Abstract
The cholinergic system plays a central role in regulating critical gastrointestinal functions, including motility, secretion, barrier and immune function. In rodent models of acute, non-infectious gastrointestinal injury, the cholinergic system functions to inhibit inflammation; however, during inflammation local expression and regulation of the cholinergic system is not well known, particularly during infectious enteritis. The objective of this study was to determine the intrinsic expression of the enteric cholinergic system in pig ileum following an acute challenge with Salmonella enterica serovar Typhimurium DT104 (S. Typhimurium). At 2 d post-challenge, a three-fold reduction in ileal acetylcholine (ACh) levels was observed in challenged animals, compared with controls. Ileal acetylcholinesterase (AChE) activity was decreased (by four-fold) while choline acetyltransferase (ChAT) expression was increased in both the ileum and mesenteric lymph nodes. Elevated ChAT found to localize preferentially to mucosa overlying lymphoid follicles of the Peyers patch in challenged pigs, with more intense labeling for ChAT in S. Typhimurium challenged pigs compared to controls. Ileal mRNA gene expression of muscarinic receptor 1 and 3 was also increased in challenged pigs, while muscarinic receptor 2 and the nicotinic receptor alpha 7 subunit gene expression were unaffected. A positive correlation was observed between ChAT protein expression in the ileum, rectal temperature, and histopathological severity in challenged animals. These data show that inflammation from S. Typhimurium challenge alters enteric cholinergic expression by down-regulating acetylcholine concentration and acetylcholine degrading enzymes while increasing acetylcholine synthesis proteins and receptors. Given the known anti-inflammatory role of the cholinergic system, the divergent expression of cholinergic genes may represent an attempt to limit tissue damage by preserving cholinergic signaling in the face of low ligand availability.
Keywords: Cholinergic, Mucosal Inflammation, Salmonella enterica Typhimurium, porcine intestine
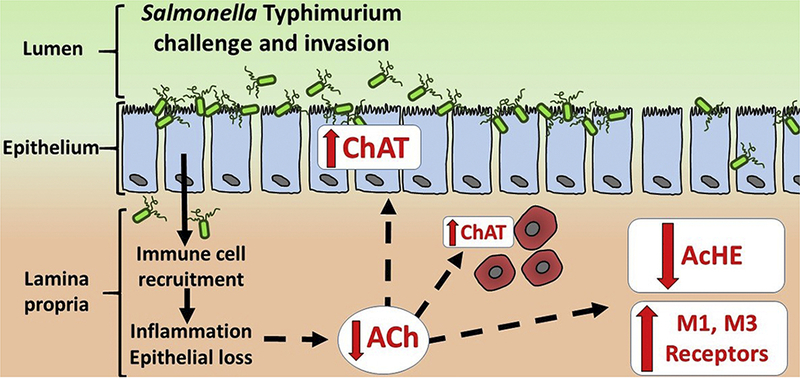
Graphical Abstract. Alterations in the enteric cholinergic system induced by Salmonella Typhimurium challenge in pigs.
Salmonella Typhimurium challenge and invasion triggers immune cell recruitment, inflammation and mucosal injury including epithelial cell loss. Subsequent reduction in acetylcholine (ACh) concentrations in the mucosa may induce a compensatory response to preserve and (or) amplify cholinergic signaling by (1) upregulating ACh synthesis via choline acetyltransferase (ChAT) in epithelial cells, lamina propria monocytes and enteric neurons (not shown) (2) down-regulating ACh degradation via suppression of acetylcholinesterase (AChE), and (3) enhancing of ACh signaling via increased expression of muscarinic M1 and M3 cholinergic receptors. Dotted lines indicate hypothesized mechanisms by which mucosal damage and inflammation may drive altered cholinergic expression.
1. Introduction
To survive infectious challenges, the host must balance pathogen inducing immunity and inflammation with responses that limit tissue damage. Therapeutic interest focuses on pathways that simultaneously limit tissue damage without comprising sterilizing immunity (Schneider and Ayres 2008; Soares et al. 2014). Due to the intimate juxtaposition of microbes and host tissue, the gastrointestinal tract is an area requiring precise balance between immunity and host tissue survival, and the cholinergic system is considered to be an important factor mediating this balancing act during enteritis (Gabanyi et al. 2016).
Cholinergic signaling via the vagus nerve is a well-established mediator of inflammation in the gastrointestinal tract (Matteoli and Boeckxstaens 2013). Several groups have shown that cholinergic function, mostly through action of the vagus nerve and nicotinic receptors, reduces gastrointestinal inflammation and prevents mucosal tissue damage in rodent models of chemical colitis (Ghia et al. 2006; Ghia et al. 2008; Mazelin et al. 1998; O’Mahony et al. 2009), endotoxemia (de Haan et al. 2013), and postoperative ileus (Matteoli et al. 2014; The et al. 2011). Further anti-inflammatory influence of the cholinergic vagal system is known by its role in promoting oral tolerance to foreign antigens (Di Giovangiulio et al. 2016).
The functional role of the cholinergic system in infectious enteritis is less well described; however, initial reports suggest that in contrast to the chemical colitis and postoperative ileus models, cholinergic signaling may enhance immunity by promoting inflammation. For example, pretreating mice with an acetylcholinesterase inhibitor prior to oral Salmonella infection increased serum inflammatory cytokine production, bacterial clearance, and host survival (Fernandez-Cabezudo et al. 2010). Another report demonstrated that cholinergic muscarinic receptors enhance T-cell pro-inflammatory activity and contribute to rapid convalescence and generation of adaptive immunity against both bacterial and parasitic infection (Darby et al. 2015). However, certain bacterial and parasitic, infectious enteritis models were also shown to result in suppressed cholinergic enteric nerve activity and attenuated release of Ach (Bercik et al. 2002; Collins et al. 1989; Galeazzi et al. 2000). Together, previous studies in rodents indicate that the cholinergic system has significant, yet divergent actions during mucosal inflammation which may depend upon the inciting stimuli.
The cholinergic system has several components that regulate the synthesis, degradation and signaling of Ach. Acetylcholine (ACh), the primary endogenous ligand inducing cholinergic signaling can be synthesized in both neuronal and non-neuronal cells. Choline acetyltransferase (ChAT), is the primary enzyme that generates acetylcholine, and it can be found in epithelia (Gareau et al. 2007), immune (Dhawan et al. 2016; Kawashima et al. 2007; Reardon et al. 2013), and neuronal cells (Vieira et al. 2017; Winston et al. 2013). Once acetylcholine is liberated into the extracellular space, it acts on two classes of cholinergic receptors, known as muscarinic and nicotinic receptors. Muscarinic receptors are g-coupled protein receptors that have up to 5 different sub-classes (Kruse et al. 2014). Nicotinic receptors are pentameric, ionotropic receptors that are formed by heterogeneous or homogenous assembly of one of several different nicotinic subunits (Albuquerque et al. 2009). Both types of receptors can be found on several different cells, mediating vastly different homeostatic functions. Finally, cholinergic signaling is terminated primarily by acetylcholinesterase (AChE), an enzyme the breaks down ACh at high efficiency and is expressed in many different cell types. While the functions of each component of the cholinergic system is well-established, how each component is dynamically during infectious challenge remain poorly defined. Considering the significant contribution of acetylcholine to gut homeostasis, understanding the expression of the cholinergic system during inflammatory challenges will provide a foundational understanding for future research and therapies. Therefore, In the present study characterized the changes in expression of the enteric mucosal cholinergic system components in pigs acutely challenged with S. Typhimurium and how these changes are correlated with tissue damage and mucosal inflammatory cytokine production.
