Abstract
It is well known that congenitally blind adults have enhanced auditory processing for some tasks. For instance, they show supra-normal capacity to perceive accelerated speech. However, only a few studies have investigated basic auditory processing in this population. In this study, we investigated if pitch processing enhancement in the blind is a domain-general or domain-specific phenomenon, and if pitch processing shares the same properties as in the sighted regarding how scores from different domains are associated. Fifteen congenitally blind adults and fifteen sighted adults participated in the study. We first created a set of personalized native and non-native vowel stimuli using an identification and rating task. Then, an adaptive discrimination paradigm was used to determine the frequency difference limen for pitch direction identification of speech (native and non-native vowels) and non-speech stimuli (musical instruments and pure tones). The results show that the blind participants had better discrimination thresholds than controls for native vowels, music stimuli, and pure tones. Whereas within the blind group, the discrimination thresholds were smaller for musical stimuli than speech stimuli, replicating previous findings in sighted participants, we did not find this effect in the current control group. Further analyses indicate that older sighted participants show higher thresholds for instrument sounds compared to speech sounds. This effect of age was not found in the blind group. Moreover, the scores across domains were not associated to the same extent in the blind as they were in the sighted. In conclusion, in addition to providing further evidence of compensatory auditory mechanisms in early blind individuals, our results point to differences in how auditory processing is modulated in this population.
Keywords: blindness, pitch perception, adaptive staircase, native and foreign vowels, speech, music
1. Introduction
Congenital blindness is one of the models used to study long-term neuroplasticity. Indeed, signs of cerebral reorganization are found in the function and the structure of the brains of individuals who never saw or lost sight at an early age. For instance, the brain areas that are traditionally devoted to visual and multisensory processing are taken over by tactile processing (Burton, Sinclair, & McLaren, 2004; Weaver & Stevens, 2007), auditory processing (Kujala, et al., 1995; Stevens & Weaver, 2009; Weaver & Stevens, 2007), or higher level functions such as language (Burton, Diamond, & McDermott, 2003; Röder, Stock, Bien, Neville, & Rösler, 2002) and memory (Amedi, Raz, Pianka, Malach, & Zohary, 2003; Bonino, et al., 2008). Changes are apparent in the functional connectivity of these brain areas (Bedny, Pascual-Leone, Dodell-Feder, Fedorenko, & Saxe, 2011; Collignon, et al., 2011; Sani, et al., 2010; Weeks, et al., 2000). The neuroplastic reorganization of a congenitally blind adult’s brain also manifests itself in changes in cortical thickness (Anurova, Renier, De Volder, Carlson, & Rauschecker, 2014; Jiang, et al., 2009; Park, et al., 2009; Voss & Zatorre, 2012), volume (Lepore, et al., 2010; Pan, et al., 2007; Park, et al., 2009; Ptito, Schneider, Paulson, & Kupers, 2008), and structural connectivity (Park, et al., 2007; Shimony, et al., 2006; Shu, Li, Li, Yu, & Jiang, 2009; Shu, Liu, et al., 2009; Yu, et al., 2007).
In addition, behavioural differences are observed in blind adults when they process auditory information. Studies have shown that some blind participants have better capacities to localize sounds and to navigate in space using sound information (Teng, Puri, & Whitney, 2012; Voss, Lepore, Gougoux, & Zatorre, 2011). In the domain of speech, where vision usually plays an important role - during speech acquisition (Kuhl, Williams, Lacerda, Stevens, & Lindblom, 1992) and in face-to-face communication (Hubbard, Wilson, Callan, & Dapretto, 2009; McNeill, 1992; Sumby & Pollack, 1954) - compensatory mechanisms in the auditory modality are also evident. Indeed, visually deprived adults are better than sighted adults in the acoustic discrimination of syllables (Hugdahl, et al., 2004) and vowels (Ménard, Dupont, Baum, & Aubin, 2009), and in the perception of words in noise (Muchnik, Efrati, Nemeth, Malin, & Hildesheimer, 1991; Niemeyer & Starlinger, 1981); they also respond faster than controls in a lexical decision task (Röder, Demuth, Streb, & Rösler, 2003). Blind adults also show stunning abilities to understand artificially accelerated speech up to a rate of 18 syllables/sec (Dietrich, Hertrich, & Ackermann, 2011, 2013; Hertrich, Dietrich, & Ackermann, 2013a, 2013b; Hertrich, Dietrich, Moos, Trouvain, & Ackermann, 2009; Moos & Trouvain, 2007; Trouvain, 2007) compared to rates of 8–10 syllables/sec in sighted controls (Trouvain, 2007). Neuroimaging studies reveal that the cerebral networks that are recruited for this kind of task differ from the recruited areas in controls with, for instance, cross-modal recruitment of visual and multisensory areas in the blind (Arnaud, Sato, Menard, & Gracco, 2013; Burton, et al., 2003; Burton, Snyder, Diamond, & Raichle, 2002; Dietrich, et al., 2013; Hertrich, et al., 2009).
Regarding pitch processing, neuroimaging studies have drawn links between, on one hand, performance on pitch processing tasks, and on the other hand, changes in the cerebral structure, e.g. cortical thickness, grey matter concentration and magnetization transfer ratio in occipital areas in blind participants (Voss, Pike, & Zatorre, 2014; Voss & Zatorre, 2012). However, a fMRI study of the processing of pitch vs. spatial properties of sounds failed at identifying cross-modal activity of occipital areas specific to pitch processing, as opposed to the spatial processing of the same sounds, (Collignon, et al., 2011) and in a MEG study on speech perception group differences between blind and sighted in pitch periodicity-correlated activity was found in the primary auditory areas of the blind and not in occipital areas (Hertrich, et al., 2013b).
The rationale behind the current study was to test if enhanced pitch processing abilities in the blind are specific to pure tones or if it extends to complex sounds such as speech sounds. Indeed, previous works have found better processing of speech for higher level tasks such as better understanding of accelerated sentences, better identification of syllables or vowel contrasts but we ignore if enhanced perception of physical properties of the sounds, such as pitch, is enhanced for speech sounds in the blind. In addition to testing enhanced pitch processing in speech sounds in the blind, we question here the impact of experience or familiarity with the stimuli (native vs. non-native) and domain (speech vs. music). To our knowledge, the question of domain specificity of the auditory advantage of the blind has not been tested before.
In summary, even though there is evidence of auditory compensation in speech processing in the blind, the full extent of these enhanced abilities is not well known. Studies have shown better pitch discrimination thresholds (Gougoux, et al., 2004; Rokem & Ahissar, 2009; Voss & Zatorre, 2012; Wan, Wood, Reutens, & Wilson, 2010) and better temporal consolidation (Stevens & Weaver, 2005) in the blind compared to controls for pure tones. There are, however, few studies that have investigated basic auditory acuity of the blind for sounds other than pure tones, so it is still unclear if this is a domain-general or domain-specific ability. Enhanced processing of basic acoustic cues in speech could contribute to the enhanced ability to process speech observed in blind adults (e.g. ultra fast speech comprehension). A challenge when comparing the processing of sounds from different categories (e.g. music, speech etc.) is the choice of comparable stimuli. In the current study, we chose to compare processing of music units (isolated instrument notes) and speech units (e.g. isolated vowels).
The objective of this study was to further define the extent of enhanced auditory processing in congenitally blind individuals. Specifically, we focused on the auditory acuity of congenitally blind adults for pitch discrimination of complex sounds coming from different acoustic domains (speech and music). We included native and non-native speech sounds to assess the impact of stimulus familiarity. The first experiment focused on the selection of a personalised set of native and non-native vowel stimuli for each participant. In the second experiment, participants underwent an adaptive pitch discrimination task on the individually selected stimuli -native and non-native vowels - as well as on non-speech sounds - instrument sounds and pure tones. The experimental design allowed for the comparison of pitch discrimination thresholds between groups (blind and control).
