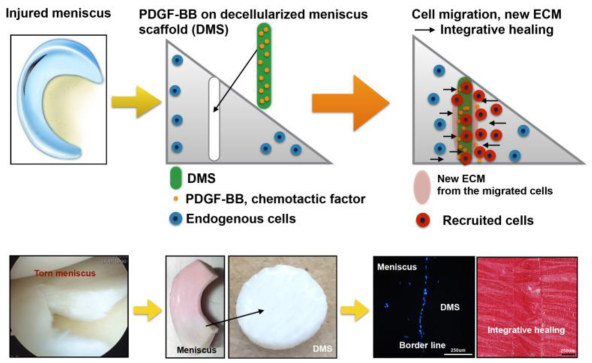
The aim of this study was to examine the potential of platelet-derived growth factor (PDGF)-coated decellularized meniscus scaffold in mediating integrative healing of meniscus tears by inducing endogenous cell migration.
Fresh bovine meniscus was chemically decellularized and covalently conjugated with heparin and PDGF-BB. In vitro PDGF release kinetics was measured. The scaffold was transplanted into experimental tears in avascular bovine meniscus explants and cultured for 2 and 4 weeks. The number migrating and proliferating cells at the borderline between the scaffold and injured explant and PDGF receptor-β (PDGFRβ) expressing cells were counted. The alignment of the newly produced ECM and collagen was analyzed by Safranin-O, picrosirius red staining, and differential interference contrast (DIC). Tensile testing of the explants was performed after culture for 2 and 4 weeks.
Heparin conjugated scaffold showed immobilization of high levels of PDGF-BB, with sustained release over 2 weeks. Insertion of the PDGF-BB treated scaffold in defects in avascular meniscus led to increased PDGFRβ expression, cell migration and proliferation into the defect zone. Safranin-O, picrosirius red staining and DIC showed tissue integration between the scaffold and injured explants. Tensile properties of injured explants treated with PDGF-BB coated scaffold were significantly higher than in the scaffold without PDGF.
In conclusion, PDGF-BB-coated scaffold increased PDGFRβ expression and promoted migration of endogenous meniscus cells to the defect area. New matrix was formed that bridged the space between the native meniscus and the scaffold and this was associated with improved biomechanical properties. The PDGF-BB-coated scaffold will be promising for clinical translation to healing of meniscus tears.
Keywords: Meniscus, PDGF, Decellularization, Heparin, Cell migration
Graphical abstract
1. Introduction
Forceful twisting, rotating, or hyper-flexion of the knee joint leads to traumatic tears of the meniscus [1]. A torn meniscus causes knee pain, swelling, stiffness, and limitations in extending the knee. Current surgical approaches to address meniscus tears are limited to suturing, and partial meniscectomy [2-4]. However meniscal tears in the inner avascular region typically do not heal spontaneously or after surgical interventions [5, 6] and represent a major risk factor for the development of knee osteoarthritis (OA) [7, 8]. The middle and inner zones of meniscus lack blood supply and have the least potential for healing [9].
Cells that have potential to promote meniscal repair and regeneration are present in the meniscus adjacent to the tear and in synovium and joint capsule [10-12]. Application of chemotactic factors to the tear site thus has potential to recruit cells that mediate repair and various approaches including gene transfer into meniscus cells or application of gene transduced cells have been pursued [13-18]. PDGF is a candidate for meniscus repair as it has strong chemotactic activity for chondrocytes and mesenchymal stem cells [19]. PDGF enhances meniscal cell proliferation and biosynthetic activity and its expression is decreased in lesions in the avascular zone [15, 20-24]. PDGF-BB binds and triggers auto-phosphorylation of the PDGFRβ. This receptor has been for targeted therapeutic manipulation of cell proliferation and extracellular matrix production [25, 26].
PDGF and other growth factors can be immobilized for sustained release on scaffolds via covalent linkage to heparin or electrostatic interactions [27-29]. Heparin has strong binding affinity for a various growth factors such as basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and PDGF-BB [30]. The present study used decellularized, heparin-conjugated meniscus as a readily available and clinically applicable scaffold for PDGF-BB immobilization and tested the ability of this scaffold to recruit endogenous cells to mediate repair of meniscus tears.
2. Materials and Methods
2.1. Meniscus explants
Fresh bovine menisci (medial and lateral) were obtained from normal knees of 18-30 months old animals (Animal Technologies Inc., Tyler, TX). The knees were harvested on the same day that the animals were slaughtered and shipped on ice for arrival in the laboratory the following day. For preparing meniscal explants, the avascular region (inner two thirds) was resected with a scalpel and cut into blocks of approximately 20 mm width. The tissue blocks were washed 3 times in DMEM with 1 % (v/v) PSF and incubated in DMEM with 10 % (v/v) calf serum (CS) (Omega Scientific Inc., Tarzana, CA) and 1 % (v/v) Penicillin-Streptomycin-Gentamycin (PSG) (Life Technologies, Carlsbad, CA) for 3 days.
2.2. Preparation of decellularized meniscus scaffold and growth factor conjugation
Disc shaped explants, 6-mm diameter and 1-mm thickness, were obtained from horizontally punctured bovine meniscus blocks using specimen needles (Fig. S1). For decellularization, the explants were sequentially incubated in a shaking incubator (300 rpm at 37 °C) with DNAse/RNAse-free water for 12 hours, 0.05 % (wt/v) trypsin-EDTA for 12 hours, washing three times with saline for 1 hour, the mixture of 2 % (v/v) aqueous Triton X-100 and 1.5 % peracetic acid for 24 hours, and 2 % (wt/v) collagenase for 4 hours. Upon completion of this sequential chemical treatment, decellularized meniscus scaffolds (DMS) were washed with distilled DNAse/RNAse-free water for 72 hours with daily media changes and stored in PBS with 0.1 % (v/v) PSG at 4 °C until use. DNA content in native meniscus was 7.75 ng/mL and in decellularized DMS 1.71 ng/mL (p=0.0014, Fig. S2.a).