Materials and Methods
2.1. Animals and experimental design
Data was generated from tissues which were collected in a previously reported experiment (Boyer et al. 2015), which was under an approved Institutional Animal care and Use Committee at North Carolina State University (protocol no. 12–051-A). As reported previously, animals used were Yorkshire-Large White piglets weaned at 16–17 days of age and housed at 8 pigs per pen with ad libitum access to water and feed. Sex was distributed equally across weaning groups. At 34 days post weaning, all piglets were transferred to isolation rooms and housed by treatment groups with continued ab libitum access to food and water. S. Typhimurium challenged pigs were orally inoculated with 3×109 colony forming units in 4mls of culture media; while uninfected controls were feed 4mls of sterile culture media. Challenged pigs were housed in separate rooms from the unchallenged controls; however the housing and environmental conditions were identical between rooms. S. Typhimurium DT104 strain culture was grown overnight at 37°C in Luria broth agar and added to 0.7% sterile saline for form final concentrations of 7.5×108 colony forming unites per mL (Boyer et al. 2015). Animals were euthanized 2 days post pathogen challenge, and collection of ileum mucosal scrapes and lymph nodes were performed and stored as previously reported (Boyer et al. 2015). The post-challenge time point (48 h) was selected to coincide with the peak acute phase responses (rectal temperature, feed intake reduction, plasma cortisol, etc.) to oral S. Typhimurium challenge in pigs as reported previously (Balaji et al. 2000).
2.2. Ileum and mesenteric lymph node protein Isolation
For SDS-PAGE and Western blot, 0.5cm3 pieces of ileal mucosa scrapes and mesenteric lymph node were collected over dry ice and homogenized in RIPA buffer (Thermo Scientific, #89900) in the presence of 1x protease inhibitor cocktail (Sigma Aldrich, #P8340) and 1x Halt Phosphatase Inhibitor (Thermo Scientific, #78420). Samples were spun at 13,300rpm at 4°C for 15min. Supernatant was collected, aliquoted and frozen at −70°C. Protein concentration was determine with Pierce BCA kit (Thermo Scientific, #23225), and samples were diluted to working a concentration of 2μg/μl.
For TNF and IL-8 ELISA and myeloperoxidase assay, samples were isolated as previously reported (Boyer et al. 2015).
2.3. SDS PAGE | Western Blot
Ileal mucosa and mesenteric lymph node protein samples were diluted to 1μg/μL in Laemmli Buffer (Bio-Rad, #161–0737) + 5% 2-mercaptoethanol and heat denatured at 70°C for 10 minutes. 10 μg of protein sample was run on a TGX-Stain Free gel (Bio-Rad #5678095). Protocol for electrophoresis, wet to wet transfer, and stain free, lane total protein quantification was performed as published in Criterion™ Precast Gels: Instruction Manual and Application Guide and Western Blot Normalization Using Image Lab™ Software (Bio-Rad). The PVDF membrane was blocked in 5% BSA at RT for 1hr prior to incubation with monoclonal 1.B3.9B3 anti- porcine ChAT antibody (Millipore Sigma #MAB5270) at a concentration of 1:1000 in 1xTBS + 5% BSA + 0.1% Tween-20 overnight at 4°C. The following morning, the blot was washed and an HRP linked anti-mouse antibody in 1xTBS + 5% BSA + 0.1% Tween-20 (Cell Signaling, #7076) at (1:1000) was incubated with the membrane for 1hr at RT. Chemiluminescence was performed using Clarity ECL (Bio-Rad, #1705060). Densitometry was performed utilizing Bio-Rad Image Lab™ Software v5.2.1 and band density was normalized to lane total protein per Western Blot Normalization Using Image Lab™ Software (Bio-Rad) protocols. Total protein image for corresponding lanes in Figure 1 are in Supplemental Figure 1.
Figure 1. Impact of S. Typhimurium challenge on acetylcholine and cholinergic enzymes in ileum mucosa.
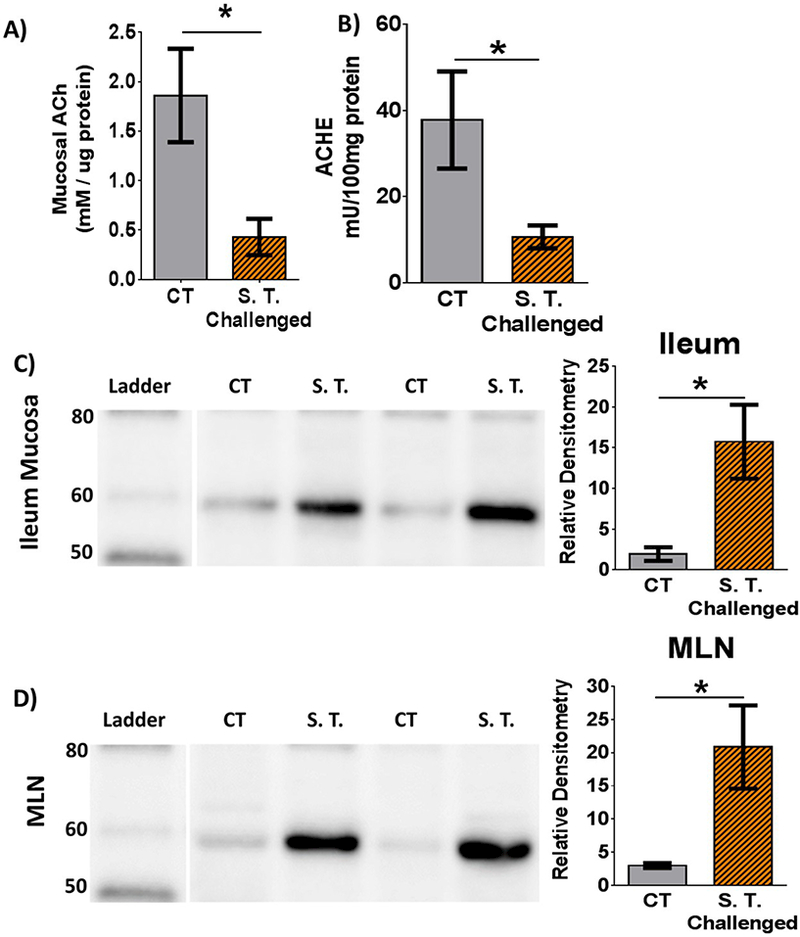
A) Acetylcholine concentration in ileal mucosa. B) Acetylcholinesterase activity ileal mucosa. C) Western blot of ileal mucosa for porcine ChAT. Prominent band is between 60 and 50kDa. Histogram quantifying relative density directly to the right. D) Western blot of mesenteric lymph node (MLN) for porcine ChAT. Respective histograms represent relative density normalized to lane total protein. CT = Control, S.T. = S. Typhimurium. Bars and SEM represented. n = 5 controls and 6 challenged per bar. Student’s t-test compared controls vs S. Typhimurium challenge. * = p<0.05.