2. Experiment 1 – Vowel identification and rating
2.1 Methods
2.1.1 Participants
Fifteen congenitally (onset of blindness during or before birth, n=12) and early (onset of blindness before 12 months, n=3) blind adults and fifteen sighted adults (control group) ranging from 24 to 64 years of age participated in this study (see Table 4 for demographic information on participants including cause and onset of blindness for blind participants). All participants were native speakers of Canadian French and self-identified as monolinguals. The two groups were matched on: age, gender, number of years of education, and number of years of formal musical training. The experiment was performed in accordance with the ethical standards in the 2004 Declaration of Helsinki and requirements of the Faculty of Medicine, McGill University. The consent form was read to the blind participants. All participants provided written consent.
Table 4.
Individual and group demographic information and results for the pitch direction identification task. Music background is defined as the number of years of formal musical training.
| Group | Subject | Age | Gender | Musical Background | Log(FDL) | Characteristics of blindness | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| French vowels | Non-native Vowels | Instruments | Pure Tone | Cause | Onset | Residual vision | |||||
| Blind | B1 | 64 | M | 0 | 0.43 | 0.29 | 0.06 | 0.52 | Cong. cataract | 0 | - |
| Blind | B2 | 47 | M | 0 | 0.61 | 0.57 | 0.66 | 0.55 | ROP: compl. blindness RE, retinal detachment LE at 7yo | 0 | Partial vision in LE until 7yo- |
| Blind | B3 | 30 | M | 0 | 1.29 | 1.14 | 0.64 | 0.42 | Leber’s cong. Amaurosis | 0 | Res. light perception |
| Blind | B4 | 60 | F | 0 | 0.42 | 0.44 | 0.23 | 0.32 | Cong. blindness | 0 | - |
| Blind | B5 | 61 | F | 0 | 0.94 | 0.79 | 0.37 | 0.54 | Retinal detachment and obstruction | 0 | - |
| Blind | B6 | 26 | M | 4.5 | 0.51 | 1.20 | 0.34 | 0.30 | Cong. microphtalmi a with malformed retina | 0 | Res. light perception |
| Blind | B7 | 46 | M | 0 | 0.22 | 0.31 | −0.29 | 0.30 | ROP | 0 | - |
| Blind | B8 | 63 | F | 8 | 0.27 | 0.40 | 0.15 | 0.33 | ROP | 0 | - |
| Blind | B9 | 41 | M | 2 | 0.64 | 0.46 | 0.47 | 0.44 | Cong. microphtalmy LE, cornea accident at 12m RE | 12m | Res. light perception |
| Blind | B10 | 47 | M | 0 | 0.58 | 0.54 | 0.44 | 0.24 | Leber’s cong. Amaurosis | 0 | Res. light perception |
| Blind | B11 | 28 | M | 2 | 0.24 | 0.28 | 0.22 | 0.21 | Retinoblastoma | 9m | - |
| Blind | B12 | 34 | F | 3 | 0.48 | 0.52 | 0.24 | 0.45 | ROP | 0 | - |
| Blind | B13 | 24 | M | 0 | 0.35 | 0.34 | 0.03 | 0.49 | Retinal detachment (genetic protein C deficiency) | 0 | - |
| Blind | B14 | 38 | F | 0 | 0.72 | 0.64 | 0.33 | 0.58 | Bilateral Retinoblastome | 10m | - |
| Blind | B15 | 53 | M | 0 | 0.79 | 0.47 | 0.91 | 0.41 | Glaucome and cong. cataract | 0 | Res. light and color perception until 15yo |
| Mean | 44 | 1.3 | 0.57 | 0.56 | 0.32 | 0.41 | |||||
| SD | 13 | - | 2.2 | 0.28 | 0.27 | 0.28 | 0.11 | ||||
| Sighted | S1 | 30 | M | 2 | 0.92 | 1.00 | 0.85 | 0.68 | |||
| Sighted | S2 | 62 | F | 0 | 0.66 | 0.65 | 1.15 | 0.58 | |||
| Sighted | S3 | 55 | M | 0 | 0.78 | 0.73 | 1.27 | 0.74 | |||
| Sighted | S4 | 47 | F | 2 | 1.33 | 1.11 | 1.38 | 1.04 | |||
| Sighted | S5 | 62 | F | 0 | 1.18 | 1.01 | 1.41 | 1.42 | |||
| Sighted | S6 | 50 | F | 0 | 1.31 | 1.30 | 1.25 | 0.94 | |||
| Sighted | S7 | 33 | M | 0 | 0.54 | 0.49 | 0.25 | 0.18 | |||
| Sighted | S8 | 30 | M | 2.5 | 0.37 | 0.24 | −0.20 | 0.16 | |||
| Sighted | S9 | 24 | M | 0 | 0.97 | 0.81 | 0.66 | 0.65 | |||
| Sighted | S10 | 24 | M | 0 | 1.22 | 1.25 | 1.10 | 1.05 | |||
| Sighted | S11 | 27 | M | 0 | 1.35 | 0.97 | 1.10 | 0.86 | |||
| Sighted | S12 | 30 | M | 6 | 0.05 | 0.04 | −0.33 | 0.29 | |||
| Sighted | S13 | 54 | F | 0.5 | 0.57 | 0.64 | 0.63 | 0.49 | |||
| Sighted | S14 | 65 | F | 0 | 0.95 | 0.77 | 0.86 | 0.85 | |||
| Sighted | S15 | 52 | M | 0 | 0.50 | 0.61 | 0.91 | 0.98 | |||
| Mean | 43 | 0.9 | 0.85 | 0.78 | 0.82 | 0.73 | |||||
| SD | 15 | 1.6 | 0.38 | 0.34 | 0.52 | 0.34 | |||||
SD = standard deviation, M = male, F = female, m = month, yo = years old, RE = right eye, LE = left eye, ROP = retinopathy of prematurity, cong. = congenital, res. = residual.
2.1.2 Experimental design
The objective of the study was to determine, for congenitally blind participants and controls, the frequency difference limen (FDL) of pitch-direction identification for native vowels, non-native vowels, instrument sounds and pure tones. The experiment was divided into two parts. The objective of the first part was to create a personalized corpus of native and non-native vowels for each participant. Participants had to identify if the vowels they heard were ‘French’ or ‘non-French’ and rate them based on their quality (see details below). Then on a second visit, participants performed the pitch-direction identification task on a personalized set of two native vowels, two non-native vowels and three non-speech sounds (pure tone, cello, piano).
Corpus creation
Stimuli
During the first visit, participants listened to a set of 108 vowels. Nine variants of each of the 6 native (French) vowels /i/, /y/, /u/, /e/, /ə/, /a/, and 9 variants of each of the 6 non-native vowels /ɨ/ /ʉ/ /ɯ/ /ʊ/ /ɤ/ and /ʌ/ (see Figure 1) were selected for this part and presented to each participant. The vowels were synthesized using the Variable Linear Articulatory Model (VLAM) (Boë & Maeda, 1998) which is based on Maeda’s model (Maeda, 1979). This program allows a precise manipulation of all acoustic features of vowel sounds, namely: duration, formant frequency bandwidth, and fundamental frequency (f0) for different ages. Here we chose to use synthesized vowels which would be produced by a 21 y.o. male, with a f0 of 112 Hz. The formant values of the ‘reference’ prototypes were chosen according to the values of reference provided in the VLAM model for each vowel category (see Table 1).
