Abstract
Background:
Stress responding is linked to drug use, but little is known about stress responses in cannabis smokers. We investigated acute stress responding in cannabis smokers as a function of trauma exposure and sex, and relationships between stress responses and cannabis relapse.
Methods:
125 healthy, non-treatment-seeking daily cannabis smokers (23F, 102M) completed the Trier Social Stress Task (TSST), a standardized laboratory stressor; subsets also completed a trauma questionnaire (n = 106) and a laboratory cannabis relapse measure (n = 54). Stress responding was assessed with heart rate (HR), salivary cortisol (CORT), and self-rated mood.
Results:
Cannabis smokers reporting at least one trauma exposure had higher CORT and anxiety overall compared to those reporting no trauma. Stress responding did not differ as a function of binary trauma exposure, although total number of exposures correlated positively with CORT and anxiety during stress. Females reported increased nervousness after stress relative to males matched to the females for cannabis and cigarette use. An interactive effect of sex and trauma on HR suggested that females with trauma exposure have increased cardiovascular stress responding relative to those without such exposure, with no differential effect in males. Stress responding did not predict laboratory cannabis relapse.
Conclusion:
We report differences in acute stress responding as a function of trauma, sex, and their interaction in a large sample of relatively homogenous cannabis smokers. Further investigation of how trauma impacts stress responding in male and female cannabis smokers, and how this relates to different aspects of cannabis use, is warranted.
Keywords: cannabis, marijuana, trauma, sex differences, stress responding, TSST
1. Introduction
Cannabis is the most widely used illicit drug internationally (UNODC, 2016). Between 2002 and 2014 there was a 28% increase in use in the US (Compton et al., 2016), with prevalence expected to continue to rise amid legal changes (Hall and Lynskey, 2016). An understanding of individual risk factors for problematic cannabis use could valuably guide prevention and intervention.
Stress exposure and dysregulated stress responding contribute to problematic use of other drugs. In rodents, exposure to acute stressors (e.g., social stress, foot shock) increases self-administration of cocaine (Goeders and Guerin, 1994; Haney et al., 1995; Miczek and Mutschler, 1996; Ramsey and van Ree, 1993), amphetamines (Vezina et al., 2002), opioids (Alexander et al., 1978; Shaham et al., 1992; Shaham et al., 1993; Shaham and Stewart, 1995), and alcohol (Pohorecky, 1990). In healthy humans, acute stress increases alcohol consumption (de Wit et al., 2003; Magrys and Olmstead, 2015; McGrath et al., 2016). Drug cravings also covary with responses to acute laboratory stress in alcohol and cocaine users (Fox et al., 2007; Fox et al., 2008; Fox et al., 2005; Sinha et al., 1999; Sinha et al., 2000; Sinha et al., 2003).
There is some evidence that dysregulated stress responding also contributes to vulnerability for drug misuse. Stress responding during withdrawal predicts relapse in alcohol-(Adinoff et al., 2005; Brady et al., 2006; Breese et al., 2011) and cocaine- (Back et al., 2010; Sinha et al., 2006) dependence. Moreover, stress-induced anxiety predicts lower engagement in aftercare following inpatient treatment in alcohol-dependent patients (Sinha, 2012). Thus, certain patterns of stress responding may increase drug taking and hinder treatment compliance.
Despite this existing research, little is known about stress responding in cannabis users. One study found that adolescents who had used cannabis ≥5x in the past year had blunted HPA-axis stress responses relative to those reporting less frequent use (van Leeuwen et al., 2011). Blunted cortisol and subjective distress ratings have similarly been observed in adult cannabis users compared to non-users (Cuttler et al., 2017). Further, social stress increased cannabis craving relative to a neutral task in cannabis users (Buckner et al., 2016; McRae-Clark et al., 2011). Thus, consistent with other drug-using groups, stress responding appears to be dysregulated in some cannabis smokers, and this may be related to clinical outcomes. To date, little is known about variability in stress responding in cannabis users.
Diverse factors may affect stress responses in cannabis smokers. Cannabis may acutely affect stress responding, given that oral THC modulates subjective stress responding (Childs et al., 2017). Adverse life experiences, such as trauma can have long-term effects on stress responding (Carpenter et al., 2007; Gutman and Nemeroff, 2003; Heim et al., 2008; McLaughlin et al., 2010; Nemeroff, 2004; Shea et al., 2005). Cannabis smokers who have experienced traumatic events may thus have altered stress responses and an increased risk for problematic cannabis use.
Stress responding also differs as a function of sex. In non-drug users, laboratory stress elicits greater heart rate and negative affect increases in women (Kelly et al., 2008; Kudielka et al., 2004; Ordaz and Luna, 2012), whereas men show higher blood pressure increases (Childs et al., 2010; Kajantie and Phillips, 2006; Kudielka and Kirschbaum, 2005; Lepore et al., 1993; Matthews et al., 2001). Findings related to sex differences in cortisol responses are inconsistent, however studies using the Trier Social Stress Task (TSST; Kirschbaum et al., 1993), a standardized stress assay, have reported heightened cortisol stress responses in males compared to females (Childs et al., 2010; Uhart et al., 2006). As noted above, social stress increases cannabis craving in cannabis users (Buckner et al., 2016; McRae-Clark et al., 2011); this effect appears to be particularly pronounced in women (Buckner et al., 2011). Women are also more likely than men to report use of cannabis for the purpose of alleviating anxiety (Cuttler et al., 2016), which can be a symptom of stress (Temple et al., 2014).
There may also be interactive effects of trauma and sex on stress responding in cannabis users. In non-drug users, trauma-exposed women display hyperactive HPA-axis and autonomic system reactivity to stress (Heim et al., 2000), whereas trauma-exposed men have blunted cortisol stress responding (Janusek et al., 2017). Such interactions may have important implications, given that trauma exposure in women predicts earlier cannabis use initiation and rapid progression to dependence (Werner et al., 2016a).
In this study, we aimed to investigate individual variability in response to standardized laboratory stress in a sample of regular cannabis smokers. We focused on differences as a function of trauma exposure and sex and their interaction. Given the possibility that stress responding may be linked to intractable cannabis use, we also investigated the relationship between acute stress responding and relapse to cannabis, as measured in a human laboratory model. We expected that: (1) cannabis smokers with trauma exposure would show greater stress reactivity than those without exposure; (2) female cannabis smokers would have greater mood and heart rate stress reactivity whereas males would have increased cortisol; and (3) cannabis smokers who relapsed to cannabis in the laboratory would show increased stress responding relative to those who remained abstinent, regardless of trauma exposure.