2.4. Acetylcholine quantification and acetylcholinesterase activity
Acetylcholine and acetylcholinesterase activity was performed on protein isolated from ileum mucosal scrapes using Amplex™ Acetylcholine/Acetlycholinesterase Assay Kit (ThermoFisher Scientific cat#A12217) as per manufacturer’s instructions.
2.5. Gene Expression Analysis
Total RNA samples were isolated from frozen intestinal mucosal scrapings using the Qiagen RNeasy Mini kit. First-strand cDNA was synthesized from 4 μg RNA using Thermo Scientific Maxima First Strand cDNA Synthesis Kit for RT-qPCR with dsDNase (Thermo Scientific, K1641) according to the manufacturer’s instructions. Semi-quantitative, real-time PCR was used to determine the relative quantities of transcripts of the genes of interest. Beta-actin (ACTB) was selected and validated as suitable internal reference genes. The relative gene expressions of cholinergic receptor muscarinic 1 (CHRM1), 2 (CHRM2), and 3 (CHRM3), cholinergic receptor nicotinic alpha 7 subunit (CHRNA7) were determined. Primer sequences for all genes are provided in Supplemental Table 1. All PCR reactions were subjected to a melt curve analysis to validate the absence of nonspecific products. The data are presented as 2- ΔΔCΤ in gene expression relative to control group, normalized to the ACTB before statistical analysis.
2.6. Correlation Analysis
Correlations were performed between mucosal acetylcholine concentrations, acetylcholinesterase activity, and the ChAT band identified on Western blot and rectal temperature, ileum histopathological scores, or ileum mucosal cytokine levels reported in a previous publication (Boyer et al. 2015). For methods on histopathology and mucosal cytokine analysis, please refer to our previous manuscript. (Boyer et al. 2015) Comparisons between ChAT and cytokines of interest was performed per each animal, and two tailed Pearson correlations were run on each group to identify any positive or negative association between ChAT expression and cytokine protein.
2.7. Immunohistochemistry and Image analysis
Sections of ileum were fixed in 10% neutral buffered formalin and paraffin embedded. Sections were prepped and immunohistochemically labeled for ChAT with B3.9B3 anti-porcine ChAT antibody (Millipore Sigma #MAB5270) at 1:100. Detection of ChAT in section was performed by using secondary anti-mouse-on-Farma HRP polymer for 30min at RT and treatment with Romulin AEC. Slides were counter stained with hematoxylin at 1:10. All sample preparation and labeling was performed by Michigan State University’s Investigative Histopathology Laboratory. Total mucosal area of ChAT positive labelling and integrated density of ChAT labelling were determined to generally assess the number of cells positive to ChAT and the intensity with which ChAT was expressed, respectively. Using ImageJ (U.S. NIH, Bethesda, MD, USA), the total area and integrated density of ChAT expression was determined by using the threshold function on the blue stack for each RGB image. Using the threshold function, we were able to assess the area and integrated density of the ChAT labeling alone, independent of the hematoxylin counter stain. The threshold was set from 0–61 and was consistently used on each image assess. The percentage area of ChAT positive staining was determined by dividing the area of ChAT positive labeling by the total area of the mucosa in the field. A total of 10 different tissue sections per slide were analyzed from each slide/animal and then average to derive the mean for individual animal.
2.8. Statistics
Two-tailed student’s t-test were performed on most data comparing unchallenged controls to S. Typhimurium challenged animals. Unless reported otherwise, all values reported are means and standard error of means. Two tailed student t-tests and Pearson correlations and figures were generated with GraphPad Prism 6, v6.04 (GraphPad Prism Software).
3. Results
3.1. Summary of S. Typhimurium clinical and histopathological findings as previously reported
In Table 1, we summarize clinical and ileal histopathological features between uninfected controls and S. Typhimurium challenged animals as previously reported (Boyer et al. 2015). S. Typhimurium challenge induced significant diarrhea and pyrexia. Histopathology scores demonstrate that S. Typhimurium induced intestinal mucosal injury with moderate to severe villus blunting, mild villus fusion, and moderate lymphoid depletion in the ileum. Table 1 summarizes the clinical and histopathological effects of the S. Typhimurium challenge in this porcine model. For representative photos of histopathological findings, see Supplemental Figure 1.
Table 1. Previously Reported Clinical and Histopathological effects of S. Typhimurium.
challenged Data in this table are summarized from a previously reported study.(Boyer et al. 2015) Fecal consistency was scored on a 4 point scale by an individual blinded to the study design (1= no diarrhea; 4= severe, profuse diarrhea). Histopathology scoring was performed as previously reported. Villus blunting was scored on a 5 point scale with 0 = 1:4 crypt to villus height and 4 = no villus present. Villus fusion and lymphoid depletion were scored on a 4 point scale with 0 = normal and 3 = severe. n=5–6 per group. pvalues generated by two tailed students t-test. For significance, y=yes; n=no.
| Control | S. typhimurium challenged | P value | ||
|---|---|---|---|---|
| Fecal Score | 1.20 | 3.33 | 0.0012 | |
| Rectal Temperature(°C) | 39.70 | 40.64 | 0.0238 | |
| Histopathology Scores | Villus Blunting | 0.00 | 2.20 | 0.0172 |
| Villus Fusion | 0.00 | 1.40 | 0.0479 | |
| Lymphoid Depletion | 0.20 | 2.00 | 0.0063 | |
3.2. S. Typhimurium challenge reduced ileal acetylcholine levels.
The immune-modulatory role of the cholinergic system is mediated primarily through the ligand acetylcholine, so we asked whether bacterial enteritis would affect ileal mucosal acetylcholine concentration. Compared with controls, S. Typhimurium challenged animals exhibited a 3-fold reduction (p=0.0142) in ileal mucosal acetylcholine concentration (Fig. 1A).
3.3. S. Typhimurium challenge down-regulates ileal mucosal acetylcholinesterase activity
Since challenged animals demonstrated reduced concentrations of acetylcholine in ileal mucosa, we reasoned that AChE, the enzyme that degrades acetylcholine, might be upregulated during enteritis. In contrast to what we expected, measurements for AChE enzymatic activity in ileal mucosa demonstrated over a 2-fold reduction in challenged animals compared with controls (Fig. 1B).
3.4. S. Typhimurium induced enhanced ChAT protein expression
Since alterations in AChE did not explain the reduction in acetylcholine concentrations, we next asked if the enzyme that produces acetylcholine, ChAT, was down-regulated in S. Typhimurium-challenged pigs compared to controls. Since there are several ChAT isoforms, we utilized Western blot to determine if there was a particular isoform that was increased following infectious enteritis. Again in contrast to our expected results, in ileal mucosa, we identified a ~60 KDa band that was increased in S. Typhimurium-challenged animals compared with controls (Fig 1C). This band corresponds to a ChAT isoform known as peripheral ChAT, which has been previously reported in porcine, non-human primate, rat, and guinea pig peripheral neurons, including the enteric nervous system.(Brehmer et al. 2004; Chiocchetti et al. 2003; Koga et al. 2013; Nakajima et al. 2000; Tooyama and Kimura 2000) No difference was found in the conical 80kDa ChAT isoform, commonly associated with the central nervous system, between challenged and control pigs (data not shown).