To immobilize PDGF-BB on DMS, heparin was conjugated to the DMS. The reactive amine groups (−NH2) on the DMS were conjugated with the carboxyl groups (−COOH) on heparin to form a covalent amide bond. This process allowed the negatively charged sulfonic groups (−SO3) in the heparin molecules to trap added PDGF-BB [30]. Heparin sodium salt 0.1 % (wt/v) (Sigma Aldrich, St. Louis, MO) was dissolved in 0.05 M 2-morpholinoethane sulfuric acid (MES) (Sigma-Aldrich) buffer (pH 5.6) containing 25 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Thermo Fisher Scientific) and 10 mM N-hydroxysuccinimide (NHS) (Sigma-Aldrich) to activate the carboxyl groups of heparin. One mL aliquots of the heparin solution were added to each DMS and incubated at room temperature for 4 hours with gentle shaking. The heparin conjugated DMS were rinsed 3 times with 0.1 M Na2HPO4, pH 7.0 and distilled water. The toluidine blue assay showed that the heparin conjugated DMS by EDC cross-linking was 33.77 fold higher than the DMS only immersed in the heparin solution (p<0.0001, Fig. S2.b).
The heparin-conjugated DMS were incubated in 0.1% (wt/v) NaN3 in PBS pH 7.0 with recombinant human PDGF-BB (Peprotech Inc., Rocky Hill, NJ) at a concentration of 200 ng/mL for 12 hours at 4 °C. After the PDGF-BB immobilization, DMS were washed 3 times with PBS and stored in PBS 1% PSG at 4 °C for up to 4 weeks. All subsequent experiments with PDGF-BB-conjugated DMS were performed at neutral pH.
2.3. Release kinetics of PDGF-BB from heparin-conjugated DMS
To remove the non-bound PDGF-BB, the scaffold was washed twice with 500 μL release media composed of PBS with 0.1 % (wt/v) BSA and 0.1 % (wt/v) NaN3. After washing, the supernatant was harvested, and the amount of PDGF-BB measured was considered as the non-absorbed PDGF-BB and used to calculate the amount of PDGF-BB bound to the scaffold (total amount of bound PDGF-BB = 200 ng – the quantity of non-absorbed PDGF-BB from the residual supernatant). To measure PDGF-BB release, replicate PDGF-BB-conjugated DMS were placed into 1 mL release media in sterile 1.5 mL tubes at 37 °C with gentle shaking at 150 rpm for up to 16 days. At each time point, all of the media was replaced with fresh release media. The harvested supernatants were centrifuged and stored at −80 °C. The cumulative amount of PDGF-BB in the release media from each sample was analyzed using a human PDGF-BB ELISA (Peprotech Inc).
2.4. In vitro culture of cells from avascular meniscus seeded on DMS
One million human avascular meniscus cells (isolated normal knee joints of a 33-year old male and 39-year old female, obtained from tissue banks) were seeded on each DMS (3 mm diameter, 6 mm height) in trans-well plates (12 multi-well plate). After two sequential applications of 50 μL cell suspension onto the top and bottom surface of the DMS, the trans-well plates were incubated at 37 °C, 5 % CO2 for 3 hours and then basal culture media or PDGF-BB (50 ng/mL) media was added. The cell-seeded DMS was transferred to 24-well plates and cultured for 24 hours.
2.5. Ex vivo culture of meniscus with experimental tear
Cylindrical explants (diameter: 12mm, height: 6mm) were cut from bovine menisci using a biopsy punch. A horizontal tear (8mm in length) was made with a blade in the center of the explant. The 6-mm diameter DMS was inserted into the defect. During the insertion, the fiber orientation of the DMS was kept identical to that of the middle part of the meniscus. The meniscus defect area in the explants without inserted DMS was sutured with a 5-0 Prolene suture (Ethicon, Blue Ash, Ohio). The explants were cultured for 2 and 4 weeks with media change every 3 days.
2.6. Histological and immunohistochemical analyses
The explants (n=5 per each group) were fixed in Z-fix (Anatech, Battle Creek MI). Paraffin-embedded sections (5-7 μm) were stained with DAPI (H-1500 Vector Laboratories, Inc.) for cell counting. For each group 6 sections were used for cell counting. The cells in 12 different regions in each section were counted. The 6 regions were near the borderline between the defect and the DMS. The percentage of cells at the borderline was calculated and compared among treatment groups. To confirm the biological activity of PDGF-BB as reflected by increased PDGFRβ expression [25, 26, 31], anti-PDGFRβ(ab107169, 1:200 dilution, Abcam) antibody was applied and detected with goat anti-rabbit IgG (A-11008, 1:500 dilution, Life Technologies). Anti-Ki67 (ab15580, 1:200 dilution, Abcam) antibody was applied and detected by goat anti-rabbit Alexa fluor 488 (ab150077, 1:200 dilution, Abcam) to detect cell proliferation induced by PDGF-BB. DAPI was used to visualize cells on the DMS. Sections were stained with Safranin-O to detect GAG and with pircrosirius red using a polarized light microscopy to detect collagen fiber alignment and interconnectivity between inserted DMS and injured explants. Safranin-O positive signal intensity relative the negative signal region was quantified by converting the positive signal to RGB stack and adjusting threshold using ImageJ [32]. Tissue interconnectivity was quantified by dividing the cumulative length of integrated segments between the meniscus explant and DMS by the total defect length in the picrosirius red stained images using ImageJ [33]. To confirm tissue interconnectivity, fully hydrated and unstained 15 μm thickness sections was examined by differential interference contrast (DIC) microscopy (Zeiss LSM 710, Carl Zeiss, Thornwood, New York). PDGF is known to increase PDGFRβ expression [25, 26, 31] and PDGFRβ positive cells were counted to confirm biological activity of PDGF-BB. Anti-β-actin (ab3280, 1:100 dilution, Abcam) antibody was applied and detected by goat anti-mouse Alexa fluor 568 (ab175473, 1:200 dilution, Abcam) to detect lamellipodia as a marker of cell migration induced by PDGF-BB [34], which was visualized at high resolution (60x) through a scanning microscope (BZ-X700, KEYENCE, Itasca, IL). Lamellipodium positive cells were counted and long and short diameters of the elongated cells were measured to compare the difference between lamellipodium positive cells and negative cells. The long diameter was divided by short diameter of each cell and the ratio was compared among experimental groups [35]. Ki67 positive cells were counted and percentaged by DAPI positive cells in the explant to determine whether cell proliferation was induced by PDGF-BB.