2. Methods
2.1. Participants
This analysis included data from cannabis smokers recruited in NYC, NY. Participants were healthy males and non-pregnant females between 18–50 years old reporting current, heavy cannabis use (defined as ≥2 cannabis cigarettes/day, ≥4 days/week). A PhD-level researcher assessed mental health status and drug use. Positive THC urine toxicology tests at all screening visits were required to biochemically verify current regular cannabis use. Estimates of number of cannabis cigarettes used were based on a rate of 1 ‘blunt’ = 2 cannabis cigarettes (Mariani et al., 2011). Participants could not: (1) be regularly (>2x/week) using other illicit drugs; (2) meet DSM-IV criteria for an Axis I disorder requiring intervention (APA, 1994); (3) be taking medication daily; (4) be seeking treatment; (5) have prior adverse cannabis effects; or (6) have a health condition contraindicating participation. Volunteers underwent psychiatric and physical examination and electrocardiogram, urinalysis, and blood panels before admission. All participants provided informed consent as approved by the New York State Psychiatric Institute (NYSPI) Institutional Review Board. Volunteers were compensated and fully debriefed at discharge.
2.2. Experimental protocol
Data were collected across 5 studies, all using an inpatient human laboratory model of cannabis withdrawal and relapse: (1) effects of cannabis cues and primes on cannabis relapse after withdrawal (not published); (2) effects of tobacco cigarette cessation versus smoking as usual on cannabis withdrawal and relapse in cigarette-smoking cannabis users (Haney et al., 2013a); (3) effects of nabilone on cannabis withdrawal and relapse (Haney et al., 2013b); (4) effects of zolpidem, alone or with nabilone, on cannabis withdrawal and relapse (Herrmann et al., 2016); and (5) effects of varenicline, alone or with nabilone, on cannabis withdrawal and relapse in cigarette smoking cannabis users (Herrmann et al., under review). For studies 2 and 5, participants also smoked at least 4 nicotine cigarettes daily.
2.2.1. Trier Social Stress Task (TSST).
Before admission and any medication administration, participants attended a single session in which they completed the TSST, a standardized assay of social stress responding (Kirschbaum et al., 1993). We aimed to test participants in their ‘normal’ daily state i.e., not acutely intoxicated by cannabis and not in withdrawal. Thus, we did not provide specific instructions regarding abstinence before the session. Acute cannabis intoxication was minimized by keeping participants in the laboratory (where they could not smoke cannabis) for at least an hour before the TSST. The TSST was conducted in the afternoon to control for diurnal cortisol variations. Baseline measurements (see Assessments) were recorded approximately 25 minutes before the TSST (see Figure 1). After baseline measurements, participants were informed that they would make a 5-minute speech outlining their job qualifications in front of a committee rating their body language for signs of stress. They were also informed that they would complete a second task with instructions provided after the speech. Participants were shown the room and alerted to a video camera that they were told was recording (no recordings were made). They were given 10 minutes to prepare (the introduction phase). During the speech, the committee (two confederates) provided minimal instruction and no encouragement. Following the speech, participants completed complex mental arithmetic for 5 minutes. They were informed of errors and asked to begin again. The TSST reliably but transiently increases markers of stress across populations (Allen et al., 2014). Following the stressor, participants remained in the laboratory for 90 minutes completing assessments.
Figure 1.
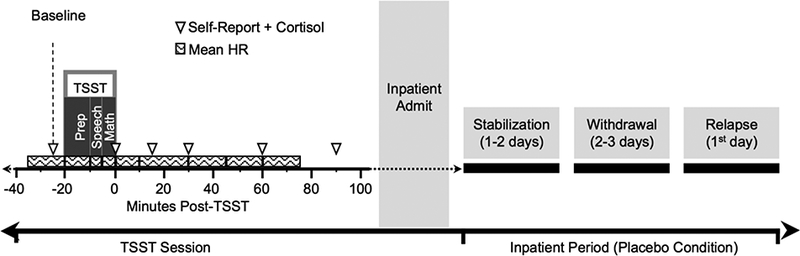
Experimental timeline of study procedures. Participants completed a single TSST outpatient session. Of these, approximately half went on to complete the protocol of cannabis withdrawal and relapse under one of five broader inpatient studies. The number of days in each phase varied depending on the study the participants were enrolled: 1–2 days in which participants received standardized administration of active cannabis (Stabilization phase), and 2–3 days in which placebo dose of self-administered cannabis could be purchased (Withdrawal phase). Relapse was buying and using cannabis on the first day that active cannabis was made available for purchase.
2.2.2. Laboratory model of cannabis withdrawal and relapse.
Following the TSST, some participants entered an inpatient study. Although the specific protocols varied, each used a version of our human laboratory model of cannabis withdrawal and relapse (Haney et al., 2008). In this model, non-treatment seeking cannabis smokers initially undergo 1–2 days of experimenter-administered cannabis comprising 18 ‘puffs’ of active cannabis (5.6–6.2% THC) per day. On the following 2–3 days, participants have the option to purchase up to 18 puffs of placebo cannabis (0.0% THC) per day for self-administration (Abstinence). They then undergo 1–4 days in which they may purchase up to 18 puffs of active (5.6–6.2% THC) cannabis/day for self-administration (Relapse). Participants are not informed about the doses, rather they sample each dose (active cannabis labeled as ‘Dose A’ and inactive as ‘Dose B’) before admission, ensuring that abstinence and relapse occur under blind conditions. Participants pay for self-administered cannabis using study earnings, and the costs are high (e.g., $9 for a single cannabis puff) in order to model treatment, where there are inherent motivations to avoid cannabis that are absent in non-treatment seekers. For the present analyses, we employed data from the first day of the relapse phase of the placebo intervention condition from each study i.e., the placebo medication condition from the nabilone, zolpidem/nabilone, and varenicline/nabilone studies; the no cue/no prime condition in the cue/prime study; and cigarette smoking as usual in the nicotine cessation study.
2.2.3. Cannabis
Placebo (0.0% THC) and active (5.6%, 5.9%, or 6.2% THC) cannabis cigarettes were provided by NIDA. They were rolled at the end and smoked through a cigarette holder. Cigarettes were frozen for storage and humidified at room temperature for 24 hours before administration. Participants smoked using a standardized, paced puffing procedure (Foltin et al., 1987).
2.3. Assessments
2.3.1. Trauma.
Trauma exposure was assessed using the Trauma Assessment for Adults - Brief Revised Version (TAA; Cusack et al., 2004; Resnick et al., 1996). The TAA is based on the Potential Stressful Events Interview from the DSM-IV field trial. The 12-item brief revised version uses a yes/no format, assessing exposure to 12 types of stressful events (e.g., combat, sexual assault, serious car accident). Some TAA items focus specifically on childhood experiences (e.g., sexual contact before age 13; coerced sexual interaction before age 18) whereas the majority of items address traumas that could happen in childhood or adulthood.