We next screened the mesenteric lymph nodes to determine if the change in cholinergic regulation extended beyond the gastrointestinal mucosa. In the mesenteric lymph nodes, a band similar in size and intensity to the ileum was found in S. Typhimurium challenged animals compared to controls (Fig 1D). In both the ileum and mesenteric lymph nodes, the expression of ChAT was significantly increased in challenged animals compared with controls (Fig 1C-D, respectively).
3.5. Cellular source of ChAT protein upregulation in S. Typhimurium challenged ileum
To gain further insight into the mechanism contributing to upregulated ChAT expression, we investigated the localization patterns of ChAT expression in ileal mucosa of control and challenged animals via immunohistochemistry. In agreement with Western blot analysis, ileum from S. Typhimurium-challenged pigs exhibited a larger area of ChAT expression (% Mucosa ChAT, Fig A-D, p=0.06). ChAT positive cells included villus and crypt epithelia (Figure 2E, F), lamina propria mononuclear cells (F) and submucosal and myenteric ganglia (not shown). Further, the % area of ChAT positive staining and intensity of staining was most pronounced within the epithelium of the Peyer’s patch follicle associated mucosa (FAM) compared to follicle free mucosa (FFM) (Figure 2G-J).
Figure 2. ChAT is elevated in epithelium and round cells of lamina propria over the Peyer’s patch following S. Typhimurium challenge.
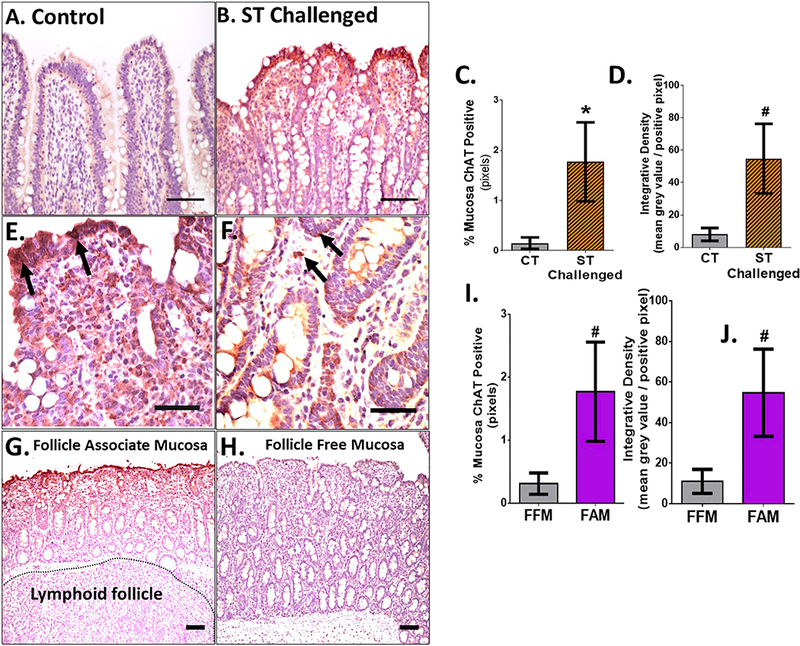
A-B: 20x images of control (A) and ST challenged (B), scale bar = 100uM. C-D: % of area of ChAT positive mucosa (C) and integrated pixel density analysis (D) between control and ST challenged pigs. E,F: 40x representative images from ileum from an S. Typhimurium challenged animal demonstrating the enhanced epithelial expression (arrows) and staining within lamina propria monocytes (E,F; arrows). G-J: 10x image of (G) follicle free mucosa (FAM) and (H) follicle associated mucosa (FAM) . In (G), note the Peyer’s patch lymphoid follicle tissue directly under the mucosa. I,J: % area ChAT positive and integrated density between (I) FFM and (J) FAM .Scale bar - 100um. CT = Control, ST = S. Typhimurium. Mann-Whitney t-test was used in (C) and (G). Students T-test used in (D) and (H). # p<0.1, * p<0.05.
3.6. Changes in cholinergic receptor gene expression following S. Typhimurium challenge.
Considering that S. Typhimurium-challenged pigs had reduced acetylcholine concentrations in ileal mucosa, we sought to determine if cholinergic receptor gene expression was altered. Cholinergic muscarinic receptors 1, 2, and 3 (CHRM1, CHRM2, and CHRM3, respectively) were selected due to their known prevalence in mediating inflammation (Hirota and McKay 2006a; Tobin et al. 2009). Utilizing real time - reverse transcription PCR with quantitation relative to control values, we determined that mRNA transcripts for CHRM1 and CHRM3 were significantly upregulated in ileal mucosa of pigs challenged with S. Typhimurium (Fig. 3). Specifically, CHRM1 expression was increased by ~ 4 fold compared with controls and CHRM3 expression was increased by ~2 fold relative to controls. There was no effect of S. Typhimurium challenge on CHRM2 expression (Fig. 3). We next measured mRNA expression of the cholinergic nicotinic alpha 7 receptor subunit (CHRNA7), which contributes to formation of the homopentameric receptor known to influence inflammatory responses (Matteoli et al. 2014; Wang et al. 2003). We found no effect of S. Typhimurium challenge on CHRNA7 expression in pig ileal mucosa (Fig. 3).
Figure 3. Impact of S. Typhimurium challenge on ileum mucosa cholinergic receptor gene expression.
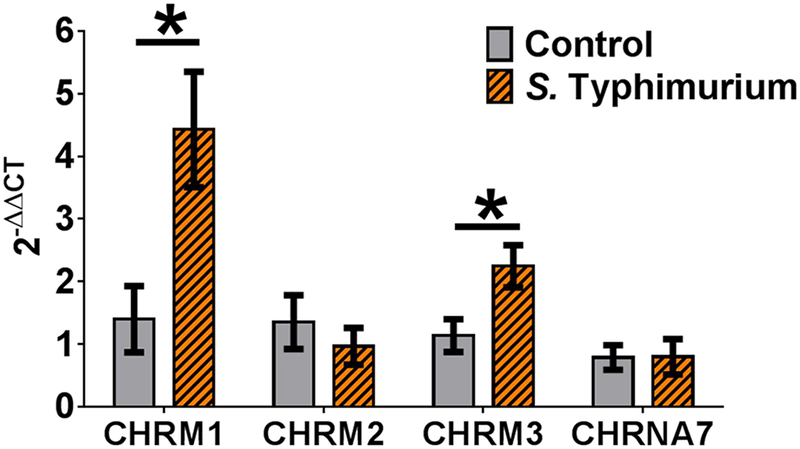
Salmonella Typhimurium enhances muscarinic receptor mRNA gene expression. mRNA gene expression was quantified utilizing two step reverse transcriptase quantitative PCR (RT-qPCR) for cholinergic muscarinic receptors 1, 2, 3 and cholinergic nicotinic receptor alpha 7 subunit (CHRM1, CHRM2, CHRM3, and CHRNA7 respectively). The data are presented as 2-ΔΔCT in gene expression relative to control group, normalized to beta-actin (ACTB) before statistical analysis. n = 6 controls and 6 challenged per bar. Student’s t-test compared controls vs S. Typhimurium challenge of each cholinergic receptor. * = p<0.05.