To assess cell death in the defect area, Live-Dead cell assay was performed by staining with calcein-AM and ethidium homodimer-1 (Thermo Fisher Scientific) (Fig. S3).
2.7. Biomechanical testing
The tensile stiffness of the injured meniscus explants cultured with DMS, or heparin-PDGF-BB-conjugated DMS was quantified by tensile testing (n=8-12 per group). After 2 and 4 weeks of culture, each explant was mounted using cyanoacrylate glue at the two ends in the mount of an UTM (Instron® Universal Testing Machine, 3342 Single Column Model, Norwood, MA) with a 500 N load cell. Each specimen was tested in tension (rate = 1 mm/min) and tensile force was monitored until the DMS separated from the repair site. The slope of the force-displacement curve before separation of the DMS was used to define the stiffness of the repair.
2.8. qRT-PCR
RNA was isolated from bovine meniscus explants and human avascular cells after 24-hour culture with PDGF-BB (0, 50, 100 ng/mL). RNA was isolated by using TRIzol Reagent (Life Technologies) and Direct-zol RNA MiniPrep Kit (Zymo research, Irvine, CA) and reverse transcribed into cDNA with PrimeScript RT Reagent Kit (Clontech Laboratories, Inc., Mountain View, CA).
Quantitative-PCR analysis was conducted on a LightCycler 480 Real-Time PCR System (Roche Diagnostics, Indianapolis, IN) with up to 45 cycles using TaqMan Gene Expression Assay probes (Life Technologies, PDGFRβ: Bt03247802_m1; VEGFA: Hs00900055_m1; GAPDH: Bt03210913_g1, Hs02758991_g1). The levels of mRNA were calculated as relative quantities in comparison to GAPDH.
2.9. Statistical analysis
Data represent mean and standard error of mean (SEM), from at least 3 to 4 replicate experiments, each performed in triplicate. The statistical significance of differences in PDGF-BB release was determined using 2-way ANOVA for multiple comparisons. In tensile testing, Mann-Whitney test was used. Differences in histological scores and values, DNA content, and Toluidine blue quantification were analyzed by unpaired t test. Fold change in gene expression was calculated using the ddCt method based on the average of technical duplicates. Results with *=p<0.05 (95% CI, confidence interval), **=p<0.01 (99% CI), ***=p<0.001 (99.9% CI), ****=p<0.0001 (99.99% CI) were considered statistically significant.
3. Results
3.1. PDGF-BB conjugation and release
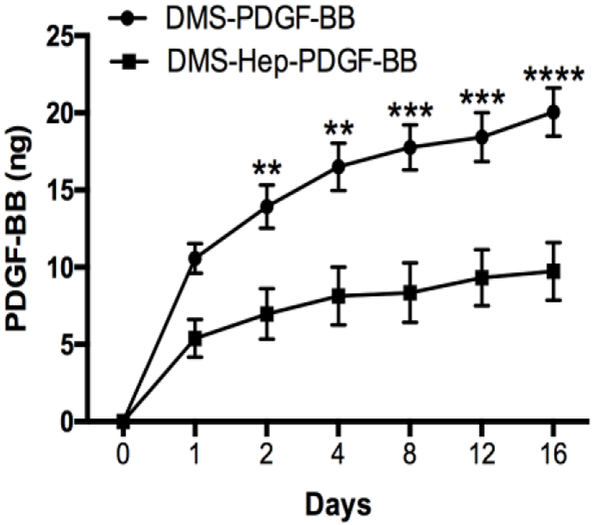
The amount of PDGF-BB that was bound to heparin-coated DMS was 86.72 % of total 200 ng PDGF-BB and 76.82 % in non-heparin coated DMS. Following an initial release of 6.22 % from heparin-coated DMS versus 13.76 % from non-heparin coated DMS during the first 24 h, there was subsequent sustained release with about 0.61 ng per 24 h during the following 16-day period (Fig. 1). The rate of release from heparin-coated DMS was on average 2.1 times slower than non-heparin coated DMS. By day 16, 5.61 % of the total amount of PDGF-BB was released from heparin-coated DMS versus 13.01 % release from the DMS without heparin. Also, there were significant differences in the amounts of PDGF-BB released at days 2, 4, 8, 12, and 16 between DMS with and without heparin.
Figure 1.
PDGF-BB release kinetics from DMS.
PDGF-BB was conjugated to DMS or heparin coated DMS and DMS was cultured at 37 °C for up to 16 days. Supernatants were collected at the indicated time points and analyzed for PDGF-BB by ELISA. Results are from 3 separate experiments. Data are shown as mean +/− standard error (SE).
3.2. Cell viability, migration and proliferation in DMS-inserted meniscus tears
Culture for up to 2 weeks of injured meniscus explants where the defect area was sutured but not inserted with DMS did not show any cell migration or fibrous connectivity in the defect area (Fig. 2b). Insertion of DMS that was not conjugated with heparin or PDGF-BB also did not induce cell migration to the defect area although the defect space was filled with DMS (Fig. 2c, Fig. S4.a-e). There was no significant difference in cell migration between DMS, heparin conjugated DMS, and PDGF-BB coated DMS without heparin conjugation (Fig. S5.a-c).
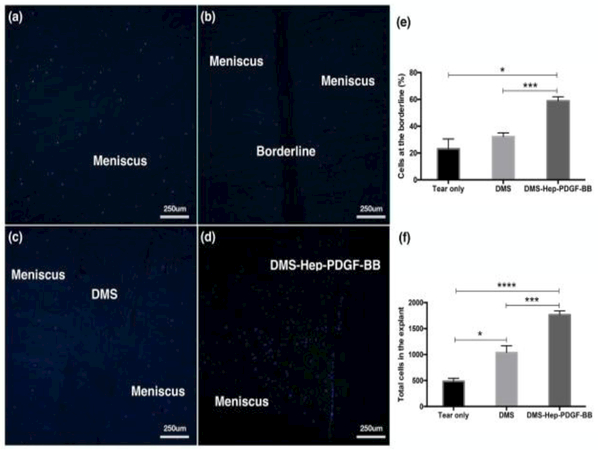
Figure 2.
Cell migration in injured meniscus explants cultured with inserted DMS.