2.3.2. Subjective stress response.
Mood state was assessed using Visual Analogue Scales (VAS; Folstein and Luria, 1973), the state component of the State Trait Anxiety Inventory (STAI; Spielberger et al., 1983) and the Profile of Mood States (POMS; McNair and Droppleman, 1971). VAS items included the following descriptors: I feel... upset, nervous, stressed, confident, and angered, with ‘Not at all’ and ‘Extremely’ as anchors. The STAI-state comprises 20 items yielding a single score. The 72-item POMS yields the following subscores: Anxious, Depressed, Angry, Vigor, Fatigued, Confused, Friendly, Elated, and Aroused. The subjective battery was completed at baseline, immediately after the TSST (0 minutes), then at 15, 30, 60 and 90 minutes.
2.3.3. Cardiovascular stress response.
Heart rate (HR) was monitored continuously using the Polar RS400 Sports Watch (Oulu, Finland). Mean HRs were calculated for the baseline period, the 10-min introduction, the 5-min speech, the 5-min arithmetic task, and then every 15 minutes up to 90 minutes.
2.3.4. Cortisol stress response.
Salivary cortisol (CORT) samples were collected using Salivette polyester swabs (Sarstedt, Germany). Following collection, the sample was stored at - 25 degrees Celsius. CORT levels were assayed by the Analytical Psychopharmacology Laboratory (PI: Thomas A. Cooper) at the Nathan Kline Institute, Orangeburg, NY. An initial pre-baseline sample was collected to reduce novelty effects; data from these samples were discarded. Samples were then collected at baseline and 0, 15, 30, 60 and 90 minutes post stressor.
2.3.5. Cannabis relapse.
Cannabis relapse was operationalized as binary (relapse/no relapse) and dimensional (number of puffs smoked) measures.
2.4. Statistical analyses
Main outcomes were physiological (CORT, HR) and subjective (STATE, VAS, POMS) variables. CORT data from two participants with medical conditions showed extreme values and were excluded from analyses. Isolated univariate outliers with z-scores greater than 3.29 were truncated to z = ±3.29 (Tabachnick and Fidell, 2013). Because ANOVAs and t-tests are relatively robust to violations of normality when not due to outliers (Gravetter and Wallnau, 2004), we retained non-normal data.
Effects of the TSST were assessed with single-factor repeated measures Analyses of Variance (ANOVAs). Main effects of time were followed-up by pairwise comparisons between each time-point and baseline measures. Group differences were analyzed with mixed ANOVAs, with trauma exposure, sex, or relapse status as the between-group factor and time as the within-group factor. In the mixed ANOVAs examining between group differences (e.g., male/female as a between-subjects variable and time point during the TSST as the within-subjects variable), a main effect of group indicates a difference between groups regardless of time point (i.e., an overall group difference irrespective of the stressor). Conversely, an interaction between group and time point indicates a differential response to the stressor between the two groups. Additionally, we conducted three-factor mixed ANOVAs with trauma exposure and sex as between-subject factors and time as the within-subject factor to examine interactions between sex and trauma exposure. Interactions were followed up with simple main effects and post-hoc pairwise comparisons. Alpha was set at 0.05 for initial ANOVAs; a corrected alpha of 0.01 was used for all post-hoc analyses. Where Mauchley’s test of sphericity indicated violation of the assumption of sphericity (p < .05), we interpreted Greenhouse-Geisser corrected degrees of freedom. Effect sizes are presented as partial η2. Correlational analyses were performed on the total number of reported past traumas and Area Under the Curve (AUC) for each stress response variable. AUCs were computed using the trapezoid formula (see(Pruessner et al., 2003). Analyses were conducted using IBM SPSS Statistics 22 (IBM, Armonk, NY).
3. Results
3.1. Participants
Table 1 shows demographic data on participants completing the TSST (N = 125), divided by trauma exposure, sex, and relapse status. Groups were well matched demographically. Nineteen participants were excluded from trauma analyses, having not completed the TAA. All females were included in the sex differences analyses. Since cigarette use is associated with TSST responses (al’Absi et al., 2003; Childs and de Wit, 2009), the subset of males included in the sex difference analyses were matched to the females for cannabis and cigarette use. Fifty-four participants completed the TSST and an inpatient study and were thus included in the relapse analyses.
Table 1.
Demographic Characteristics
| Trauma Exposure | Sex Differences | Cannabis Relapsea |
||||
|---|---|---|---|---|---|---|
| Yes | No | Female | Male | Yes | No | |
| N | 68 (10 F) |
38 (10 F) |
23b | 23b | 32 (4 F) | 22 (4 F) |
| Race (White/Black/other) |
9/55/4 | 7/30/1 | 3/19/1 | 4/17/2 | 6/25/1 | 3/19/0 |
| Ethnicity (Hispanic/non- Hispanic) |
18/50 | 12/26 | 4/19 | 12/11 | 8/24 | 4/18 |
| Age | 32.3 ± 8.4 |
30.9 ± 7.3 |
29.6 ± 8.4 |
31.3 ± 8.5 |
30.0 ± 7.4 |
29.4 ± 8.8 |
| Education (years) | 12.3 ± 1.3 |
12.4 ± 1.4d |
12.3 ± 1.3g |
12.4 ± 1.5 |
12.5 ± 1.1 |
12.4 ± 1.0 |
| Cannabis days/week | 6.9 ± 0.5 |
6.7 ± 0.6 |
6.8 ± 0.4 |
6.7 ± 0.8 |
6.9 ± 0.2 |
6.9 ± 0.3 |
| Cannabis ‘blunts’/day | 4.5 ± 3.1 |
4.4 ± 3.7 |
5.4 ± 4.9 |
5.0 ± 3.6 |
4.3 ± 2.6 |
4.1 ± 3.3 |
| Age first cannabis use | 14.3 ± 3.9e |
14.7 ± 2.0 |
15.0 ± 2.5 |
14.6 ± 3.4g |
14.9 ± 3.9 |
13.6 ± 3.3 |
| Age regular cannabis use |
17.6 ± 5.8 |
16.6 ± 3.8 |
17.2 ± 5.0 |
17.6 ± 5.1 |
16.5 ± 5.6h |
16.5 ± 4.7 |
| Years regular cannabis use |
14.0 ± 7.4 |
14.8 ± 7.9 |
13.1 ± 9.1 |
13.7 ± 8.1 |
12.8 ± 6.5 |
12.7 ± 8.0 |
| Daily cigarette smokers (n) |
64 | 36 | 21 | 21 | 30 | 16 |
| Cigarettes per day | 9.6 ± 6.3 |
10.0 ± 7.7 |
9.6 ± 5.1 |
10.4 ± 7.8 |
9.3 ± 5.0 |
7.2 ± 3.9 |
| Alcohol drinkers (n) | 63 | 29 | 15 | 22 | 27 | 18 |
| Alcohol days/week | 1.6 ± 2.2 |
1.1 ± 0.9f |
1.6 ± 1.9 |
2.2 ± 2.9 |
1.2 ± 1.3i |
1.3 ± 1.2 |
| Alcoholic drinks/occasion |
3.2 ± 2.4 |
3.1 ± 2.1f |
2.7 ± 1.1c |
4.4 ± 3.0c |
3.3 ± 2.3 i |
3.8 ± 2.8 |
Note: Data presented as means (± SD), except where otherwise specified.