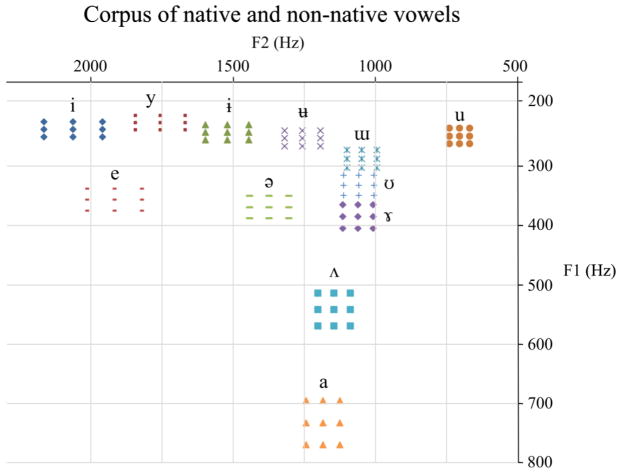
Figure 1.
Native and non-native vowels used in identification and rating task. Nine tokens of each of 6 native (i y u e ə a) and 6 non-native (ɨ ʉ ɯ ʊ ɤ ʌ) vowels were presented to participants in an identification and rating task. For each vowel category, eight variants were then synthesized around the reference prototype by modifying the values of the first two formants in 5% steps.
Table 1.
Formant Fi and bandwidth Bi values, in hertz, of ‘reference’ stimuli /a/, /e/, /ə/, /ɤ/, /i/, /ɨ/, /ɯ/, /u/, /ʊ/, /ʉ/, /ʌ/, and /y/ synthesized for the perceptual experiment. F0=112Hz and duration is 600ms for all stimuli. Prototypical values are based on the monophthong oral vowels of the world’s languages, reported in UPSID (UCLA Phonological Segments Inventory Database, Maddieson, 1986).
| Vowel | F1 | F2 | F3 | F4 | F5 | B1 | B2 | B3 | B4 | B5 |
|---|---|---|---|---|---|---|---|---|---|---|
| /i/ | 247 | 2062 | 3372 | 3896 | 4528 | 78 | 13 | 61 | 154 | 340 |
| /y/ | 236 | 1756 | 2122 | 3410 | 4159 | 88 | 40 | 19 | 19 | 30 |
| /ɨ/ | 252 | 1521 | 2139 | 3191 | 3898 | 103 | 24 | 20 | 20 | 28 |
| /ʉ/ | 262 | 1257 | 1915 | 2906 | 3806 | 90 | 30 | 16 | 17 | 31 |
| /ɯ/ | 296 | 1048 | 2119 | 3469 | 3984 | 80 | 18 | 16 | 19 | 19 |
| /u/ | 258 | 705 | 2002 | 3175 | 3647 | 97 | 31 | 15 | 17 | 26 |
| /ʊ/ | 340 | 1059 | 2069 | 3311 | 3932 | 67 | 18 | 16 | 20 | 21 |
| /e/ | 364 | 1922 | 2509 | 3548 | 4154 | 48 | 40 | 148 | 67 | 145 |
| / / | 376 | 1374 | 2012 | 3036 | 3866 | 57 | 26 | 21 | 21 | 40 |
| /ɤ/ | 392 | 1062 | 2014 | 3192 | 3908 | 56 | 19 | 17 | 21 | 24 |
| /ʌ/ | 546 | 1146 | 1998 | 3080 | 3627 | 39 | 55 | 31 | 31 | 120 |
| /a/ | 734 | 1185 | 2241 | 3716 | 4169 | 38 | 45 | 55 | 137 | 118 |
For each vowel category, eight variants were then synthesized around the ‘reference’ prototype by modifying the values of the first two formants by 5% steps. The only acoustic differences between the 9 variants of one vowel category were the values of the first two formants F1 and F2 (see Figure 1).
Procedure
Participants listened to the 108 randomly presented vowels using Presentation® software (Version 18.1, Neurobehavioral Systems, Inc., Berkeley, CA, www.neurobs.com) through headphones. Control participants wore blindfolds to avoid visual distraction.
Participants were asked to identify whether the presented vowel was French or ‘not French’, and in the case of a French vowel to rate the vowel quality on a scale from 1 to 7. The duration of the task was about 10 minutes.
2.1.3 Analyses
The participants’ task was to identify the vowels by giving a verbal answer to the investigator. For the analysis of the identification results, we classified the answers into 8 categories: /i/, /y/, /u/, /e/, /ə/, /a/, /ε/, /o/. Indeed, to simplify the task for the participant and keep it short we did not ask them to distinguish between the French categories /ø/, /ə/, or /œ/, so all these answers were grouped together during the analysis of the identification results. Likewise, the answers /a/ and /ɑ/, and /o/ or /ɔ/ were grouped together.
2.2 Results
2.2.1 Vowel identification and quality rating
We obtained identification and quality ratings of the 108 vowels for each participant. Overall, the variants of the native vowels (/i/, /y/, /u/, /e/, /ə/ and /a/) were correctly identified and received high ratings (see Table 2). However, the variant of the native vowels that received the highest rating varied among the participants. For each of the native variants, we calculated the percentage of participants who rated it as one of the best variants of the category. On average, the percentage of agreement was 49.7% ± 14.2 [min=20.0% max =83.3%].
Table 2.
Percentage of identification of native vowels and average rating for correctly identified vowels. Averages are calculated over the 9 variants of each vowel category for all participants. Note that /ø/, /ə/, and /œ/ answers were grouped together as well as /o/ and /ɔ/ Rating of the quality of pronunciation of correctly identified native vowels. The rating scale was between 1 (bad pronunciation) and 7 (good pronunciation).
| % of identification as
|
Average rating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| /i/ | /y/ | /u/ | /e/ | /ə/, /ø/, or /œ/ | /a/ | /ε/ | /o/ or /ɔ/ | Non-native | ||
| /i/ | 97.8 | 0.4 | 0 | 0.4 | 0 | 0.7 | 0 | 0 | 0.4 | 6.1 |
| /y/ | 0.4 | 98.5 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 | 6.1 |
| /u/ | 0 | 0 | 95.6 | 0 | 0 | 0 | 0 | 0 | 4.4 | 5.9 |
| /e/ | 0 | 0 | 0 | 96.3 | 0 | 0 | 3.3 | 0 | 0.4 | 5.8 |
| /ə/ | 0.4 | 0 | 0 | 0.4 | 95.6 | 0 | 0.4 | 0 | 3.3 | 4.8 |
| /a/ | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 5.9 |
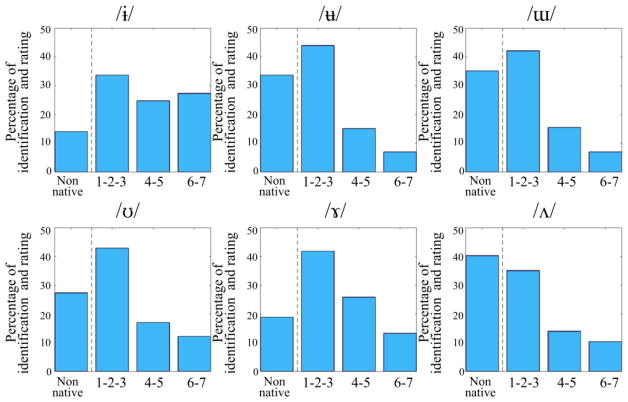
The variants of the non-native vowels (/ɨ/, /ʉ/, /ɯ/, /ʊ/, /ɤ/, and /ʌ/) were either correctly identified as non-native vowels or as /ə/ (see Table 3) except for /ɨ/, which was most often identified as /y/. When non-native vowels were identified as native vowels, they received a low rating (see Figure 2).