DAPI stained sections of explants cultured for 2 weeks (n=3-6 per group, 10x).
a. Native non-injured meniscus.
b. Injured meniscus cultured without DMS.
c. Injured meniscus cultured with DMS.
d. Injured meniscus cultured with DMS-Hep-PDGF-BB.
e. Graph with numbers of cells at the borderline.
f. Graph with total cell numbers in the explant.
Data represent the mean of 6-8 values from 3 separate experiments.
Insertion of the DMS that was conjugated with heparin and PDGF-BB (DMS-Hep-PDGF-BB) into the meniscus tears led to migration of meniscus cells to the defect zone (Fig. 2d, Fig. S4.f-j). There was alignment of cells at the border between the meniscus tissue and the inserted DMS. Most of the recruited cells were in the defect space but some cells migrated into the DMS (Fig. S5.d).
Cell counting of DAPI stained sections revealed that DMS-Hep-PDGF-BB induced significantly higher cell density (59±3 %) at the borderline between DMS and meniscus than the DMS not conjugated with Hep or PDGF (32±3 %) (Fig. 2e). Total cell numbers in the DMS-Hep-PDGF-BB group (1766±73) were significantly increased compared with tear only (485±57) or DMS only groups (1039±129) (Fig. 2f).
Analysis of cell viability by live/dead stain showed that there were dead cells at the edge of the cut and there were fewer dead cells in the samples that were inserted with DMS, in particular with DMS-Hep-PDGF-BB as compared to defects that were only sutured and had no DMS inserted (Fig. S3).
Sections from the same meniscus explants were also stained with PDGFRβ antibody. PDGFRβ expression is seen in most cells in human meniscus in the vascular zone (Fig. S6a, b). However exogenous PDGF-BB induced PDGFRβ expression in the avascular zone (Fig. S6c, d, e). Moreover, PDGFRβ mRNA expression was increased by PDGF-BB treatment in the avascular meniscus (Fig. S7a). In cultured bovine meniscus, PDGF-BB-coated DMS induced a significant increase in the number of PDGFRβ positive cells throughout the explants (Fig. 3d). DMS-Hep-PDGF-BB (92±3 %) induced significantly higher numbers of PDGFRβ-positive cells in the defect area than the DMS inserted group (50±6 %), and the sutured meniscus group without DMS (22±7 %) (Fig. 3e). However, there was no significant difference in PDGFRβ-positive cells between DMS, heparin conjugated DMS, and PDGF-BB coated DMS without heparin conjugation (Fig. S8.a-c).
Figure 3.
PDGFRβ positive cells in injured meniscus explants.
Anti-PDGFRβ stained sections of explants cultured for 2 weeks (n=3-6 per group, 40x).
a. Native non-injured meniscus.
b. Injured meniscus cultured without DMS.
c. Injured meniscus cultured with DMS.
d. Injured meniscus cultured with DMS-Hep-PDGF-BB.
e. Graph with numbers of migrated cells.
Data represent the mean of 6-8 values from 3 separate experiments.
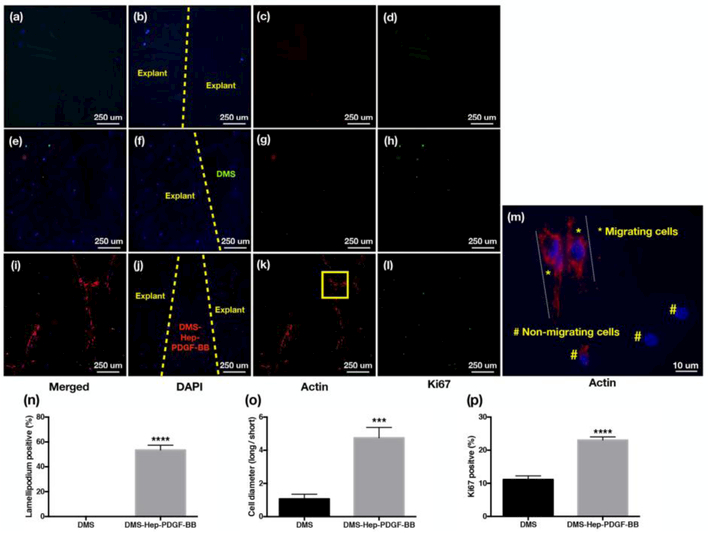
The injured meniscus explants without DMS (Fig. 4a-d) and with inserted DMS (no Hep or PDGF-BB) (Fig. 4e-h) showed weak β-actin, and Ki67 staining. The DMS-Hep-PDGF-BB inserted group showed more DAPI positive cells in the defect region and more cells were positive for β-actin and Ki67 (Fig. 4i-l). The higher resolution images of β-actin-stained DMS-Hep-PDGF-BB inserted explants showed significantly higher lamellipodium positive cells with elongated cell diameter indicative of migrating cells. Ki67 positive cells representing proliferating cells were also increased (Fig. 4m-p).
Figure 4.
Migratory and proliferating cells in injured meniscus explants.
DAPI, β–actin and Ki67 stained sections of explants cultured for 2 weeks (n=3-6 per group).
a-d. Injured meniscus cultured without DMS: (a) Merged; (b) DAPI; (c) β–actin; (d) Ki67.
e-h. Injured meniscus cultured with DMS: (e) Merged; (f) DAPI; (g) β–actin (h) Ki67.
i-l. Injured meniscus cultured with DMS-Hep-PDGF-BB: (i) Merged; (j) DAPI; (k) β–actin; (1) Ki67.
m. β–actin staining of injured meniscus cultured with DMS-Hep-PDGF-BB. The elongated actin subunits (lamellipodia) are strongly stained lamella and are indicative of cell migration. Non-migrating cells show negative actin staining or short actin structures. The area marked by a yellow square in panel k is shown at higher magnification in panel m.
n. Graph with % lamellipodium positive cells.
o. Graph with the ratio of cell diameter (long/short diameter).
p. Graph with Ki67 positive cells.