F = female
Relapsed to cannabis in a human laboratory model of cannabis withdrawal and relapse.
Males for sex difference analyses were selected from total sample of males to match females on amounts of cannabis use and cigarette smoking.
Difference between Females vs. Males (p < .05).
n = 37 due to missing data.
n = 67 due to missing data.
n = 28 due to missing data.
n = 22 due to missing data.
n = 31 due to missing data.
n = 26 due to missing data.
3.2. Stress responding in the sample overall
As expected, the TSST increased indicators of stress in the overall sample. There was a main effect of time on HR (F(3.2, 361.0) = 215.7, p < .001, partial η2 = .66), such that HR increased from baseline during stress, then subsided to baseline within 15 minutes. There was a main effect of time on CORT (F(2.7, 296.1) = 28.2, p < .001, partial η2 = .21), with cortisol highest at baseline and 15 minutes after the stressor and decreasing thereafter. Main effects of time were observed across all subjective variables (all p values < .01), except VAS ‘angered’. STAI state anxiety increased after the TSST and remained somewhat elevated, whereas VAS ‘upset’, ‘stressed’, and ‘nervous’, as well as POMS ‘anxious’, ‘depressed’, ‘confused’, and ‘angry’ increased immediately after the TSST, returning to baseline by completion of the session.
3.3. Stress responding as a function of trauma
There was a main effect of trauma on CORT (F(1, 94) = 4.4, p = .039, partial η2 = .045), with cannabis smokers who reported any trauma having higher salivary cortisol overall, relative to those who denied trauma exposure, with no difference in cortisol stress responding (i.e., no interaction between trauma and time; Figure 2). Compared to those without trauma, participants endorsing any trauma also had higher overall VAS ‘stress’ (F(1, 93) = 7.4, p = .008, partial η2 = .07), POMS ‘anxiety’ (F(1, 97) = 6.2, p = .015, partial η2 = .06), STAI state ‘anxiety’ (F(1, 103) = 4.6, p = .034, partial η2= .04), and POMS ‘fatigue’ (F(1, 102) = 6.3, p = .013, partial η2 = .06), again with no interaction between trauma and time, indicating differential stress responding (Figure 2). There were no other main effects of trauma and no interactive effects between trauma and time, indicating that stress responding did not differ with trauma assessed as a binary variable. However, correlations revealed small-moderate positive relationships between the total number of traumas endorsed and AUCs for CORT r = .22, p = .034, POMS ‘anxiety’ r = .31, p = .001, POMS ‘fatigue’ r = .25, p = .009, and POMS ‘confused’ r = .20, p = .038.
Figure 2.
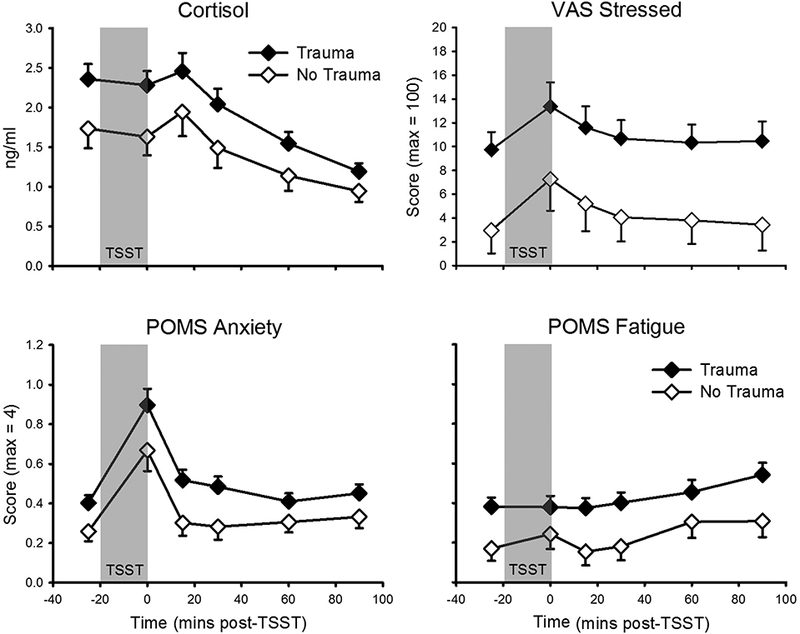
TSST responses as a function of trauma. Top Left: Salivary cortisol (no trauma: n = 35; trauma: n = 61). Top Right: Visual Analogue Scale (VAS) ‘stress’ (no trauma: n = 35; trauma: n = 60). Bottom Left: Profile of Mood States (POMS) ‘anxiety’ (no trauma: n = 38; trauma: n = 61). Bottom Right: POMS ‘fatigue’; (no trauma: n = 37; trauma: n = 67). TSST (introduction + speech + mental arithmetic) appears as gray-filled region. Data presented are means and standard errors. There were main effects of trauma group on all four variables presented, with no differential stress responding.
3.4. Stress responding as a function of sex
There was a main effect of sex on HR (F(1, 41) = 7.8, p = .008, partial η2 = .16), such that females exhibited higher HRs than males overall, with no interactions between sex and time indicating differential stress responses (Figure 3). Men had greater overall POMS ‘arousal’ scores (F(1, 44) = 8.6, p = .005, partial η2 = .16), with female arousal levels decreasing relative to males at the end of the session (Figure 2). There was an interaction of sex and time on VAS ‘nervous’ (F(5, 190) = 5.2, p < .001, partial η2 = .12), such that in females but not males, nervousness increased after the stressor (Figure 3). There was an interaction between sex and time on STAI state ‘anxiety’ scores (F(5, 215) = 3.7, p = .012, partial η2 = .08). Although STAI anxiety increased in females following the TSST, no individual time point comparisons reached statistical significance. There was an interaction between sex and time on POMS ‘fatigue’ (F(5, 215) = 2.6, p = .024, partial η2 = .06); again, no individual time point comparisons reached significance. There were no other main effects of sex or interactive effects of sex and time.