Table 3.
Percentage of identification of non-native vowels. Averages are calculated over the 9 variants of each vowel category for all participants. Note that /ø/, /ə/, and /œ/ answers were grouped together as well as /o/ and /ɔ/
| % of identification as: | Non native | /i/ | /y/ | /u/ | /e/ | /ə/ (or /ø/, or /œ/ | /a/ | /ε/ | /o/ or /ɔ/ |
|---|---|---|---|---|---|---|---|---|---|
| /ɨ/ | 14.1 | 0 | 74.1 | 0 | 0 | 11.9 | 0 | 0 | 0 |
| /ʉ/ | 33.7 | 0 | 23.3 | 0.7 | 0 | 42.2 | 0 | 0 | 0 |
| /ɯ/ | 35.2 | 0 | 1.1 | 7.8 | 0 | 54.8 | 0 | 0 | 1.1 |
| /ʊ/ | 27.4 | 0 | 0.4 | 0.7 | 0 | 65.9 | 0 | 0 | 5.2 |
| /ɤ/ | 18.9 | 0 | 0 | 0 | 0 | 72.6 | 2.6 | 0 | 5.9 |
| /ʌ/ | 40.4 | 0 | 0 | 0 | 0 | 40 | 18.9 | 0.4 | 0.4 |
Figure 2.
Identification of non-native stimuli. Non-native vowels were either identified as non-native vowels or identified as native vowels and were given low ratings, except for /ɨ/, which was also recognized as a good /y/.
The proportion of non-native answers varied across individuals. On average, participants identified 16 ± 13 [min=0, max=41] stimuli as non-native. It should be noted, however, that participants who never or rarely identified vowels as non-native instead identified them as variants of the French vowel /ə/ with a low rating.
2.2.2 Selection of native and non-native stimuli
The objective of the identification and rating task was to select personalized native and non-native variants for each participant.
The French vowels that were best identified and received the highest ratings were /i/, /a/, and /y/. /y/ was used for the practice block of the pitch processing task and /i/ and /a/ were chosen for the test blocks.
The vowels /ɯ/ and /ɤ/ were selected as the non-native categories of the pitch task because questionnaires revealed that many participants also had English as a second language, and contrary to /ʊ/ and /ʌ/ they are not produced in English. The vowel /ʉ/ wasn’t chosen because despite being more often identified as non-native than /ɤ/ it was also often identified as two different French categories /y/ and /ə/.
For each participant, the best variant of each of the native vowels /i/, and /a/, was defined as one of the vowels that was correctly identified and received the highest rating. For all participants, at least one variant of each of these two vowel categories received a rating of 5, 6 or 7 except for one participant for whom the highest rating for the vowel /a/ was 4 and one participant whose highest rating for the vowel /a/ was 2.
For each participant, the best variant of each of the non-native stimuli, /ɯ/ and /ɤ/, was one of the non-native vowels identified as ‘non-French’ or one among those that received the lowest rating. For all participants, at least one variant of each of these vowels was identified as non-native (n=41) or received a rating of 1 (n=13), 2 (n=2), or 3 (n=3), except for one participant whose lowest rating of the variants of the vowel /ɤ/ was 4.
3. Experiment 2 - Pitch-direction identification task
3.1 Methods
3.1.1 Stimuli
For the pitch-direction identification task, stimuli were chosen according to the results of the identification and rating task. For each participant, we selected a set comprising the best exemplar of /i/ and /a/ and the best exemplar (i.e. ‘non-native’ labeling or lowest rating) of non-native vowels /ɯ/ and /ɤ/. As a result, each participant had his or her own set of native and non-native vowels.
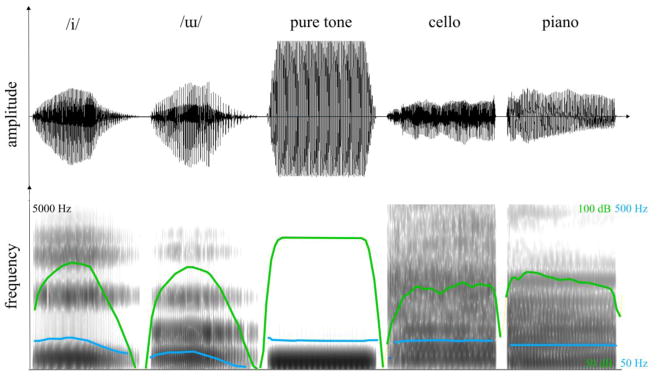
To create the ‘non-speech’ stimuli, one pure tone was synthesised (f0= 110Hz, duration = 600ms, ramps of 10 ms). The instrument notes (cello and piano) were synthesized using Sibelius 7.5 (Avid) and the sound database Sibelius 7.0. The mean-energy intensity of all stimuli was adjusted so that the loudness, evaluated using the Loudness Toolbox (Genesis S.A based on Zwicker and Fastl (1999)), was between 21 and 22 sones. Figure 3 presents ome spectrogram examples.
Figure 3.
Signal (top) and spectrogram (bottom) representations of examples of stimuli used in the FDL task. Pitch and intensity contours are shown in blue and green respectively. Note that the pure tone appears to be clipped but this is an artifact due to the limited time resolution of the display window.
3.1.2 Adaptive procedure
Frequency difference limens (FDLs) for pitch-direction identification were measured for each condition, using a two-interval, forced-choice procedure with a three-down, one-up tracking algorithm, estimating the 83.15 percent-correct point (García-Pérez, 1998). At each trial, two successive stimuli were presented. The only differences between both stimuli was the value of the fundamental frequency and the intensity (see below). The listeners were instructed to focus on the stimuli pitch and their task was to indicate if the pitch of the second stimulus was going up or down compared to the first stimulus. For each trial, a reference fundamental frequency was randomly chosen in the interval 100–120Hz varying by steps of 0.01 Hz. The sign of the f0 shift and the order of presentation was randomly chosen at each trial (i.e. the probability of having a higher pitch interval was 50%). The inter-stimulus interval was 300ms and the inter-trial interval was 400ms. Before the test, participants received training with feedback for as many trials as they needed to understand the instructions. The training was done with a French vowel that was not presented during the actual test (i.e. the vowel /y/).
There were 7 conditions in total per participant (two native vowels, two non-native vowels, two instrument sounds, one pure tone). Each condition was presented within a block. The 7 blocks were randomized across speakers.
The stimuli were presented using a staircase procedure (Presentation, NBS) and we followed the recommendations of Garcia-Perez (2000, 2011) to optimize the convergence of the trials. For a down step, the size of the new shift was divided by 1.41 and for an up step it was multiplied by 1.6. Before the first reversal, a 1up-1down procedure was used. The number of reversals to end the block was set to 16 in addition to the first reversal, which corresponded to an average of 82 trials (3 to 4 min) per block.
Because we used an adaptive design with step sizes in a non-integer ratio, the shifts needed for each trial could not be determined in advance. Therefore, the shifted stimuli were synthesized during the experiment during the inter-trial interval. The duration of this sequence (processed during the inter-trial interval) was about 100ms. In addition to the controlled shift of pitch, a random shift of intensity (comprised between −3.5 dB and 3.5 dB Mathias, Micheyl, and Bailey (2010)) was applied independently to both stimuli of the pair to prevent the use of the inherent loudness difference (due to the f0 manipulation) to complete the task.
3.1.3 Analyses
The FDL was estimated as the average of the stimulus level at the last 16 reversal points and was transformed using a base-10 logarithmic transformation because a visual inspection of the histograms of the FDL data revealed that the data were not normally distributed. A mixed ANCOVA was carried out to assess: 1/ the effect of the between-group factor ‘group’ (blind vs. sighted), 2/ the effect of the within-group factor ‘stimulus category’ (native, non-native vowels, music instruments, and pure tones), and 3/ the interaction of both factors (group and stimulus category) on the FDL.