a-l: 20x; m: 60x
3.3. ECM formation in the injured meniscus explants
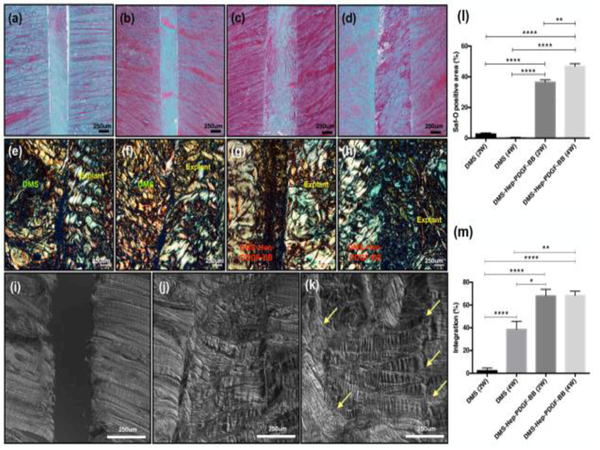
Safranin-O and picrosirius red staining showed tissue integration between DMS and injured explants (Fig. 5a-h). The Safranin-O positive area assessed by image analysis in the DMS-Hep-PDGF-BB group after 2 weeks (34±2 %) and 4 weeks-culture (47±2 %) was significantly higher than in the DMS group after 2 weeks (3±1 %) and 4 weeks-culture (0.3±0.3 %) (Fig. 5l). The interconnectivity area between the inserted DMS and injured bovine meniscus explant in DMS-Hep-PDGF-BB group after 2 weeks (68±6 %) and 4 weeks-culture (68±4 %) was significantly higher than in the DMS group after 2 weeks (2±2 %) and 4 weeks-culture (39±7 %) (Fig. 5m). In the differential interference contrast (DIC) imaging of DMS-Hep-PDGF-BB inserted meniscus, collagen fibers covered the space between DMS and injured meniscus explant (Fig. 5k). However, explants without DMS and explants with DMS (no heparin or PDGF) showed no ECM connecting with the meniscus (Fig. 5i-j).
Figure 5.
ECM formation in the injured meniscus explants.
a-b. Safranin-O staining: Injured meniscus cultured with DMS for 2 and 4 weeks.
c-d. Safranin-O staining: Injured meniscus cultured with DMS-Hep-PDGF-BB for 2 and 4 weeks.
e-f. Picrosirius red staining: Injured meniscus cultured with DMS for 2 and 4 weeks.
g-h. Picrosirius red staining: Injured meniscus cultured with DMS-Hep-PDGF-BB for 2 and 4 weeks.
i-k. Differential interference contrast (DIC) imaging: (i) Injured meniscus without DMS; (j) with DMS; (k) with DMS-Hep-PDGF-BB.
l-m. Safranin-O positive stained area (% of total area) and integration % between DMS and explant assessed by pircrosirius red staining and shown as % integrated interface of total interface area.
3.4. Mechanical properties of the injured meniscus explants after DMS insertion
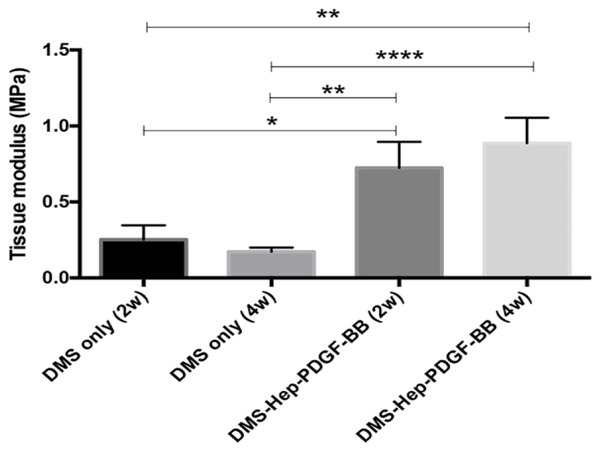
Tensile properties were compared between explants inserted with DMS or DMS-Hep-PDGF-BB after culture for 2 and 4 weeks. Explants inserted with DMS-Hep-PDGF-BB showed significantly higher tissue moduli (0.73 MPa after 2 weeks, 0.89 MPa after 4 weeks) than DMS inserted explants (0.25 MPa after 2 weeks, 0.17 MPa after 4 weeks) (Fig. 6).
Figure 6.
Mechanical properties of the injured meniscus explants cultured with DMS.
Injured explants were inserted with DMS or DMS-Hep-PDGF-BB and cultured for 2 and 4 weeks. Tensile properties were measured by pulling to failure. Data represent the mean +/− standard error (SE) of 7-10 values from 3 separate experiments.
4. Discussion
The goal of the present study was to develop a growth factor-conjugated scaffold that can be readily applied to recruit endogenous cells that promote repair of meniscus tears. The present scaffold is not intended to be used for partial or larger meniscus defects. We selected DMS as this is a biocompatible material and can be readily manufactured for clinical use. The decellularization process with chemical and proteolytic enzyme treatment decreased DNA content by 78% (1.13±0.03ng DNA/mg dry weight), similar to commercialized ECM scaffold products (1.71±0.01ng DNA/mg dry weight) [36]. This process is essential as DNA content is correlated with host immune reactions [36, 37].
Prior studies about growth factor-conjugated scaffolds including natural polymers such as collagen, gelatin, demineralized bone matrix, and synthetic polymers have shown the feasibility of growth factor immobilization for cell recruitment and tissue repair [38-43].
Studies about insertion of membranes into the experimental meniscus tears have also been reported. The insertion of a collagenase-releasing nanofibrous scaffold showed enhanced cell infiltration by loosening the dense meniscus explant [44]. However, there was no integration at the edge occupied by the scaffold. A multi-laminated collagenous biomaterial was conductive for cell repopulation with host meniscal elements [45].
Scaffolds for meniscus repair in clinical applications need not only to promote endogenous cell recruitment but also have mechanical properties to resist shear and compressive stresses in the knee joint. DMS has been used as a scaffold [46-50], having similar mechanical properties as human meniscus [51] but there is no study about growth factor immobilized DMS for endogenous cell recruitment in meniscus.
During decellularization the dense bovine meniscus was modified by proteolytic enzyme treatment to facilitate subsequent cell infiltration. The decreased tissue density enhanced cell infiltration and new tissue formation by the recruited cells at the junction between tissue and DMS.