Figure 3.
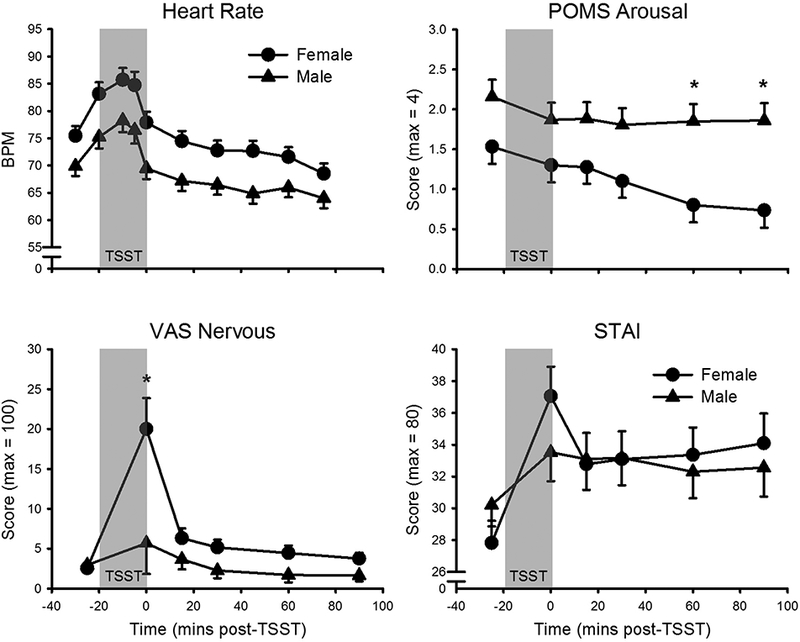
TSST responses as a function of sex. Top Left: Heart rate (female: n = 21; male: n = 22). Top Right: Profile of Mood States (POMS) ‘arousal’ (female: n = 23; male: n = 23). Bottom Left: Visual Analogue Scale (VAS) ‘nervous’ (female: n = 20; male: n = 20). Bottom Right: State Trait Anxiety Inventory (STAI) state score (female: n = 22; male: n = 23). TSST (introduction + speech + mental arithmetic) appears as gray-filled region. Data presented are means and standard errors. There were main effects of sex on heart rate and POMS ‘arousal’. There were interactive effects of sex and time on POMS ‘arousal’, VAS ‘nervous’, and STAI state. Data presented are means and standard errors. *p < .01.
3.5. Stress responding as a function of the interaction between trauma and sex
Among the men and women matched for cigarette and cannabis use, there was interaction effect between trauma exposure, sex, and time on HR (F(9, 324) = 5.5, p < .001, partial η2 = .13). Follow-up analysis revealed an interaction between trauma status and time in females (F(9, 144) = 2.8, p = .004, partial η2 = .15), but not in males, with no main effects of trauma in either sex. The interactive effect in females appears due to increased HR stress responding among females who endorsed trauma relative to those with no trauma history (Figure 4); however, differences between groups did not reach significance at any individual time points, potentially due to the small sample of females when split into two groups (no trauma: n = 8; trauma: n = 10 due to missing data). There were no other interactive effects of sex and trauma exposure.
Figure 4.
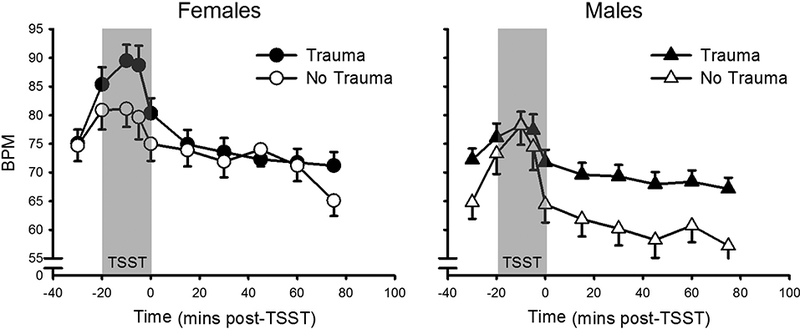
Heart rate response to the TSST as a function of trauma and sex. Left: Females (no trauma: n = 8; trauma: n = 10). Right: Males (no trauma: n = 7; trauma: n = 15). TSST (introduction + speech + mental arithmetic) appears as gray-filled region. Data presented are means and standard errors. There was an interaction of trauma and time in females but not males.
3.6. Stress responding as a function of cannabis relapse
There was a main effect of relapse (yes/no) on VAS ‘stressed’ (F(1, 47) = 4.2, p = .047, partial η2 =.08). Participants who relapsed reported lower stress overall, with no differential stress responding (i.e., no interaction between relapse status and time; Figure 5). There was also a main effect on ‘upset’ (F(1, 46) = 4.1, p = .048, partial η2 = .08), such that participants who relapsed were less upset overall, without differences in stress responding. There were no other main effects of relapse status or interactions between relapse status and time. Correlational analyses showed no relationships between the number of puffs smoked and AUCs across stress variables.
Figure 5.
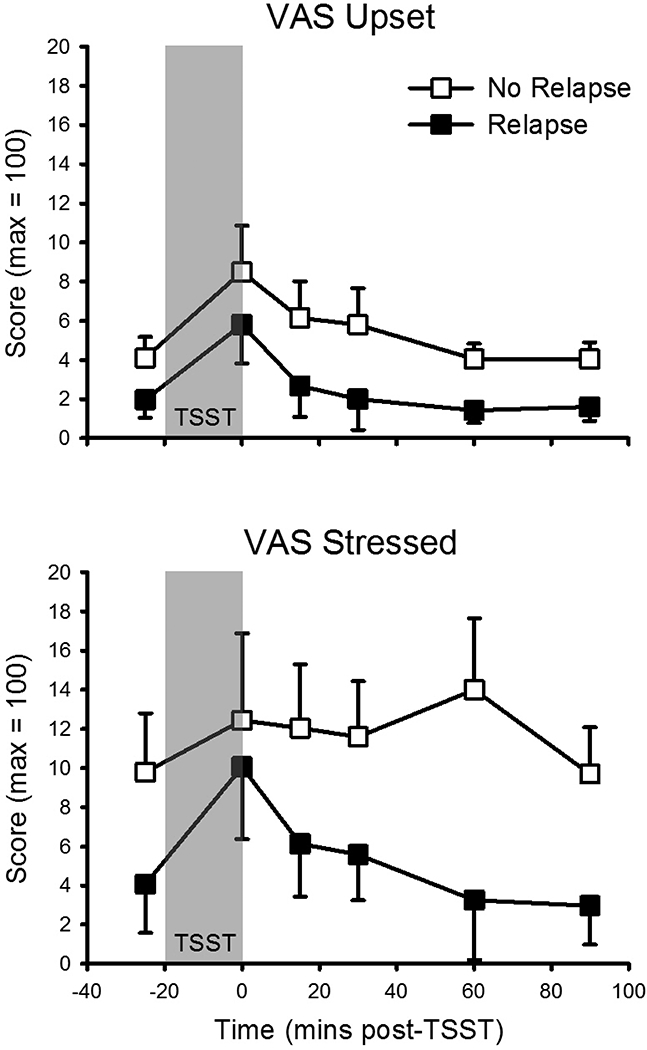
TSST responses as a function of relapse to cannabis use in a laboratory model of cannabis withdrawal and relapse. Top: Visual Analogue Scale (VAS) ‘upset’ (no relapse: n = 20; relapse: n = 28). Bottom: VAS ‘stressed’ (no relapse: n = 20; relapse: n = 29). TSST (introduction + speech + mental arithmetic) appears as gray-filled region. Data presented are means and standard errors. There were main effects of relapse group, with no differential stress responding.