3.7. Correlation of mucosal ileal ChAT expression with clinical symptoms and histopathology
Since S. Typhimurium challenge influences several enzymes and receptors of the cholinergic system, we asked whether ileal mucosal ChAT expression was correlated with symptom severity and histopathological findings. Correlation analysis of ChAT expression intensity between rectal temperatures were performed separately for both controls and S. Typhimurium challenged animals. In controls, there was no correlation between ChAT expression and rectal temperature (r=0.1363, p=0.8270), (Fig 4A). However, in S. Typhimurium challenged pigs, a significant, positive correlation was found between ileum ChAT expression and rectal temperatures (r=0.8985, p=0.0149), (Fig. 4B).
Figure 4. Correlation of ChAT with body temperature and ileum histopathology.
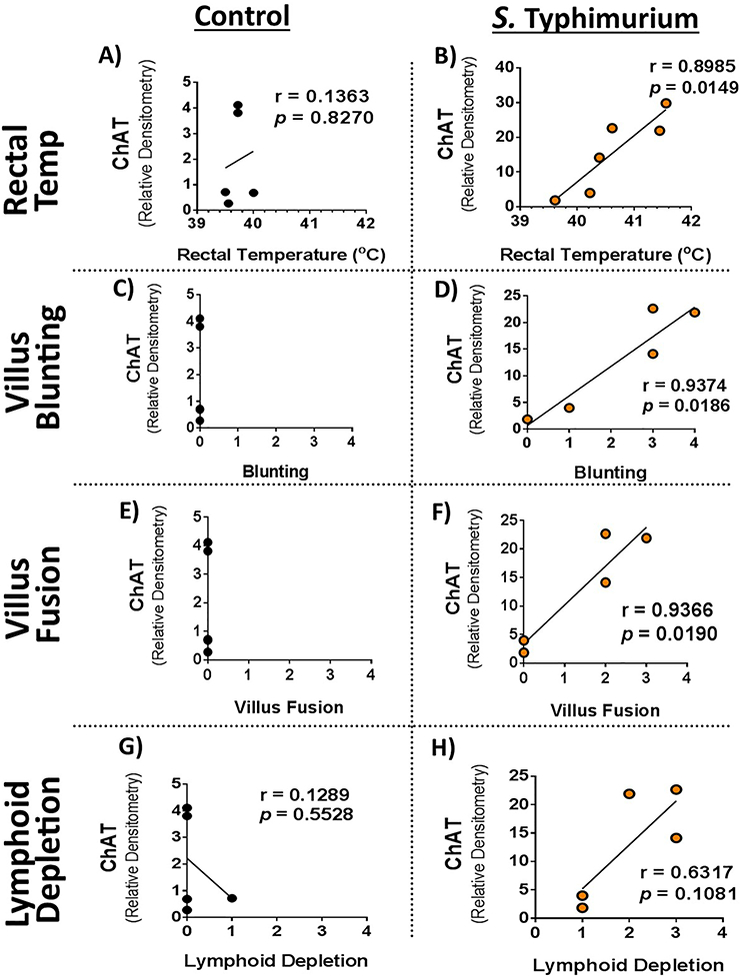
Rectal temperatures and histopathological scores were correlated with ileum mucosal ChAT expression from Figure 1. Correlations were performed independently on controls A, C, E, G) and S. Typhimurium challenge B, D, F, H) pigs. Pearson correlations were performed between mucosal ChAT and rectal temperature A, B); Villus Blunting C, D); Villus Fusion E, F); and lymphoid depletion G, H). Pearson correlation, r values and p values reported on figures.
Correlations between ileal mucosal ChAT expression and ileum histopathological scores for villus blunting, villus fusion, and lymphoid depletion were also performed separately for both controls and S. Typhimurium challenged animals. There was no villus blunting or villus fusion found in control pigs; therefore, no correlation could be performed (Fig 4C and 4E). While one control pig was found to have mild lymphoid depletion, overall there was no correlation between ileum ChAT expression and lymphoid depletion in controls was found (r=0.1289, p= 0.5528), (Fig 4G). However, in S. Typhimurium challenged pigs, mucosal ChAT expression positively correlated with villus blunting (r=0.9374, p=0.0185) and villus fusion (r=0.9366, p-0.0190), (Fig 4D and 4F, respectively). Also, ileal mucosal ChAT expression had a trending positive correlation with lymphoid depletion; however, the relationship was not significant (r=0.6317, p=0.1081), (Fig 4H). A negative correlation was found between AChE and lymphoid depletion in S. Typhimurium-challenged animals (r = −0.9296, p<0.0222, data not shown).
3.8. Correlation of mucosal ileum ChAT expression with mucosal ileum cytokine expression
Given the positive correlation between ileal mucosal ChAT expression and rectal temperature and histopathology in S. Typhimurium challenged animals, we next asked if mucosal ChAT expression correlated with inflammatory cytokine production. No correlation between mucosal ChAT expression and TNF, IL-8, or myeloperoxidase (MPO) expression was found in controls (Fig 5A, 5C, 5E). However, in S. Typhimurium challenged pigs, a trend towards a positive correlation was found between ileum mucosal ChAT expression and ileum mucosal TNF expression, (r=0.7354, p=0.0955), (Fig 5B). No significant correlation was found between ChAT and IL-8 or MPO in S. Typhimurium challenged animals (Fig 5D and 5F). We also performed correlation analysis between acetylcholine concentrations or AChE activity with ileum mucosal cytokine levels which showed no significant correlations (data not shown).
Figure 5. Correlation of ChAT with ileal mucosal cytokines.
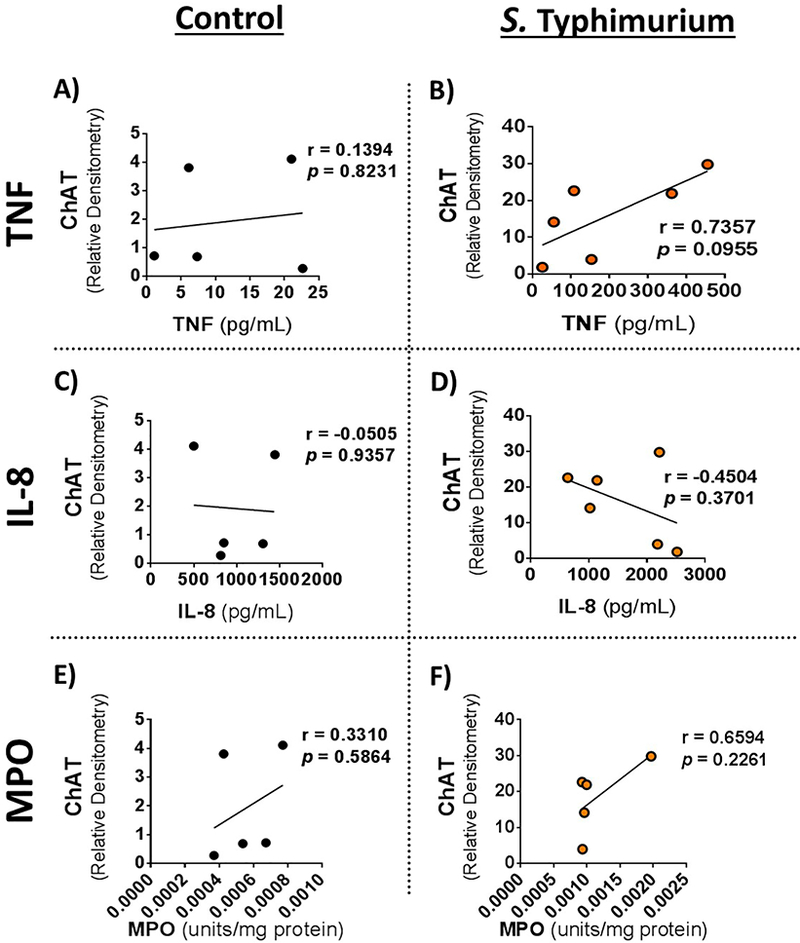
Mucosal cytokine protein expressions were correlated with ileal mucosal ChAT expression. Ileal mucosal ChAT and cytokine correlations from A, C, E) controls and B, D, F) Salmonella Typhimurium challenge. ChAT correlation with mucosal TNF A, B); IL8 C,D); and MPO E,F). Cytokine protein levels were determine previously by ELISA and protein expression of ChAT, determined in Figure 1, from Western blot. Pearson correlation, r values and p values reported on figures. Each point represents an individual animal. R and p values for two tailed Pearson correlation were analyzed and are reported on each figure.