We chose a two-step DMS conjugation, first with heparin and then with PDGF-BB. This resulted in retention of PDGF-BB at similar levels as used in clinical applications where PDGF-BB conjugated grafts (0.3mg/mL) have been used in dental and orthopedic surgeries [52-55]. Heparin has sites for covalent binding of PDGF [30] and heparin conjugated DMS provides prolonged PDGF release. Two mechanisms appear to be involved in PDGF release from heparin conjugated DMS. PDGF that was not bound or bound with low affinity to heparin may account for the initial release [56]. The sustained release of the PDGF is thought to be mediated by the enzymatic modification of the heparin-bound PDGF [57].
The PDGF-conjugated DMS was biologically active after insertion into meniscus explants as directly demonstrated by increased PRGFRβ expression on cells adjacent to the experimental tear. This was associated with cell migration to the PDGF-BB-conjugated DMS in the meniscus defect. Cell migration was not observed with DMS that was not coated with PDGF-BB. In addition, cells with β-actin rearrangement to lamellipodia characteristic of migratory cells were highest in the samples that were inserted with PDGF-BB-coated DMS. PDGF-BB also increased proliferation of cells at the defect area.
At the early points after PDGF-BB treatment, the expression of PDGFRβ and VEGFA were increased in avascular meniscus tissue explant and cells (Fig. S7b). The VEGF-mediated neovascularization is essential to the healing of injured tissues [58]. VEGF in cultured vascular meniscal cells was higher than in avascular meniscal cells. Also, VEGF was detected mainly around injured areas of the meniscus [21, 59]. VEGF expression induced by PDGF-BB treatment may modulate the meniscus healing process in the avascular zone.
Previous studies showed that PDGF/PDGFR signaling is involved on defining phenotype and regulating function of endothelial progenitor cells, or mesenchymal stem cells [26, 60]. PDGFβ receptor positive cells have been reported to include stem/progenitor populations [61] and the present results indicate that these cell populations in meniscus are recruited and/or activated by PDGF-BB. We did not characterize the phenotype of the migrated cells, but immature or meniscus progenitor cells exhibit migratory activity [62].
The cells that were recruited by PDGF-BB to the defect area produced new extracellular matrix and this increased interconnectivity between the PDGF-BB coated DMS and defect region with new ECM. PDGF is not only chemotactic but also enhances synthesis of fibrocartilage matrix components such as GAG and collagens [63, 64]. Moreover, there was increased proliferation of the endogenous cells that were recruited to the PDGF-BB coated DMS compared with DMS without PDGF-BB.
The enhanced interconnectivity between meniscus and DMS was associated with improved biomechanical property as indicated by increased tissue modulus. Interestingly, the tissue modulus of the DMS only inserted explant significantly increased after 4-week culture compared to 2-week culture. This may be due to release of endogenous bioactive molecules from the DMS [65].
5. Conclusions
This study shows that heparin-conjugated DMS showed strong immobilization of PDGF-BB, which was released slowly. PDGF-BB coated DMS promoted migration of endogenous meniscus cells to the defect area and into the scaffold. New matrix was formed that bridged the space between the native meniscus and the scaffold and this was associated with improved biomechanical properties. The PDGF-BB coated DMS is a promising approach for integrative healing of the meniscus tears.
Supplementary Material
Statement of Significance.
Meniscus tears are the most common injury of the knee joint. The most prevalent forms that occur in the inner third typically do not spontaneously heal and represent a major risk factor for the development of knee osteoarthritis. The goal of this project was to develop an approach that is readily applicable for clinical use.
We selected a natural and readily available decellularized meniscus scaffold and conjugated it with PDGF, which we had previously found to have strong chemotactic activity for chondrocytes and progenitor cells.
The present results show that insertion of the PDGF-conjugated scaffold in defects in avascular meniscus led to endogenous cell migration and proliferation into the defect zone with tissue integration between the scaffold and injured explants and improved tensile properties.
This PDGF-conjugated scaffold will be promising for a translational approach to healing of meniscus tears.
Acknowledgments
This work was supported by the National Institutes of Health, Grant AG007996. The authors thank Emily Lee, and Nicholas Glembotski for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Authors have no conflicts of interest to disclose.
References
- [1].Sanders TG, Medynski MA, Feller JF, Lawhorn KW, Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury, Radiographics 20 Spec No (2000) S135–51. [DOI] [PubMed] [Google Scholar]
- [2].Cavanaugh JT, Killian SE, Rehabilitation following meniscal repair, Curr Rev Musculoskelet Med 5(1) (2012) 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hagino T, Ochiai S, Watanabe Y, Senga S, Wako M, Ando T, Sato E, Haro H, Complications after arthroscopic knee surgery, Arch Orthop Trauma Surg 134(11) (2014) 1561–4. [DOI] [PubMed] [Google Scholar]
- [4].Tuman J, Haro MS, Foley S, Diduch D, All-inside meniscal repair devices and techniques, Expert Rev Med Devices 9(2) (2012) 147–57. [DOI] [PubMed] [Google Scholar]
- [5].Arnoczky SP, Warren RF, The microvasculature of the meniscus and its response to injury. An experimental study in the dog, Am J Sports Med 11(3) (1983) 131–41. [DOI] [PubMed] [Google Scholar]
- [6].McNulty AL, Guilak F, Integrative repair of the meniscus: lessons from in vitro studies, Biorheology 45(3-4) (2008) 487–500. [PMC free article] [PubMed] [Google Scholar]
- [7].Heckmann TP, Barber-Westin SD, Noyes FR, Meniscal repair and transplantation: indications, techniques, rehabilitation, and clinical outcome, J Orthop Sports Phys Ther 36(10) (2006) 795–814. [DOI] [PubMed] [Google Scholar]
- [8].Hasan J, Fisher J, Ingham E, Current strategies in meniscal regeneration, J Biomed Mater Res B Appl Biomater 102(3) (2014) 619–34. [DOI] [PubMed] [Google Scholar]