4. Discussion
We examined (1) acute stress responding in cannabis smokers as a function of trauma exposure, sex, and their interaction, and (2) the relationship between stress responding and relapse to cannabis use as measured in the laboratory. Trauma-exposed cannabis smokers had higher overall salivary cortisol and negative affect than non-exposed smokers, with no differential stress response. Although individuals reporting any trauma did not respond differently to the stressor compared to those without trauma (i.e., a binary measure), we did observe small-medium positive correlations between the number of traumatic exposures and global measurements (AUCs) of cortisol and negative mood responses. Comparing females with males matched for cannabis and cigarette use, we observed differential subjective stress responses, with females, but not males, reporting nervousness after the TSST. Moreover, there was evidence that HR stress responses varied as a function of trauma in females, but not males, an effect that was due to heightened HR stress responding in trauma-exposed females. Finally, there was some indication that, during the TSST, participants who subsequently relapsed to cannabis had lower overall distress relative to those who did not go on to relapse, with no differences in stress responding. These last findings should be interpreted cautiously, given the marginal significance (p = 0.047/0.048) and small effect size (partial η2 = .08).
Previous studies in non-drug users showed that women report more negative emotions following stress than men (Kelly et al., 2008; Kirschbaum et al., 1999). Similarly, we found that female cannabis smokers reported nervousness levels more than 3x those of males after the TSST. While research in healthy subjects indicates greater cortisol reactivity in men and more pronounced HR responses in women (Kirschbaum et al., 1999; Kudielka et al., 2004), we found no sex differences in cardiovascular or cortisol stress responding. Dampened cortisol responses to cannabis administration have been documented among frequent, but not occasional, cannabis users (D’Souza et al., 2008), suggesting that physiological stress responding may be blunted in regular cannabis users, as appears to be the case in some other drug users (Adinoff et al., 2005; al’Absi et al., 2005; Lovallo et al., 2000; Tsuda et al., 1996). Such blunting may have masked sex differences in physiological stress reactivity in these cannabis users.
Trauma exposure also alters stress response systems, potentially leading to increased risk for developing substance use disorders (Heim and Nemeroff, 2001; Mullen et al., 1996). In females, past trauma has been linked to heightened autonomic response to social stress (Heim et al., 2000). This was also apparent in these female cannabis smokers, with an indication of heightened HR stress reactivity in those endorsing trauma, an effect not observed in males. Prior research indicates that trauma exposure in females is linked to earlier initiation (Werner et al., 2016b) and greater severity of cannabis use (Lipschitz et al., 2000; Vetter et al., 2008). Individuals who show hypersensitivity to stress may be motivated to use cannabis for coping (Hyman and Sinha, 2009). Thus, female cannabis smokers with trauma exposure may be an important population for future investigation (i.e., examination of whether heightened stress reactivity mediates relationships between trauma exposure and onset and severity of cannabis use in women).
Observed correlations between the number of traumas and global measurements (AUC) of physiological and emotional stress responding suggest compounding effects of multiple traumatic experiences on stress system functioning, regardless of sex. While lifetime exposure to traumatic events is linked to anxiety, depression, and posttraumatic stress disorder (PTSD;(Suliman et al., 2009; Vrana and Lauterbach, 1994), to our knowledge, this is the first time trauma exposure in non-PTSD adults has been associated with acute social stress responding. Moreover, we observed these relationships in a homogenous sample of cannabis smokers with no other acute psychiatric disorders. Thus, trauma exposure may have clinically-relevant implications in cannabis users, even in the absence of the psychopathology that can follow traumatic experiences.
Contrary to our hypotheses, we did not observe increased stress responding in those who subsequently relapsed in the laboratory. Thus, these data do not support a role for acute stress responding in the relationship between trauma exposure and clinical outcomes in cannabis smokers. However, relapse occurs after withdrawal, and our participants were not in withdrawal during the stressor. It is possible that stress responding during withdrawal might be predictive of relapse. A further clinically-important direction for research would thus be to explore the acute effects of stress during withdrawal on relapse to cannabis use, given evidence for increased stress-potentiated consumption of alcohol in alcohol dependent participants (Thomas et al., 2011). Moreover, participants were non-treatment seekers and our laboratory relapse model has not been validated as a predictor of relapse in clinical populations, although it is affected by many of the same factors that alter cannabis use in the natural ecology (Haney, 2009; Haney and Spealman, 2008).
This study has some limitations. First, because we aimed to assess stress responding in cannabis smokers’ ‘normal’ state, participants were not asked to abstain before the stressor. Thus, these data cannot speak to the effects of cannabis intoxication or withdrawal on stress responding. Moreover, although participants remained in the laboratory for approximately an hour prior to the TSST and no participant displayed overt signs of intoxication, we cannot fully rule out the possibility that individual participants may have been experiencing some residual intoxication from cannabis smoked prior to their arrival at the laboratory. Stress responding in regular cannabis smokers in relation to acute and residual intoxication is an important area for further research. Second, to enable recruitment of a large sample who completed both the TSST and the laboratory relapse model, relapse data were taken from different study protocols, which varied somewhat. Although these variations were small, it is theoretically possible that this may have increased non-systematic variability, potentially reducing signal detection capacity in the relapse analyses. Third, as an initial study, we did not comprehensively assess trauma exposure details such as age of onset and duration. This also would be expected to add variability to the data, such that the actual effects of trauma (more comprehensively assessed) on stress reactivity in cannabis smokers may be greater than those observed. Further, there is evidence that different types of stress and trauma exposure may differentially affect women and men in relation to drug use (McKee et al., 2003; Oberleitner et al., 2015; Smith et al., 2016). Thus, trauma effects on stress responding in cannabis smokers as a function of different types of trauma and the developmental period in which trauma occurred represent an important direction for future research. Fourth, the majority (94%) of the cannabis smokers included in these analyses were also cigarette smokers, largely because two of five studies from which data were taken were focused on comorbid cigarette and cannabis users. Thus, findings may be most relevant to the approximately 50% of regular cannabis smokers who also smoke cigarettes, who appear to represent a particularly clinically-relevant group of cannabis smokers to study (Haney et al., 2013a). Finally, our results were obtained in a sample of non-treatment-seeking daily cannabis smokers without psychiatric or physiological illness, which may limit the generalizability of the findings to the broader population of cannabis smokers.