3. Discussion
4.1. S. Typhimurium challenge in pigs alters the expression of cholinergic proteins involved in ACh synthesis, receptor signaling and degradation
Results from the present study showed that acute oral S. Typhimurium challenge in pigs resulted in significant alterations in the expression of cholinergic system markers characterized by reduction in ileal mucosal ACh concentrations, down-regulation of acetylcholine degrading enzyme activity, AChE, and upregulation of acetylcholine synthesis enzyme, ChAT. Cholinergic muscarinic receptor 1 and 3 expression was also upregulated in S. Typhimurium challenged pigs.
To date, nematode-induced enteritis in rodents has been the most commonly utilized animal model to study pathogen-mediated modulation of the enteric cholinergic system, and many of these studies focused on the cholinergic myenteric plexus and motility. Commonly demonstrated across these studies, and very similar to our data, are that parasitic enteritis acutely induces reduced production and release of acetylcholine (Collins et al. 1989; Davis et al. 1998), elevated ChAT activity and expression (Davis et al. 1998; Palmer and Koch 1995), and diminished AChE activity (Davis et al. 1998; Palmer and Koch 1995) in the small intestine. Only a few studies exist reporting the impact of bacterial and viral infection on the enteric cholinergic system following chronic infections. In one study, Helicobacter pylori and Herpes Simplex Virus-1 infection were shown to induce chronic down-regulation of acetylcholine release, suggesting that like acute infection as observed in the present study, chronic infections may also act to suppress acetylcholine availability (Bercik et al. 2002; Brun et al. 2010). Similar to the acute infectious enteritis models, an acute chemical ileitis model of 2,4,6- trinitrobenzenesulfonic acid (TNBS) resulted in impaired ACh release with paradoxical increased ChAT expression when compared to controls (Vieira et al. 2017). Chemical colitis models utilizing TNBS and dextran sodium sulfate (DSS) have been shown to impact ChAT expression, though the effects were dichotomous, with TNBS reducing ChAT expression and DSS enhancing ChAT expression (Winston et al. 2013). In the present study, our results demonstrated similar paradoxical expression of impaired ACh production, with increased ChAT expression and reduced AChE function following acute bacterial enteritis. Therefore, previous reports and our data here suggest a highly conserved cholinergic response to acute intestinal injury across animal species and etiological agents.
4.2. Potential benefits and consequences of dynamic, enteric cholinergic expression changes during pathogen challenge
As mentioned above, the present study and previous work in rodent models support evidence for a conserved cholinergic response to intestinal injury characterized by a reduction in ACh levels and alterations in cholinergic system components involved in Ach synthesis and muscarinic receptor expression, and decreased ACh degredation. Together, this response may represent a compensatory mechanism by the host to preserve and recover cholinergic signaling in the face of diminished ACh levels. The functional significance of this response is unclear at this time but might represent a beneficial response to limit intestinal mucosal injury. In support of this, cholinergic signaling through muscarinic receptor subtype 3 was shown to limit intestinal tissue damage in response to acute chemical colitis (Hirota and McKay 2006b). In the presence of inflammatory cytokines, TNF and INF-γ, muscarinic signaling preserved barrier function and limited release of neutrophil chemotactic cytokine IL-8, (Khan et al. 2015) demonstrating a protective role of cholinergic-mediated muscarinic signaling on the intestinal barrier potentially via control of the inflammatory milieu. Additionally, endotoxin induced upregulation of B lymphocyte ChAT expression was shown to negatively regulate neutrophil influx through action of acetylcholine on endothelium muscarinic receptors (Reardon et al. 2013). Likewise, down-regulation of mucosal AChE expression in response to endotoxin challenge was also shown to be necessary to limit acute cytokine production (Shaked et al. 2009). Increased cholinergic, muscarinic signaling, via muscarinic receptors may also play a role in adaptive immune responses. When challenged with enteric nematode infection, muscarinic receptor 3 knock out mice demonstrated an attenuated ability to generate humoral cytokines necessary to upregulate mucus production or alter motility, and this attenuated response resulted in increased worm and egg burden compared to wild type infected controls.(McLean et al. 2016) Further, muscarinic receptor 3 deficient mice infected with the enteric pathogen Citrobacter rodentium also demonstrated an impaired pathogen clearance (McLean et al. 2015) potentially linked with impaired adaptive immunity. Featuring a more direct role of muscarinic receptor signaling in adaptive immunity, muscarinic receptor 3 deficient lymphocytes demonstrated reduced cytokine production to cholinergic agonists with impaired memory immune responses and reduced activation and cytokine production in response to both humoral and cell mediated immunity inducing pathogens (Darby et al. 2015).
ACh can also bind and signal via α 7 nicotinic receptors, which have been more intensively studied in rodent chemical colitis models as major anti-inflammatory mechanism. In murine pathogen challenge models, α 7 nicotinic receptor genetic deficiency resulted in enhanced neutrophil recruitment which coincided with improved bacterial clearance compared with control animals (Giebelen et al. 2008). Pharmacological blockade of nicotinic receptors was shown to reduce intestinal villus damage induced by invasive Shigella infection (Svensson et al. 2004). In the present study, unlike muscarinic receptors, we did not observe significant changes in the expression of ileal α 7 nicotinic receptors in response to S. Typhimurium challenge. However, lack of changes in expression of α 7 nicotinic receptors following S. Typhimurium challenge does not necessarily imply an insignificant role for this receptor. For example, based on the preponderance of literature showing an inhibitory role of α 7 nicotinic receptors in immune activation, an unchanged expression level during pathogen challenge may facilitate bacterial clearance. Related to this, the reduced concentration of ACh in S. Typhimurium challenged pigs in the present may be an effort by the host to selectively activate muscarinic receptors over nicotinic receptors, since muscarinic receptors are known to have a much higher affinity for ACh compared with nicotinic receptors (Ahmad et al. 1987; Kellar et al. 1985; Purohit and Grosman 2006).