The number of years of formal musical training was also included in the analysis as a control variable.
Pearson product-moment correlation coefficients were computed to assess the relationship across the scores for the four categories of stimuli within each group. Bonferroni correction was applied to control for multiple comparisons.
Finally, a mixed ANOVA was carried out on the accuracy scores to assess the effect of the within-group factors ‘intensity direction’ (up, down) and ‘pitch direction’ (up, down) and the effect of the between-group factor ‘group’ (blind, sighted).
3.2 Results
3.2.1 Pitch-direction identification task
The objective of this experiment was to determine the FDL of pitch-direction identification for native vowels, non-native vowels, musical instruments, and pure tones for our two groups. The values of the pitch-direction identification thresholds for the four categories of stimuli are reported in Table 4.
We ran a t-test to determine whether there was a difference in the number of years of formal musical training between the two groups. There was no statistical difference when taking into account all participants (t(28) = 0.585, p = 0.563) or only the participants with some formal musical training (t(8)=0.8982, p = 0.3953).
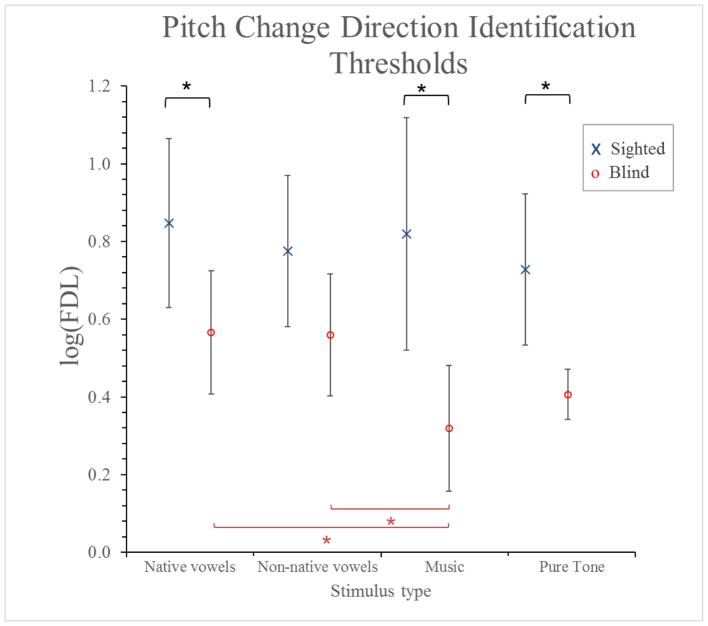
A two-way ANCOVA was conducted that examined the effect of stimulus category and group on the FDL of pitch direction identification (see Figure 4). The number of years of formal musical training was entered as a covariate and its effect was significant (p = 0.035). All results described below are corrected for this effect. There was a statistically significant interaction between the effects of group and stimulus category on the FDL, F (3,84) = 3.066, p = 0.032.
Figure 4.
FDL for pitch direction identification for different types of auditory stimuli for blind and sighted participants. Error bars represent 95% confidence intervals.
Simple main effects analysis showed that blind participants had lower thresholds than controls for music stimuli (p < 0.001), pure tones (p = 0.018), and French vowels (p = 0.039), but not for non-native vowels (p = 0.121). Stimulus category had an effect only within the blind group, whose thresholds were smaller for music stimuli than for both native (p = 0.005) and non-native vowels (p = 0.004). Within the control group, stimulus category had no significant effect.
3.2.2 Correlation between the scores
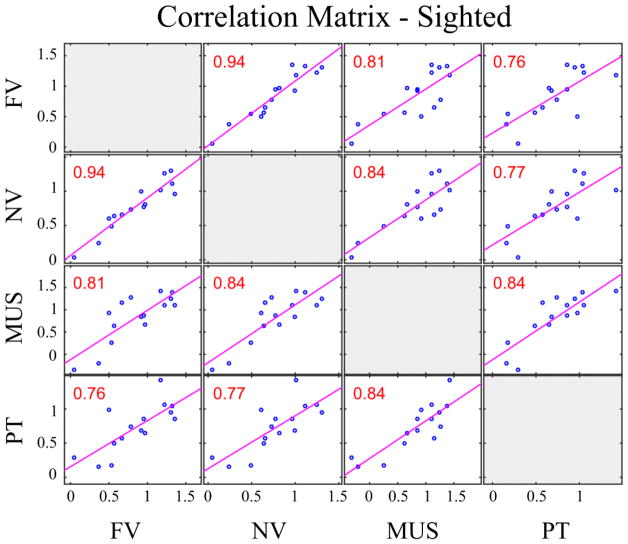
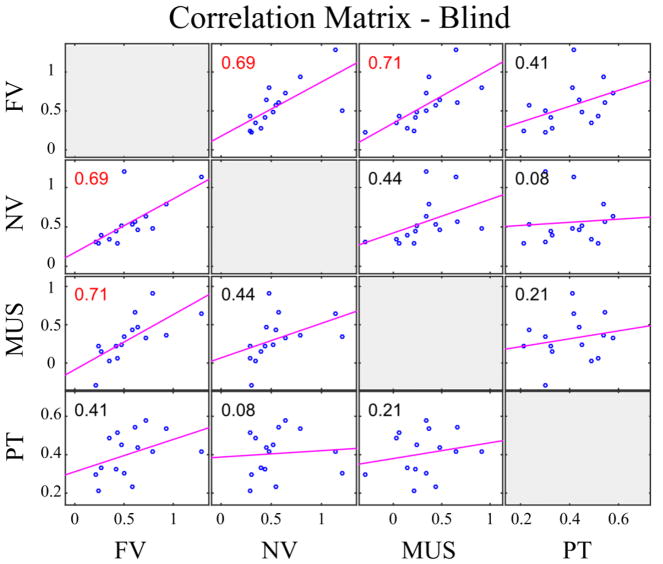
Pearson product-moment correlation coefficients were computed to assess the relationship across the scores for the four categories of stimuli within each group. The Bonferroni-corrected p-value is p = 0.05/6 = 0.00833. Overall, there was a strong positive correlation between all pairs of variables (r > 0.75 and p < 0.002, R2> 57% for all pairs) within the sighted group. However, within the blind group, only the scores for French and non-native vowels, and French vowels and instrument sounds were significantly correlated (r = 0.6883, p = 0.0046, R2 =47.4%; r = 0.7066, p = 0.0032, R2 =49.9%). The other pairs yielded weaker correlations which did not reach significance (r = [0.086–0.439], p > 0.1, R2 = [0.6% – 19%]). The correlation results are summarized in Figures 5 and 6.
Figure 5.
Correlation matrix between FDL thresholds of sighted participants. The figure shows the correlation between the log-transformed FDLs for French vowels (FV), non-native vowels (NV), pure tones (PT) and music instruments (MUS) for sighted participants. Pearson’s linear correlation coefficients are shown for each pair; red color indicates significant correlation corrected with Bonferroni adjustment (p<0.0083).
Figure 6.
Correlation matrix between FDL thresholds of blind participants. The figure shows the correlation between the log-transformed FDLs for French vowels (FV), non-native vowels (NV), pure tones (PT) and music instruments (MUS) for blind participants. Pearson’s linear correlation coefficients are shown for each pair; red color indicates significant correlation corrected with Bonferroni adjustment (p<0.0083).
3.2.3 Effect of intensity and pitch directions on pitch direction identification accuracy
For each trial, in addition to the pitch shift that was applied using the adaptive procedure, a shift of intensity was randomly applied to prevent the participants to use the loudness difference to judge the pitch direction.