Limitations notwithstanding, these data suggest that variability in responding to acute social stress as a function of sex, trauma exposure and the interaction between them can be detected, even in a homogenous sample of healthy cannabis smokers. Although we did not identify a relationship between acute stress responding and cannabis relapse in the laboratory, results indicate that further investigation of the impact of trauma exposure in male and female cannabis smokers, and how this relates to different aspects of cannabis use, is warranted.
Highlights.
Stress responding did not differ with trauma exposure (yes/no) in cannabis smokers.
Total traumas correlated positively with cortisol and anxiety during stress.
Female cannabis smokers reported more nervousness after stress than did males.
Stress responding did not predict cannabis relapse in the human laboratory.
Acknowledgements
The authors would like to thank participants for completing the study.
Role of Funding Source
This work was supported by the National Institute on Drug Abuse (Haney: DA19239, DA09236, DA031005; Hien: DA035161; Bedi: DA034877). The funding source had no role in the study design, data collection, analysis or interpretation, writing of the report, or in the decision to submit the article for publication.
Footnotes
Contributors
TC conducted all data analysis and drafted the abstract, introduction, results and discussion. VR wrote the methods section and contributed to revising the overall manuscript. DH contributed to the data analysis plan and to revising the manuscript for important intellectual content. GB supervised TC in the data analysis and drafting of the manuscript, and revised the manuscript for important intellectual content. MH designed the study and oversaw all data collection. She contributed to the data analytic plan and to revising the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Conflict of Interest
The authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S, 2005. Suppression of the HPA axis stress-response: Implications for relapse. Alcohol Clin. Exp. Res 29, 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, 2005. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacol. (Berl.) 181, 107–117. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B, 2003. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol. Biochem. Behav 74, 401–410. [DOI] [PubMed] [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF, 1978. The effect of housing and gender on morphine self-administration in rats. Psychopharmacol. (Berl.) 58, 175–179. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, 2014. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev 38, 94–124. [DOI] [PubMed] [Google Scholar]
- APA, 1994. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association: MD, US. [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT, 2010. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 106, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK, 2006. Cold pressor task reactivity: Predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin. Exp. Res 30, 938–946. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M, 2011. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol. Ther 129, 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Silgado J, Schmidt NB, 2011. Marijuana craving during a public speaking challenge: Understanding marijuana use vulnerability among women and those with social anxiety disorder. J. Behav. Ther. Exp. Psychiatry 42, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Ecker AH, Jeffries ER, 2016. Cannabis craving in response to laboratory-induced social stress among racially diverse cannabis users: The impact of social anxiety disorder. J. Psychopharmacol 30, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH, 2007. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 62, 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H, 2009. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacol. (Berl.) 203, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Dlugos A, de Wit H, 2010. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiol. 47, 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Lutz JA, de Wit H, 2017. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend. 177, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Han B, Jones CM, Blanco C, Hughes A, 2016. Marijuana use and use disorders in adults in the USA, 2002–14: Analysis of annual cross-sectional surveys. Lancet Psychiatry 3, 954–964. [DOI] [PubMed] [Google Scholar]
- Cusack KJ, Frueh BC, Brady KT, 2004. Trauma history screening in a community mental health center. Psychiatr. Serv 55, 157–162. [DOI] [PubMed] [Google Scholar]
- Cuttler C, Mischley LK, Sexton M, 2016. Sex differences in cannabis use and effects: A cross-sectional survey of cannabis users. Cannabis Cannabinoid Res. 1, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, Spradlin A, Nusbaum AT, Whitney P, Hinson JM, McLaughlin RJ, 2017. Blunted stress reactivity in chronic cannabis users. Psychopharmacol. (Berl.) 234, 2299–2309. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J, 2008. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacol. 33, 2505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Soderpalm AH, Nikolayev L, Young E, 2003. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin. Exp. Res 27, 1270–1277. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R, 1973. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol. Med 3, 479–486. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD, 1987. Marijuana and cocaine interactions in humans: Cardiovascular consequences. Pharmacol. Biochem. Behav 28, 459–464. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R, 2007. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin. Exp. Res 31, 395–403. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R, 2008. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacol. 33, 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R, 2005. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 30, 880–891. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF, 1994. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacol. (Berl.) 114, 63–70. [DOI] [PubMed] [Google Scholar]
- Gravetter FJ, Wallnau LB, 2004. Statistics for the Behavioral Sciences. Wadsworth/Thomson Learning, Belmont, CA. [Google Scholar]
- Gutman DA, Nemeroff CB, 2003. Persistent central nervous system effects of an adverse early environment: Clinical and preclinical studies. Physiol. Behav 79, 471–478. [DOI] [PubMed] [Google Scholar]
- Hall W, Lynskey M, 2016. Evaluating the public health impacts of legalizing recreational cannabis use in the United States. Addiction 111, 1764–1773. [DOI] [PubMed] [Google Scholar]
- Haney M, 2009. Self-administration of cocaine, cannabis and heroin in the human laboratory: Benefits and pitfalls. Addict. Biol 14, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW, 2013a. Predictors of marijuana relapse in the human laboratory: Robust impact of tobacco cigarette smoking status. Biol. Psychiatry 73, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW, 2013b. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacol. 38, 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW, 2008. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacol. (Berl.) 197, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV, 1995. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 698, 46–52. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R, 2008. Controversies in translational research: Drug self-administration. Psychopharmacol. (Berl.) 199, 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB, 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB, 2000. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284, 592–597. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB, 2008. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 33, 693–710. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, Foltin RW, Haney M, 2016. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacol. (Berl.) 233, 2469–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Sinha R, 2009. Stress-Related Factors in Cannabis Use and Misuse: Implications for Prevention and Treatment. J. Subst. Abuse. Treat 36, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusek LW, Tell D, Gaylord-Harden N, Mathews HL, 2017. Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: An epigenetic link. Brain Behav. Immun 60, 126–135. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI, 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL, 2008. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J. Behav. Ther. Exp. Psychiatry 39, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH, 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med 61, 154–162. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol. 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C, 2004. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: The impact of age and gender. Int. J. Behav. Med 11, 116–121. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: A review. Biol. Psychol 69, 113–132. [DOI] [PubMed] [Google Scholar]