Together, results from our study with S. Typhimurium challenge in pigs and the literature support a critical role of the cholinergic response in balancing a robust immune responses required for pathogen clearance and development of adaptive immunity while at the same time limiting excessive inflammation and loss of intestinal barrier integrity. Therefore, conditions that result in suppression or hyper-active enteric cholinergic tone could have significant consequences for host immunity and survival.
4.3. Increased ChAT expression and localization to the Peyer’s patch epithelium in S. Typhimurium challenged pigs
Choline acetyltransferase (ChAT) has been shown to be expressed in neuronal and non-neuronal cell types. To gain more insight into the potential role of upregulated ileal ChAT expression in S. Typhimurium-challenged, investigated the localization of ChAT expression via IHC. As expected, we identified ChAT-positive cells within submucosal and myenteric neuronal ganglia and lamina propria which was increased in challenged pigs. Unexpectedly, we found the greatest intensity of ChAT-positive cells within the epithelium covering the Peyer’s patch lymphoid mucosa. The mechanism for upregulation of ChAT, particularly in the Peyer’s patch epithelium in challenged pigs remains to be determined. S. Typhimurium is an intracellular bacterial pathogen which establishes infection by transportation through microfold cells (M cells) over the Peyer’s patches in the ileum or through invasion of absorptive ileum enterocytes (Broz et al. 2012; Meyerholz et al. 2002). After crossing the intestinal epithelial barrier, S. Typhimurium enters the lamina propria where the pathogen is phagocytosed by macrophage and dendritic cells of the immune system. The pathogen resides and replicates within these leukocytes, permitting dissemination to other organs such as the mesenteric lymph nodes (Broz et al. 2012; Rieger et al. 2015). Therefore, the focal pattern of ChAT localization observed in challenged pigs in present study follows the pathway of S. Typhimurium invasion. Others have shown that bacterial products such as LPS upregulate ChAT expression in mucosal associated lymphoid tissue, peritoneal B cells, macrophage, and dendritic cells (Kawashima et al. 2007; Reardon et al. 2013). Specifically in line with the current study, S. Typhimurium infection was shown to elicit induction of intestinal CD4+ Th17 lymphocytes, (Raffatellu et al. 2008) a group of cells that has recently been shown to by ChAT positive (Dhawan et al. 2016). Therefore we hypothesize that the specific ChAT localization patterns of Peyer’s patch epithelium revealed in the present study is critical in the modulation of bacterial invasion and subsequent modulation of innate and adaptive immune response.
4.4. Summary
In summary, while the vagal cholinergic input has been shown to be an important key modulator of gut inflammatory responses, results from the present study demonstrate that the intrinsic cholinergic system, which includes neuronal and non-neuronal cell types, is dynamically regulated in response to S. Typhimurium challenge. The precise cause, cellular source, and significance of these cholinergic responses to overall host defense and immunity remains to be elucidated; however, our data establishes a foundation for future mechanistic research aiming to better understand the role of the enteric cholinergic system in mediating a balance between immune activation required for pathogen clearance and host immunity and the prevention of excessive inflammation and epithelial barrier damage.
Supplementary Material
Supplemental Table 1: PCR forward and reverse primers
Supplemental Figure 1. H&E stained sections of porcine control and S.Typhimurium challenged pigs as published previously.(Boyer et al. 2015) Section highlight differences displayed in Table 1 of previously published data. Not the villus blunting and fusion in the S. Typhimurium challenged pigs (B).
Highlights.
Typhimurium challenge in pigs resulted in a marked suppression of acetylcholine (Ach) levels in the ileum mucosa which coincided with a paradoxical upregulation of choline acetyltransferase (ChAT) and muscarinic receptor subtypes 1 (M1) and 3 (M3).
Upregulated ChAT expression in S. Typhimurium-challenged pigs was localized predominantly in the ileal Peyer’s patch epithelium.
Ileal ChAT expression was positively correlated with rectal temperature, and histopathological severity in S. Typhimurium challenged pigs
Funding Sources
This work was supported by U.S. National Institutes of Health grants R01 HD072968 (to AJM), K08 DK097462 (to AJM), R03 DK097462 (to AJM). This project was also supported by Agriculture and Food Research Initiative Competitive Grant no. 2017–67015-26673 from the USDA National Institute of Food and Agriculture (to AJM).
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad A, Gordon RK, and Chiang PK (1987), ‘A microtechnique for quantification of detergent- solubilized muscarinic and nicotinic acetylcholine receptors using a semi-automated cell harvestor’, FEBS Lett, 214 (2), 285–90. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, et al. (2009), ‘Mammalian nicotinic acetylcholine receptors: from structure to function’, Physiol Rev, 89 (1), 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji R, et al. (2000), ‘Acute phase responses of pigs challenged orally with Salmonella typhimurium’, J Anim Sci, 78 (7), 1885–91. [DOI] [PubMed] [Google Scholar]
- Bercik P, et al. (2002), ‘Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection’, Gastroenterology, 123 (4), 1205–15. [DOI] [PubMed] [Google Scholar]
- Boyer PE, et al. (2015), ‘Early-life dietary spray-dried plasma influences immunological and intestinal injury responses to later-life Salmonella typhimurium challenge’, Br J Nutr, 113 (5), 783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer A, et al. (2004), ‘Co-expression pattern of neuronal nitric oxide synthase and two variants of choline acetyltransferase in myenteric neurons of porcine ileum’, J Chem Neuroanat, 27 (1), 33–41. [DOI] [PubMed] [Google Scholar]
- Broz P, Ohlson MB, and Monack DM (2012), ‘Innate immune response to Salmonella typhimurium, a model enteric pathogen’, Gut Microbes, 3 (2), 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun P, et al. (2010), ‘Herpes simplex virus type 1 infection of the rat enteric nervous system evokes small-bowel neuromuscular abnormalities’, Gastroenterology, 138 (5), 1790–801. [DOI] [PubMed] [Google Scholar]
- Chiocchetti R, et al. (2003), ‘Evidence that two forms of choline acetyltransferase are differentially expressed in subclasses of enteric neurons’, Cell Tissue Res, 311 (1), 11–22. [DOI] [PubMed] [Google Scholar]
- Collins SM, et al. (1989), ‘Impaired acetylcholine release from the myenteric plexus of Trichinella- infected rats’, Am J Physiol, 257 (6 Pt 1), G898–903. [DOI] [PubMed] [Google Scholar]
- Darby M, et al. (2015), ‘The M3 muscarinic receptor is required for optimal adaptive immunity to helminth and bacterial infection’, PLoS Pathog, 11 (1), e1004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Masella J, and Blennerhassett MG (1998), ‘Acetylcholine metabolism in the inflamed rat intestine’, Exp Neurol, 152 (2), 251–8. [DOI] [PubMed] [Google Scholar]
- de Haan JJ, et al. (2013), ‘Lipid-rich enteral nutrition regulates mucosal mast cell activation via the vagal anti-inflammatory reflex’, American journal of physiology. Gastrointestinal and liver physiology, 305 (5), G383–91. [DOI] [PubMed] [Google Scholar]
- Dhawan S, et al. (2016), ‘Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity’, Am J Physiol Gastrointest Liver Physiol, 311 (5), G920–G33. [DOI] [PubMed] [Google Scholar]