We wanted to test the effect of the direction of the pitch change and the intensity change on the accuracy scores to test if the there was an impact of direction of the intensity and pitch shifts within or across group. A mixed ANOVA with the accuracy score as the dependent variable revealed no significant three way interaction among pitch direction * intensity direction * group (F(1,28) = 0.424, p = 0.520). The pitch direction*group and the intensity direction*group interactions were not significant (F(1,28) =1.646, p = 0.210; F(1,28)=0.478, p = 0.495). There was a significant pitch direction*intensity direction interaction with the scores being better when the intensity shift was positive for negative pitch shift, and when the intensity shift was negative shift for positive pitch shift (F(1,28) = 15.658, p < 0.001). Finally the main effects of intensity direction, pitch direction, and group were not significant (F(1,28) = 0.057, p = 0.814; F(1,28) = 1.670, p = 0.207; F(1,28) = 0.809, p = 0.376).
4. Discussion
The aim of the current study was to better understand the impact of visual deprivation on the auditory sensitivity of congenitally blind persons. In particular, we wanted to determine the extent of the auditory compensation for processing low level acoustic cues (pitch) in complex sounds. The questions we addressed were: (1) is the enhanced sensitivity for pitch variation in congenitally blind a domain-general or specific ability and is it modulated by stimulus familiarity?; (2) Are the characteristics of pitch processing the same as those observed in sighted and, specifically, do we find in the blind a) an advantage for musical stimuli compared to speech stimuli? b) a high degree of association between the scores from the different domains?
In summary, the results from this study indicate that the blind show a domain-general advantage over sighted individuals. In addition to enhanced pitch perception abilities, the results seem to indicate differences between blind and sighted participants in how the pitch thresholds for stimuli from different domains are associated with each other.
4.1 Enhanced Processing in the Congenitally Blind
Previous evidence indicates that congenitally blind adults show a certain degree of auditory compensation in tasks involving pitch perception. Blind adults are better at indicating pitch change direction for pure tones (Gougoux, et al., 2004; Rokem & Ahissar, 2009; Voss & Zatorre, 2012; Wan, et al., 2010), and at simple and transposed melody tasks (Voss & Zatorre, 2012). Pitch processing in the blind may rely on different neural mechanisms, such as intra and cross-modal plasticity that result in larger pitch periodicity-correlated activity in auditory areas and differential lateralization effects (Hertrich, et al., 2013b), recruitment of occipital areas during pitch processing (Collignon, et al., 2011; Ross, Olson, & Gore, 2003), and changes in the structure of visual areas associated with enhancement of pitch perception (Voss, et al., 2014; Voss & Zatorre, 2012). Our analysis revealed that the blind are better than the sighted participants for native vowels, instrument sounds and for pure tones. Even though the blind have better thresholds for non-native vowels as well, this difference only approached significance. These results are in line with phonetic behavioral studies conducted with the same blind cohort. In a series of production and perception experiments, it has been shown that the lack of vision influenced the articulatory movements used to implement phonemes: blind participants used smaller lip displacement than their sighted peer to produced vowel contrasts, but compensated using larger tongue displacements (Ménard, et al., 2009; Ménard, Leclerc, & Tiede, 2014; Ménard, et al., 2013).
As for the impact of stimulus type on perception thresholds, in a previous study on sighted participants, we showed an advantage for pitch processing of musical stimuli compared to vocal stimuli, regardless of the music education background of the participants (Arnaud et al. 2018, in prep). What is surprising here is that we replicate this result for the blind group but not for the current sighted group. Indeed, in the sighted group of the current study, no difference was found between thresholds for different stimulus types. In fact, for a part of the sighted group, we observed unusually higher thresholds for instrument sounds compared to vocal sounds. Methodologically, minor differences exist between the studies. The inter-trial interval was 400ms here and varied between 600ms and 1s in the previous study. The instrument stimuli were natural recordings in the first study and were synthetic in the current study. Finally, the adaptive staircase was stopped after 16 reversals here (18 in the previous study). However, these differences in the methods are minor and we believe they can’t explain the discrepancy in the results.
What could explain the results, however, is the age of the participants. In the first study, participants’ ages ranged from 18 to 35 years old, whereas in the current study control participants were chosen to match the blind group, whose ages ranged from 25 to 65 years. In order to evaluate whether age was a significant factor in the current participants, we calculated the ratio between the music difference limen and the speech difference limen for each participant and ran a correlation analysis with age within each group. One sighted participant was excluded from the analysis because his ratio was lower than the mean minus three standard deviations. The correlation on the controls (n=14) showed a high and significant positive association of age and the music to speech ratio (r=0.60, p = 0.024, see supplemental figure). That is, the older the participant, the higher the music difference limen compared to the speech difference limen. What is more surprising is that when we ran the same analysis within the blind group, we did not find an age-related effect (r=0.022, p = 0.937, see supplemental figure). It therefore seems that aging does not affect auditory perception in the blind in the same way as in the sighted. This finding is consistent with the conclusions of Gordon-Salant and Friedman (2011) in their study of speech perception in older blind and sighted participants. In that study, the authors showed that whereas older blind and sighted participants did not differ for normal-rate speech perception in quiet, the older blind individuals were better at perceiving time-compressed speech and speech in noise than older sighted people. In fact, the older blind participants processed speech similarly to the younger sighted controls. The authors propose that “greater attention to auditory information, which is the primary means of receiving spoken information by blind adults, may reduce the expected age-related decline in auditory temporal processing”. It appears that the apparent decline in sensitivity to pitch with age is offset in the blind due to the long-term reliance on the auditory system to compensate for the loss of vision. Moreover, while there are methodological differences as noted above in our previous study participants (Arnaud et al. 2018, in prep), the blind participants in the current study performed much better than the younger adults and comparable to the musicians. In conclusion, our results confirm that congenitally blind individuals show an advantage for pitch perception in pure tones, and extend these results to familiar speech sounds and instrument sounds and show a significantly better threshold for music stimuli than vocal stimuli, similar to younger sighted subjects.
4.2 Pitch perception processes
In addition to the comparison of pitch sensitivity, we also compared the characteristics of pitch processing in the two groups to determine (1) if the intensity shifts were used differently between the groups, and, (2) if the thresholds for the different types of stimuli were correlated. During the task, in addition to the pitch shift that was applied in an adaptive way, a random intensity shift was applied on each trial to prevent the use of the perceived intensity shift (caused by the pitch shift) to identify the pitch direction of change. Indeed, in this frequency range, a positive change in intensity can be perceived as a negative shift in pitch (and vice versa). No main effect was found for the pitch shift direction or the intensity shift direction overall, indicating that no direction was easier than the other. As expected, analyses demonstrated that when the direction of the intensity shift was in the opposite direction of the pitch shift, it had a positive impact on the score. More importantly, no interaction with the group was found, indicating that the blind and sighted did not differ in their strategies to use intensity as a cue for pitch change.
We then tested the degree of association across the scores for the different types of stimuli. In our first study, and in the current study, all sighted participants showed a very strong and significant correlation between performance on all stimuli (all correlation coefficients in a [0.77–0.98] range), indicating that the sensitivity thresholds for pitch change for different types of stimuli rely on shared perceptual/cognitive processes.