- [9].Mauck RL, Burdick JA, From repair to regeneration: biomaterials to reprogram the meniscus wound microenvironment, Ann Biomed Eng 43(3) (2015) 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scotti C, Hirschmann MT, Antinolfi P, Martin I, Peretti GM, Meniscus repair and regeneration: review on current methods and research potential, Eur Cell Mater 26 (2013) 150–70. [DOI] [PubMed] [Google Scholar]
- [11].Makris EA, Hadidi P, Athanasiou KA, The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration, Biomaterials 32(30) (2011) 7411–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, Tabuchi T, Koga H, Tsuji K, Sekiya I, Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs, Osteoarthritis Cartilage 23(6) (2015) 1007–17. [DOI] [PubMed] [Google Scholar]
- [13].Cucchiarini M, McNulty AL, Mauck RL, Setton LA, Guilak F, Madry H, Advances in combining gene therapy with cell and tissue engineering-based approaches to enhance healing of the meniscus, Osteoarthritis Cartilage 24(8) (2016) 1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esparza R, Gortazar AR, Forriol F, Cell study of the three areas of the meniscus: effect of growth factors in an experimental model in sheep, J Orthop Res 30(10) (2012) 1647–51. [DOI] [PubMed] [Google Scholar]
- [15].Lin H, Chen B, Sun W, Zhao W, Zhao Y, Dai J, The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds, Biomaterials 27(33) (2006) 5708–14. [DOI] [PubMed] [Google Scholar]
- [16].Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA, The effect of cytokines on the proliferation and migration of bovine meniscal cells, Am J Sports Med 27(5) (1999) 636–43. [DOI] [PubMed] [Google Scholar]
- [17].Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM, Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB), J Orthop Res 13(2) (1995) 201–7. [DOI] [PubMed] [Google Scholar]
- [18].Davies NH, Schmidt C, Bezuidenhout D, Zilla P, Sustaining neovascularization of a scaffold through staged release of vascular endothelial growth factor-A and platelet-derived growth factor-BB, Tissue Eng Part A 18(1-2) (2012) 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mishima Y, Lotz M, Chemotaxis of human articular chondrocytes and mesenchymal stem cells, J Orthop Res 26(10) (2008) 1407–12. [DOI] [PubMed] [Google Scholar]
- [20].Tumia NS, Johnstone AJ, Platelet derived growth factor-AB enhances knee meniscal cell activity in vitro, Knee 16(1) (2009) 73–6. [DOI] [PubMed] [Google Scholar]
- [21].Ruiz Iban MA, Comellas Melero N, Martinez-Botas J, Ortiz A, Diaz Heredia J, Growth factor expression after lesion creation in the avascular zone of the meniscus: a quantitative PCR study in rabbits, Arthroscopy 30(9) (2014) 1131–8. [DOI] [PubMed] [Google Scholar]
- [22].Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF, Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro, In Vitro Cell Dev Biol Anim 41(8-9) (2005) 305–10. [DOI] [PubMed] [Google Scholar]
- [23].Imler SM, Doshi AN, Levenston ME, Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis, Osteoarthritis Cartilage 12(9) (2004) 736–44. [DOI] [PubMed] [Google Scholar]
- [24].Lietman SA, Hobbs W, Inoue N, Reddi AH, Effects of selected growth factors on porcine meniscus in chemically defined medium, Orthopedics 26(8) (2003) 799–803. [DOI] [PubMed] [Google Scholar]
- [25].Marx M, Perlmutter RA, Madri JA, Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis, J Clin Invest 93(1) (1994) 131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J, Qin Z, Wang Q, Wang K, Lu W, Liu J, Huang L, Over-expression of PDGFR-beta promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway, PLoS One 7(2) (2012) e30503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wissink MJ, Beernink R, Pieper JS, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J, Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation, Biomaterials 22(2) (2001) 151–63. [DOI] [PubMed] [Google Scholar]
- [28].You I, Kang SM, Byun Y, Lee H, Enhancement of blood compatibility of poly(urethane) substrates by mussel-inspired adhesive heparin coating, Bioconjug Chem 22(7) (2011) 1264–9. [DOI] [PubMed] [Google Scholar]
- [29].Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA, Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix, Proc Natl Acad Sci U S A 110(12) (2013) 4563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee J, Yoo JJ, Atala A, Lee SJ, The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration, Biomaterials 33(28) (2012) 6709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, Lindblom P, Norlin J, Betsholtz C, Heuchel R, Welsh M, Claesson-Welsh L, Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation, Blood 108(6) (2006) 1877–86. [DOI] [PubMed] [Google Scholar]
- [32].Ishak MF, See GB, Hui CK, Abdullah A, Saim L, Saim A, Idrus R, The formation of human auricular cartilage from microtic tissue: An in vivo study, Int J Pediatr Otorhinolaryngol 79(10) (2015) 1634–9. [DOI] [PubMed] [Google Scholar]
- [33].Pabbruwe MB, Esfandiari E, Kafienah W, Tarlton JF, Hollander AP, Induction of cartilage integration by a chondrocyte/collagen-scaffold implant, Biomaterials 30(26) (2009) 4277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK, WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase, J Biol Chem 280(23) (2005) 21748–55. [DOI] [PubMed] [Google Scholar]
- [35].Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P, Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force, J Cell Biol 201(7) (2013) 1069–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gilbert TW, Freund JM, Badylak SF, Quantification of DNA in biologic scaffold materials, J Surg Res 152(1) (2009) 135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Crapo PM, Gilbert TW, Badylak SF, An overview of tissue and whole organ decellularization processes, Biomaterials 32(12) (2011) 3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Freymann U, Endres M, Goldmann U, Sittinger M, Kaps C, Toward scaffold-based meniscus repair: effect of human serum, hyaluronic acid and TGF-ss3 on cell recruitment and re-differentiation, Osteoarthritis Cartilage 21(5) (2013) 773–81. [DOI] [PubMed] [Google Scholar]