- Di Giovangiulio M, et al. (2016), ‘Vagotomy affects the development of oral tolerance and increases susceptibility to develop colitis independently of the alpha-7 nicotinic receptor’, Mol Med, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cabezudo MJ, et al. (2010), ‘Cholinergic stimulation of the immune system protects against lethal infection by Salmonella enterica serovar Typhimurium’, Immunology, 130 (3), 388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabanyi I, et al. (2016), ‘Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages’, Cell, 164 (3), 378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeazzi F, et al. (2000), ‘Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent’, Am J Physiol Gastrointest Liver Physiol, 278 (2), G259– 65. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, and Perdue MH (2007), ‘Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability’, American journal of physiology.Gastrointestinal and liver physiology, 293 (1), G198–203. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, and Collins SM (2008), ‘Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression’, J Clin Invest, 118 (6), 2209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia JE, et al. (2006), ‘The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model’, Gastroenterology, 131 (4), 1122–30. [DOI] [PubMed] [Google Scholar]
- Giebelen IA, et al. (2008), ‘Deficiency of alpha7 cholinergic receptors facilitates bacterial clearance in Escherichia coli peritonitis’, J Infect Dis, 198 (5), 750–7. [DOI] [PubMed] [Google Scholar]
- Hirota CL and McKay DM (2006a), ‘Cholinergic regulation of epithelial ion transport in the mammalian intestine’, Br J Pharmacol, 149 (5), 463–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota CL and McKay DM (2006b), ‘M3 muscarinic receptor-deficient mice retain bethanechol-mediated intestinal ion transport and are more sensitive to colitis’, Can J Physiol Pharmacol, 84 (11), 1153–61. [DOI] [PubMed] [Google Scholar]
- Kawashima K, et al. (2007), ‘Expression and function of genes encoding cholinergic components in murine immune cells’, Life Sci, 80 (24–25), 2314–9. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, et al. (1985), ‘High-affinity binding of [3H]acetylcholine to muscarinic cholinergic receptors’, J Neurosci, 5 (6), 1577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MR, et al. (2015), ‘Activation of muscarinic cholinoceptor ameliorates tumor necrosis factor- alpha-induced barrier dysfunction in intestinal epithelial cells’, FEBS Lett, 589 (23), 3640–7. [DOI] [PubMed] [Google Scholar]
- Koga T, et al. (2013), ‘Immunoreactivity for Choline Acetyltransferase of Peripheral-Type (pChAT) in the Trigeminal Ganglion Neurons of the Non-Human Primate Macaca fascicularis’, Acta Histochem Cytochem, 46 (2), 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, et al. (2014), ‘Muscarinic acetylcholine receptors: novel opportunities for drug development’, Nature reviews.Drug discovery, 13 (7), 549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G and Boeckxstaens GE (2013), ‘The vagal innervation of the gut and immune homeostasis’, Gut, 62 (8), 1214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, et al. (2014), ‘A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen’, Gut, 63 (6), 938–48. [DOI] [PubMed] [Google Scholar]
- Mazelin L, et al. (1998), ‘Protective role of vagal afferents in experimentally-induced colitis in rats’, J Auton Nerv Syst, 73 (1), 38–45. [DOI] [PubMed] [Google Scholar]
- McLean LP, et al. (2016), ‘Type 3 muscarinic receptors contribute to intestinal mucosal homeostasis and clearance of Nippostrongylus brasiliensis through induction of TH2 cytokines’, Am J Physiol Gastrointest Liver Physiol, 311 (1), G130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean LP, et al. (2015), ‘Type 3 Muscarinic Receptors Contribute to Clearance of Citrobacter rodentium’, Inflamm Bowel Dis, 21 (8), 1860–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, et al. (2002), ‘Early epithelial invasion by Salmonella enterica serovar Typhimurium DT104 in the swine ileum’, Vet Pathol, 39 (6), 712–20. [DOI] [PubMed] [Google Scholar]
- Nakajima K, et al. (2000), ‘Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats’, J Chem Neuroanat, 18 (1–2), 31–40. [DOI] [PubMed] [Google Scholar]
- O’Mahony C, et al. (2009), ‘Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer’, Am J Physiol Regul Integr Comp Physiol, 297 (4), R1118–26. [DOI] [PubMed] [Google Scholar]
- Palmer JM and Koch TR (1995), ‘Altered neuropeptide content and cholinergic enzymatic activity in the inflamed guinea pig jejunum during parasitism’, Neuropeptides, 28 (5), 287–97. [DOI] [PubMed] [Google Scholar]
- Purohit Y and Grosman C (2006), ‘Estimating binding affinities of the nicotinic receptor for low- efficacy ligands using mixtures of agonists and two-dimensional concentration-response relationships’, J Gen Physiol, 127 (6), 719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, et al. (2008), ‘Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut’, Nat Med, 14 (4), 421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon C, et al. (2013), ‘Lymphocyte-derived ACh regulates local innate but not adaptive immunity’, Proc Natl Acad Sci U S A, 110 (4), 1410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger J, et al. (2015), ‘Enhancement of immunohistochemical detection of Salmonella in tissues of experimentally infected pigs’, Eur J Histochem, 59 (3), 2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS and Ayres JS (2008), ‘Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases’, Nat Rev Immunol, 8 (11), 889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked I, et al. (2009), ‘MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase’, Immunity, 31 (6), 965–73. [DOI] [PubMed] [Google Scholar]
- Soares MP, Gozzelino R, and Weis S (2014), ‘Tissue damage control in disease tolerance’, Trends Immunol, 35 (10), 483–94. [DOI] [PubMed] [Google Scholar]
- Svensson L, Bergquist J, and Wenneras C (2004), ‘Neuromodulation of experimental Shigella infection reduces damage to the gut mucosa’, Microbes Infect, 6 (3), 256–64. [DOI] [PubMed] [Google Scholar]
- The F, et al. (2011), ‘Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus’, Br J Pharmacol, 163 (5), 1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin G, Giglio D, and Lundgren O (2009), ‘Muscarinic receptor subtypes in the alimentary tract’, J Physiol Pharmacol, 60 (1), 3–21. [PubMed] [Google Scholar]
- Tooyama I and Kimura H (2000), ‘A protein encoded by an alternative splice variant of choline acetyltransferase mRNA is localized preferentially in peripheral nerve cells and fibers’, J Chem Neuroanat, 17 (4), 217–26. [DOI] [PubMed] [Google Scholar]
- Vieira Catia, et al. (2017), ‘Post-inflammatory Ileitis Induces Non-neuronal Purinergic Signaling Adjustments of Cholinergic Neurotransmission in the Myenteric Plexus’, Frontiers in Pharmacology, 8 (811). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. (2003), ‘Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation’, Nature, 421 (6921), 384–8. [DOI] [PubMed] [Google Scholar]
- Winston JH, Li Q, and Sarna SK (2013), ‘Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn’s colitis and ulcerative colitis’, Am J Physiol Gastrointest Liver Physiol, 305 (4), G295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: PCR forward and reverse primers
Supplemental Figure 1. H&E stained sections of porcine control and S.Typhimurium challenged pigs as published previously.(Boyer et al. 2015) Section highlight differences displayed in Table 1 of previously published data. Not the villus blunting and fusion in the S. Typhimurium challenged pigs (B).