In the blind, we see a strong association between familiar and non-familiar speech sounds (r=0.69) and between familiar speech sounds and instrument sounds (r=0.71) but less than for the sighted group. The other associations were weak (correlation coefficients in the [0.08–0.41] range) and not statistically significant. The score for pure tones was not correlated with the scores for the three other types of sounds. One reason for this result might be due to the pure tone threshold data distribution shape, which is characterized by a smaller dispersion than for the other scores (standard deviation 2 to 3 times smaller than for the other scores in the blind group). It appears that the discrimination thresholds for the pure tones are much less variable than for the complex sounds. This could be due to the blind reaching a ceiling in the ability to process pitch in pure tones. The variations in the strength of association between the scores in the blind suggest a difference in the strategies used to process sounds coming from different acoustic domains. The blind might be more flexible in the way they recruit additional resources to process auditory information.
In our previous study, amateur musicians were better than non-musicians in pitch change direction discrimination and demonstrated a positive correlation with training experience in the amateur musicians. Whereas differences in musical training between blind and sighted groups could explain differences in the enhanced auditory perception processes that are observed in studies in the blind, (Collignon, et al., 2011; Rokem & Ahissar, 2009; Voss, et al., 2014; Voss & Zatorre, 2012; Wan, et al., 2010), to control for the effect of musical training, the groups of control and blind participants in the current study were matched for musical training, and the number of years of musical training was entered as a covariate in the analysis.
The present results show a global advantage of the blind over sighted participants, which agrees with studies on compensatory effects in the blind population, that are likely due to the cross-modal plasticity reported in neuroimaging studies. Whereas the blind develop enhanced auditory acuity through neural reorganization, this acuity does not seem to be a result of musical training. In conclusion, this study extended the results of enhanced pitch perception for pure tones in the blind to more complex sounds, including speech and instrument sounds. The results point to additional differences between the groups, namely possible differences in how age impacts auditory perception in sighted and blind adults.
Supplementary Material
Effect of Age on Music to Speech ratio in sighted and blind participants. In the sighted group, one outlier was removed.
Highlights.
We tested music and speech pitch discrimination in early blind and sighted adults.
Blind participants had lower discrimination thresholds for native speech and music.
The association of pitch thresholds from different domains differed for the groups.
Early visual deprivation enhances pitch discrimination ability.
Acknowledgments
This work was supported by grants to VG from the Natural Sciences and Engineering Research Council of Canada (238540-2012), and the National Institutes of Health (NIDCD R01-DC012502); and LA was supported by a Graduate Scholar Stipend from the Center for Research on Brain, Language and Music (CRBLM). The CRBLM is funded by the Government of Quebec via the Fonds de Recherche Nature et Technologies and Société et Culture.
The authors would like to thank Mélinda Maysounave, Thomas Granger, and Don Nguyen for their assistance with the blind participants, Jill Vandermeerschen for her help with the statistical analysis as well as all participants who took part in the experiments. We would also like to thank Dr. Shari Baum for her helpful and constructive comments which improved this manuscript.
Abbreviations
- FDL
frequency difference limen
- f0
fundamental frequency
- F1
first formant
- F2
second formant
- dB
decibel
- Hz
Hertz
- ms
millisecond
Footnotes
Conceived and designed the experiments: LA LM VG. Performed the experiments and analyzed the data: LA. Wrote the paper: LA LM VG.
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci. 2003;6:758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Anurova I, Renier LA, De Volder AG, Carlson S, Rauschecker JP. Relationship Between Cortical Thickness and Functional Activation in the Early Blind. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud L, Sato M, Menard L, Gracco VL. Repetition suppression for speech processing in the associative occipital and parietal cortex of congenitally blind adults. PLoS One. 2013;8:e64553. doi: 10.1371/journal.pone.0064553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc Natl Acad Sci U S A. 2011;108:4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boë L-J, Maeda S. Modélisation de la croissance du conduit vocal. Journées d’Études Linguistiques, La voyelle dans tous ses états. 1998:98–105. [Google Scholar]
- Bonino D, Ricciardi E, Sani L, Gentili C, Vanello N, Guazzelli M, Vecchi T, Pietrini P. Tactile spatial working memory activates the dorsal extrastriate cortical pathway in congenitally blind individuals. Arch Ital Biol. 2008;146:133–146. [PubMed] [Google Scholar]
- Burton H, Diamond JB, McDermott KB. Dissociating cortical regions activated by semantic and phonological tasks: a FMRI study in blind and sighted people. J Neurophysiol. 2003;90:1965–1982. doi: 10.1152/jn.00279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG. Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum Brain Mapp. 2004;23:210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Diamond JB, Raichle ME. Adaptive changes in early and late blind: a FMRI study of verb generation to heard nouns. J Neurophysiol. 2002;88:3359–3371. doi: 10.1152/jn.00129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon O, Vandewalle G, Voss P, Albouy G, Charbonneau G, Lassonde M, Lepore F. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proc Natl Acad Sci U S A. 2011;108:4435–4440. doi: 10.1073/pnas.1013928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S, Hertrich I, Ackermann H. Why do blind listeners use visual cortex for understanding ultra-fast speech? The Journal of the Acoustical Society of America. 2011;129:2494. [Google Scholar]
- Dietrich S, Hertrich I, Ackermann H. Ultra-fast speech comprehension in blind subjects engages primary visual cortex, fusiform gyrus, and pulvinar -- a functional magnetic resonance imaging (fMRI) study. BMC neuroscience. 2013;14:74. doi: 10.1186/1471-2202-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez MA. Optimal setups for forced-choice staircases with fixed step sizes. Spatial vision. 2000;13:431–448. doi: 10.1163/156856800741306. [DOI] [PubMed] [Google Scholar]
- García-Pérez MA. A cautionary note on the use of the adaptive up–down method. The Journal of the Acoustical Society of America. 2011;130:2098–2107. doi: 10.1121/1.3628334. [DOI] [PubMed] [Google Scholar]
- García-Pérez MA. Forced-choice staircases with fixed step sizes: asymptotic and small-sample properties. Vision Res. 1998;38:1861–1881. doi: 10.1016/s0042-6989(97)00340-4. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Friedman SA. Recognition of rapid speech by blind and sighted older adults. J Speech Lang Hear Res. 2011;54:622–631. doi: 10.1044/1092-4388(2010/10-0052). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougoux F, Lepore F, Lassonde M, Voss P, Zatorre RJ, Belin P. Neuropsychology: pitch discrimination in the early blind. Nature. 2004;430:309. doi: 10.1038/430309a. [DOI] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, Ackermann H. How can audiovisual pathways enhance the temporal resolution of time-compressed speech in blind subjects? Front Psychol. 2013a;4:530. doi: 10.3389/fpsyg.2013.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, Ackermann H. Tracking the speech signal--time-locked MEG signals during perception of ultra-fast and moderately fast speech in blind and in sighted listeners. Brain Lang. 2013b;124:9–21. doi: 10.1016/j.bandl.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, Moos A, Trouvain J, Ackermann H. Enhanced speech perception capabilities in a blind listener are associated with activation of fusiform gyrus and primary visual cortex. Neurocase. 2009;15:163–170. doi: 10.1080/13554790802709054. [DOI] [PubMed] [Google Scholar]