- Lepore SJ, Allen KA, Evans GW, 1993. Social support lowers cardiovascular reactivity to an acute stressor. Psychosom. Med 55, 518–524. [DOI] [PubMed] [Google Scholar]
- Lipschitz DS, Rasmusson AM, Anyan W, Cromwell P, Southwick SM, 2000. Clinical and functional correlates of posttraumatic stress disorder in urban adolescent girls at a primary care clinic. J. Am. Acad. Child Adolesc. Psychiatry 39, 1104–1111. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ, 2000. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin. Exp. Res 24, 651–658. [PubMed] [Google Scholar]
- Magrys SA, Olmstead MC, 2015. Acute stress increases voluntary consumption of alcohol in undergraduates. Alcohol Alcohol. 50, 213–218. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Brooks D, Haney M, Levin FR, 2011. Quantification and comparison of marijuana smoking practices: Blunts, joints, and pipes. Drug Alcohol Depend. 113, 249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF, 2001. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychol. 20, 403–410. [PubMed] [Google Scholar]
- McGrath E, Jones A, Field M, 2016. Acute stress increases ad-libitum alcohol consumption in heavy drinkers, but not through impaired inhibitory control. Psychopharmacol. (Berl.) 233, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM, 2003. Sex differences in the effects of stressful life events on changes in smoking status. Addiction 98, 847–855. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, Koenen KC, 2010. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depress. Anxiety 27, 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DLM, Droppleman L, 1971. Profile of Mood States. Educational and Industrial Testing Service, San Diego. [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT, 2011. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacol. (Berl.) 218, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, 1996. Activational effects of social stress on IV cocaine self administration in rats. Psychopharmacol. (Berl.) 128, 256–264. [DOI] [PubMed] [Google Scholar]
- Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP, 1996. The long-term impact of the physical, emotional, and sexual abuse of children: A community study. Child Abuse Negl. 20, 7–21. [DOI] [PubMed] [Google Scholar]
- Nemeroff CC, 2004. Early-life adversity, CRF dysregulation, and vulnerability to mood and anxiety disorders. Psychopharmacol. Bull 38, 14–20. [PubMed] [Google Scholar]
- Oberleitner LM, Smith PH, Weinberger AH, Mazure CM, McKee SA, 2015. Impact of exposure to childhood maltreatment on transitions to alcohol dependence in women and men. Child maltreat. 20, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S, Luna B, 2012. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology 37, 1135–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA, 1990. Interaction of ethanol and stress: research with experimental animals--an update. Alcohol Alcohol. 25, 263–276. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH, 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM, 1993. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain Res. 608, 216–222. [DOI] [PubMed] [Google Scholar]
- Resnick H, Falsetti S, Kilpatrick D, Freedy J, 1996. Assessment of rape and other civilian trauma-related post-traumatic stress disorder: Emphasis on assessment of potentially traumatic events In Stressful Life Events (ed. Miller T). International Universities Press, Madison. [Google Scholar]
- Shaham Y, Alvares K, Nespor SM, Grunberg NE, 1992. Effect of stress on oral morphine and fentanyl self-administration in rats. Pharmacol. Biochem. Behav 41, 615–619. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Klein LC, Alvares K, Grunberg NE, 1993. Effect of stress on oral fentanyl consumption in rats in an operant self-administration paradigm. Pharmacol. Biochem. Behav 46, 315–322. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J, 1995. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacol. (Berl.) 119, 334–341. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M, 2005. Child maltreatment and HPA axis dysrégulation: Relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology 30, 162–178. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2012. How does stress lead to risk of alcohol relapse? Alcohol Res. 34, 432–440. [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S, 1999. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacol. (Berl.) 142, 343–351. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin L-R, O’Malley SS, 2000. Psychological stress, drug-related cues and cocaine craving. Psychopharmacol. (Berl.) 152, 140–148. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ, 2006. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry 63, 324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ, 2003. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacol. (Berl.) 170, 62–72. [DOI] [PubMed] [Google Scholar]
- Smith BC, Armelie AP, Boarts JM, Brazil M, Delahanty DL, 2016. PTSD, depression, and substance use in relation to suicidality risk among traumatized minority lesbian, gay, and bisexual youth. Arch. Suicide Res. 20, 80–93. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G, 1983. Manual for the State-Trait Anxiety Inventory Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Suliman S, Mkabile SG, Fincham DS, Ahmed R, Stein DJ, Seedat S, 2009. Cumulative effect of multiple trauma on symptoms of posttraumatic stress disorder, anxiety, and depression in adolescents. Compr. Psychiatry 50, 121–127. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, 2013. Using Multivariate Statistics. Pearson, Boston. [Google Scholar]
- Temple EC, Driver M, Brown RF, 2014. Cannabis use and anxiety: Is stress the missing piece of the puzzle? Front. Psychiatry 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE, 2011. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacol. (Berl.) 218, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C, 1996. Cigarette smoking and psychophysiological stress responsiveness: Effects of recent smoking and temporary abstinence. Psychopharmacol. (Berl.) 126, 226–233. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin P-I, Wand GS, 2006. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology 31, 642–652. [DOI] [PubMed] [Google Scholar]
- UNODC, 2016. World Drug Report 2016. United Nations: New York. [Google Scholar]
- van Leeuwen AP, Creemers HE, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC, 2011. Hypothalamic-pituitary-adrenal axis reactivity to social stress and adolescent cannabis use: The TRAILS study. Addiction 106, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Vetter S, Rossegger A, Rossler W, Bisson JI, Endrass J, 2008. Exposure to the tsunami disaster, PTSD symptoms and increased substance use - an Internet based survey of male and female residents of Switzerland. BMC Public Health 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N, 2002. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J. Neurosci 22, 4654–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana S, Lauterbach D, 1994. Prevalence of traumatic events and post-traumatic psychological symptoms in a nonclinical sample of college students. J. Trauma Stress 7, 289–302. [DOI] [PubMed] [Google Scholar]
- Werner KB, McCutcheon VV, Agrawal A, Sartor CE, Nelson EC, Heath AC, Bucholz KK, 2016a. The association of specific traumatic experiences with cannabis initiation and transition to problem use: Differences between African-American and European-American women. Drug Alcohol Depend. 162, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner KB, Sartor CE, McCutcheon VV, Grant JD, Nelson EC, Heath AC, Bucholz KK, 2016b. Association of specific traumatic experiences with alcohol initiation and transitions to problem use in European American and African American women. Alcohol Clin. Exp. Res 40, 2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]