- [39].Schmidt MB, Chen EH, Lynch SE, A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair, Osteoarthritis Cartilage 14(5) (2006) 403–12. [DOI] [PubMed] [Google Scholar]
- [40].Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ, Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep, Sci Transl Med 6(266) (2014) 266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liang F, Yen SL, Imahiyerobo T, Sanborn L, Yen L, Yen D, Nazarian S, Jedrzejewski B, Urata M, Hammoudeh J, Three-Dimensional Cone Beam Computed Tomography Volumetric Outcomes of rhBMP-2/Demineralized Bone Matrix versus Iliac Crest Bone Graft for Alveolar Cleft Reconstruction, Plast Reconstr Surg 140(4) (2017) 767–774. [DOI] [PubMed] [Google Scholar]
- [42].Lee KW, Lee JS, Jang JW, Shim YB, Lee KI, Tendon-bone interface healing using an injectable rhBMP-2-containing collagen gel in a rabbit extra-articular bone tunnel model, J Tissue Eng Regen Med 11(5) (2017) 1435–1441. [DOI] [PubMed] [Google Scholar]
- [43].Lee KW, Lee JS, Kim YS, Shim YB, Jang JW, Lee KI, Effective healing of chronic rotator cuff injury using recombinant bone morphogenetic protein-2 coated dermal patch in vivo, J Biomed Mater Res B Appl Biomater 105(7) (2017) 1840–1846. [DOI] [PubMed] [Google Scholar]
- [44].Qu F, Lin JM, Esterhai JL, Fisher MB, Mauck RL, Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair, Acta Biomater 9(5) (2013) 6393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gastel JA, Muirhead WR, Lifrak JT, Fadale PD, Hulstyn MJ, Labrador DP, Meniscal tissue regeneration using a collagenous biomaterial derived from porcine small intestine submucosa, Arthroscopy 17(2) (2001) 151–9. [DOI] [PubMed] [Google Scholar]
- [46].Lakes EH, Matuska AM, McFetridge PS, Allen KD, Mechanical Integrity of a Decellularized and Laser Drilled Medial Meniscus, J Biomech Eng 138(3) (2016) 4032381. [DOI] [PubMed] [Google Scholar]
- [47].Azhim A, Ono T, Fukui Y, Morimoto Y, Furukawa K, Ushida T, Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering, Conf Proc IEEE Eng Med Biol Soc 2013 (2013) 6953–6. [DOI] [PubMed] [Google Scholar]
- [48].Stapleton TW, Ingram J, Fisher J, Ingham E, Investigation of the regenerative capacity of an acellular porcine medial meniscus for tissue engineering applications, Tissue Eng Part A 17(1-2) (2011) 231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stabile KJ, Odom D, Smith TL, Northam C, Whitlock PW, Smith BP, Van Dyke ME, Ferguson CM, An acellular, allograft-derived meniscus scaffold in an ovine model, Arthroscopy 26(7) (2010) 936–48. [DOI] [PubMed] [Google Scholar]
- [50].Stapleton TW, Ingram J, Katta J, Knight R, Korossis S, Fisher J, Ingham E, Development and characterization of an acellular porcine medial meniscus for use in tissue engineering, Tissue Eng Part A 14(4) (2008) 505–18. [DOI] [PubMed] [Google Scholar]
- [51].Abdelgaied A, Stanley M, Galfe M, Berry H, Ingham E, Fisher J, Comparison of the biomechanical tensile and compressive properties of decellularised and natural porcine meniscus, J Biomech 48(8) (2015) 1389–96. [DOI] [PubMed] [Google Scholar]
- [52].Deshpande A, Koudale SB, Bhongade ML, A comparative evaluation of rhPDGF-BB + beta-TCP and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in humans, Int J Periodontics Restorative Dent 34(2) (2014) 241–9. [DOI] [PubMed] [Google Scholar]
- [53].Nevins M, Kao RT, McGuire MK, McClain PK, Hinrichs JE, McAllister BS, Reddy MS, Nevins ML, Genco RJ, Lynch SE, Giannobile WV, Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial, J Periodontol 84(4) (2013) 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Margolis DJ, Morris LM, Papadopoulos M, Weinberg L, Filip JC, Lang SA, Vaikunth SS, Crombleholme TM, Phase I study of H5.020CMV.PDGF-beta to treat venous leg ulcer disease, Mol Ther 17(10) (2009) 1822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Solchaga LA, Hee CK, Roach S, Snel LB, Safety of recombinant human platelet-derived growth factor-BB in Augment((R)) Bone Graft, J Tissue Eng 3(1) (2012) 2041731412442668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].King WJ, Krebsbach PH, Growth factor delivery: how surface interactions modulate release in vitro and in vivo, Adv Drug Deliv Rev 64(12) (2012) 1239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lee K, Silva EA, Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, J R Soc Interface 8(55) (2011) 153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lu Z, Furumatsu T, Fujii M, Maehara A, Ozaki T, The distribution of vascular endothelial growth factor in human meniscus and a meniscal injury model, J Orthop Sci 22(4) (2017) 715–721. [DOI] [PubMed] [Google Scholar]
- [59].Becker R, Pufe T, Kulow S, Giessmann N, Neumann W, Mentlein R, Petersen W, Expression of vascular endothelial growth factor during healing of the meniscus in a rabbit model, J Bone Joint Surg Br 86(7) (2004) 1082–7. [DOI] [PubMed] [Google Scholar]
- [60].Gharibi B, Ghuman MS, Hughes FJ, Akt- and Erk-mediated regulation of proliferation and differentiation during PDGFRbeta-induced MSC self-renewal, J Cell Mol Med 16(11) (2012) 2789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hori Y, Fukumoto M, Kuroda Y, Enrichment of putative pancreatic progenitor cells from mice by sorting for prominin1 (CD133) and platelet-derived growth factor receptor beta, Stem Cells 26(11) (2008) 2912–20. [DOI] [PubMed] [Google Scholar]
- [62].Muhammad H, Schminke B, Bode C, Roth M, Albert J, von der Heyde S, Rosen V, Miosge N, Human migratory meniscus progenitor cells are controlled via the TGF-beta pathway, Stem Cell Reports 3(5) (2014) 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Howard D, Shepherd JH, Kew SJ, Hernandez P, Ghose S, Wardale JA, Rushton N, Release of growth factors from a reinforced collagen GAG matrix supplemented with platelet rich plasma: Influence on cultured human meniscal cells, J Orthop Res 32(2) (2014) 273–8. [DOI] [PubMed] [Google Scholar]
- [64].Hoben GM, Willard VP, Athanasiou KA, Fibrochondrogenesis of hESCs: growth factor combinations and cocultures, Stem Cells Dev 18(2) (2009) 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rothrauff BB, Shimomura K, Gottardi R, Alexander PG, Tuan RS, Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix, Acta Biomater 49 (2017) 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.