- Hubbard AL, Wilson SM, Callan DE, Dapretto M. Giving speech a hand: gesture modulates activity in auditory cortex during speech perception. Hum Brain Mapp. 2009;30:1028–1037. doi: 10.1002/hbm.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Ek M, Takio F, Rintee T, Tuomainen J, Haarala C, Hamalainen H. Blind individuals show enhanced perceptual and attentional sensitivity for identification of speech sounds. Brain Res Cogn Brain Res. 2004;19:28–32. doi: 10.1016/j.cogbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhu W, Shi F, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Thick visual cortex in the early blind. J Neurosci. 2009;29:2205–2211. doi: 10.1523/JNEUROSCI.5451-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Kujala T, Huotilainen M, Sinkkonen J, Ahonen AI, Alho K, Hamalainen MS, Ilmoniemi RJ, Kajola M, Knuutila JE, Lavikainen J. Visual cortex activation in blind humans during sound discrimination. Neurosci Lett. 1995;183:143–146. doi: 10.1016/0304-3940(94)11135-6. [DOI] [PubMed] [Google Scholar]
- Lepore N, Voss P, Lepore F, Chou YY, Fortin M, Gougoux F, Lee AD, Brun C, Lassonde M, Madsen SK, Toga AW, Thompson PM. Brain structure changes visualized in early- and late-onset blind subjects. Neuroimage. 2010;49:134–140. doi: 10.1016/j.neuroimage.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S. An articulatory model of the tongue based on a statistical analysis. The Journal of the Acoustical Society of America. 1979;65:S22–S22. [Google Scholar]
- Mathias SR, Micheyl C, Bailey PJ. Stimulus uncertainty and insensitivity to pitch-change direction. The Journal of the Acoustical Society of America. 2010;127:3026–3037. doi: 10.1121/1.3365252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill D. Hand and mind: What gestures reveal about thought. University of Chicago Press; 1992. [Google Scholar]
- Ménard L, Dupont S, Baum SR, Aubin J. Production and perception of French vowels by congenitally blind adults and sighted adults. J Acoust Soc Am. 2009;126:1406–1414. doi: 10.1121/1.3158930. [DOI] [PubMed] [Google Scholar]
- Ménard L, Leclerc A, Tiede M. Articulatory and Acoustic Correlates of Contrastive Focus in Congenitally Blind Adults and Sighted Adults. Journal of Speech, Language, and Hearing Research. 2014;57:793–804. doi: 10.1044/2014_JSLHR-S-12-0395. [DOI] [PubMed] [Google Scholar]
- Ménard L, Toupin C, Baum SR, Drouin S, Aubin J, Tiede M. Acoustic and articulatory analysis of French vowels produced by congenitally blind adults and sighted adults. The Journal of the Acoustical Society of America. 2013;134:2975. doi: 10.1121/1.4818740. [DOI] [PubMed] [Google Scholar]
- Moos A, Trouvain J. Comprehension of ultra-fast speech - Blind vs. “normally hearing” persons. 16th International Congress of Phonetic Sciences; Saarbrucken, Germany. 2007. pp. 677–680. [Google Scholar]
- Muchnik C, Efrati M, Nemeth E, Malin M, Hildesheimer M. Central auditory skills in blind and sighted subjects. Scand Audiol. 1991;20:19–23. doi: 10.3109/01050399109070785. [DOI] [PubMed] [Google Scholar]
- Niemeyer W, Starlinger I. Do the blind hear better? Investigations on auditory processing in congenital or early acquired blindness. II. Central functions. Audiology. 1981;20:510–515. doi: 10.3109/00206098109072719. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Wu G, Li CX, Lin F, Sun J, Lei H. Progressive atrophy in the optic pathway and visual cortex of early blind Chinese adults: A voxel-based morphometry magnetic resonance imaging study. Neuroimage. 2007;37:212–220. doi: 10.1016/j.neuroimage.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Park HJ, Jeong SO, Kim EY, Kim JI, Park H, Oh MK, Kim DJ, Kim SY, Lee SC, Lee JD. Reorganization of neural circuits in the blind on diffusion direction analysis. Neuroreport. 2007;18:1757–1760. doi: 10.1097/WNR.0b013e3282f13e66. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee JD, Kim EY, Park B, Oh MK, Lee S, Kim JJ. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage. 2009;47:98–106. doi: 10.1016/j.neuroimage.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Ptito M, Schneider FC, Paulson OB, Kupers R. Alterations of the visual pathways in congenital blindness. Exp Brain Res. 2008;187:41–49. doi: 10.1007/s00221-008-1273-4. [DOI] [PubMed] [Google Scholar]
- Röder B, Demuth L, Streb J, Rösler F. Semantic and morpho-syntactic priming in auditory word recognition in congenitally blind adults. Language and Cognitive Processes. 2003;18:1–20. [Google Scholar]
- Röder B, Stock O, Bien S, Neville H, Rösler F. Speech processing activates visual cortex in congenitally blind humans. Eur J Neurosci. 2002;16:930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- Rokem A, Ahissar M. Interactions of cognitive and auditory abilities in congenitally blind individuals. Neuropsychologia. 2009;47:843–848. doi: 10.1016/j.neuropsychologia.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Ross DA, Olson IR, Gore JC. Cortical plasticity in an early blind musician: an fMRl study. Magnetic resonance imaging. 2003;21:821–828. doi: 10.1016/s0730-725x(03)00103-6. [DOI] [PubMed] [Google Scholar]
- Sani L, Ricciardi E, Gentili C, Vanello N, Haxby JV, Pietrini P. Effects of visual experience on the human MT+ functional connectivity networks: an fMRI study of motion perception in sighted and congenitally blind individuals. Frontiers in Systems Neuroscience. 2010:4. doi: 10.3389/fnsys.2010.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N, Li J, Li K, Yu C, Jiang T. Abnormal diffusion of cerebral white matter in early blindness. Hum Brain Mapp. 2009;30:220–227. doi: 10.1002/hbm.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N, Liu Y, Li J, Li Y, Yu C, Jiang T. Altered anatomical network in early blindness revealed by diffusion tensor tractography. PLoS One. 2009;4:e7228. doi: 10.1371/journal.pone.0007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Weaver K. Auditory perceptual consolidation in early-onset blindness. Neuropsychologia. 2005;43:1901–1910. doi: 10.1016/j.neuropsychologia.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Stevens AA, Weaver KE. Functional characteristics of auditory cortex in the blind. Behav Brain Res. 2009;196:134–138. doi: 10.1016/j.bbr.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby WH, Pollack I. Visual Contribution to Speech Intelligibility in Noise. Journal of the Acoustical Society of America. 1954;26:212–215. [Google Scholar]
- Teng S, Puri A, Whitney D. Ultrafine spatial acuity of blind expert human echolocators. Exp Brain Res. 2012;216:483–488. doi: 10.1007/s00221-011-2951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouvain J. On the comprehension of extremely fast synthetic speech 2007 [Google Scholar]
- Voss P, Lepore F, Gougoux F, Zatorre RJ. Relevance of spectral cues for auditory spatial processing in the occipital cortex of the blind. Front Psychol. 2011;2:48. doi: 10.3389/fpsyg.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P, Pike B, Zatorre RJ. Evidence for both compensatory plastic and disuse atrophy-related neuroanatomical changes in the blind. Brain. 2014;137:1224–1240. doi: 10.1093/brain/awu030. [DOI] [PubMed] [Google Scholar]
- Voss P, Zatorre RJ. Occipital cortical thickness predicts performance on pitch and musical tasks in blind individuals. Cereb Cortex. 2012;22:2455–2465. doi: 10.1093/cercor/bhr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Wood AG, Reutens DC, Wilson SJ. Early but not late-blindness leads to enhanced auditory perception. Neuropsychologia. 2010;48:344–348. doi: 10.1016/j.neuropsychologia.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Weaver KE, Stevens AA. Attention and sensory interactions within the occipital cortex in the early blind: an fMRI study. J Cogn Neurosci. 2007;19:315–330. doi: 10.1162/jocn.2007.19.2.315. [DOI] [PubMed] [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, Hallett M, Rauschecker JP. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Shu N, Li J, Qin W, Jiang T, Li K. Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. Neuroimage. 2007;36:411–417. doi: 10.1016/j.neuroimage.2007.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of Age on Music to Speech ratio in sighted and blind participants. In the sighted group, one outlier was removed.