Abstract
Approximately 40% of all patients with ovarian cancer in Japan are aged ≥65 years. The aim of the present study was to evaluate the differences in prognosis and prognostic factors between elderly and younger patients with epithelial ovarian cancer. A total of 114 patients with International Federation of Gynecology and Obstetrics (FIGO) stage I–IV ovarian cancer who were initiated on primary treatment at the Osaka City University Hospital (Osaka, Japan) were included in this study. Patient characteristics, treatment outcome and prognosis were compared between elderly (aged ≥65 years) and younger patients, and the prognostic factors associated with overall survival were evaluated by univariate and multivariate analyses. The most common histological type in younger patients was clear cell carcinoma (33.8%) vs. serous carcinoma in elderly patients (44.1%), with a significant difference in the distribution of histological type (P=0.006). Complete resection was achieved in 56.2% of younger patients compared with 32.4% of elderly patients (P=0.03). The rates of standard primary treatment were comparable (56.7% of younger vs. 50.0% of elderly patients). Overall and disease-free survival did not differ significantly between the two groups. Multivariate analyses identified FIGO stage and standard primary therapy as prognostic factors in younger patients and performance status in elderly patients. Age was not an independent significant prognostic factor among patients with ovarian cancer. Therefore, performance status, rather than age, should be considered when selecting the optimal treatment for elderly patients based on objective assessment.
Keywords: chemotherapy, elderly, ovarian cancer, prognosis, survival
Introduction
Ovarian cancer has the highest mortality rate among gynecological tumors worldwide (1). The incidence of ovarian cancer in Japan has been increasing over the past 20 years, with an estimated ~10,400 new cases of ovarian cancer diagnosed and 4,800 ovarian cancer-related deaths in 2017 (2). Epithelial ovarian cancer (EOC) is the most common histological type of ovarian cancer, and the standard primary treatment (SPT) is primary debulking surgery (PDS), followed by six cycles of combination chemotherapy with paclitaxel and a platinum-containing drug, administered every 3 weeks (3–5).
Approximately 40% of all patients with ovarian cancer in Japan are aged ≥65 years (2), and the rapidly aging population suggests that the number of elderly women requiring treatment for ovarian cancer is likely to increase. In general, elderly patients are likely to have multiple comorbidities, poor performance status (PS), and require social and family support for their daily activities, which means that they may not be able to adapt to standard treatments. Elderly patients with ovarian cancer have thus been therapeutically undertreated compared with younger patients (6–8). Elderly patients are also more likely to be excluded from clinical trials due to comorbidities, polypharmacy, poor functional status and fragility; therefore, evidence for suitable treatment strategies for elderly patients is lacking.
Previously reported prognostic factors for ovarian cancer include International Federation of Gynecology and Obstetrics (FIGO) stage, histological subtype and grade, volume of the residual tumor after surgical resection, PS and age (9). However, the prognostic effect of age remains controversial (10), and the difference in prognostic factors between elderly and younger patients is not evident.
The aim of the present study was to compare the survival of elderly and younger patients with EOC, and determine the differences in prognostic factors between the two groups.
Patients and methods
Inclusion criteria
This retrospective study included consecutive patients with epithelial ovarian, fallopian tubal or peritoneal cancers, who were initiated on primary treatment for ovarian cancer at our institution between January 2008 and December 2011. All patients with stage I–IV disease according to the FIGO staging classification were included, whereas all patients with borderline tumors were excluded.
Clinicopathological characteristics
Patient data were obtained from electronic medical charts at our institution and included demographic, surgical, pathological, therapeutic and survival information. The clinicopathological characteristics recorded included age at diagnosis, body mass index, PS, histological type, FIGO stage, nodal status, albumin, hemoglobin and biomarker (CA-125) levels, and the therapeutic characteristics included neoadjuvant chemotherapy, surgery, intraoperative blood loss, operative time, residual tumor, SPT, recurrence status and secondary debulking surgery. ‘No residual tumor’ was defined as the absence of any macroscopic residual tumor at the end of PDS. SPT was defined as completing at least six cycles of adjuvant chemotherapy after surgery, except for stage 1A and 1B patients; SPT in cases with stage 1A and 1B disease was defined as PDS without adjuvant chemotherapy. Adjuvant chemotherapy mainly involved six cycles of combination chemotherapy with paclitaxel and a platinum-containing drug as the basic regimen, although this could vary depending on the patient's condition.
Overall survival (OS) was calculated as the time from the start of primary therapy to the date of death, and disease-free survival (DFS) was calculated as the time from the start of primary therapy to the date of recurrence or death. Patients who remained alive were censored on the date of the last follow-up.
Data collection
Study data were collected and managed using the Research Electronic Data Capture (REDCap) electronic tool hosted at Osaka City University Graduate School of Medicine (11). REDCap is a secure, web-based application designed to support data capture for research studies.
Patient groups
The registered patients were divided into two groups: An elderly group aged ≥65 years and a younger group aged <65 years. Patient characteristics, treatment outcome and prognosis were compared between the two groups. Univariate and multivariate analyses were also performed to identify and compare prognostic factors in the overall study population, and in elderly and younger patients.
Statistical analysis
Median values were compared between the two age groups as appropriate using the Mann-Whitney U test, and categorical variables were compared using the χ2 test. Kaplan-Meier survival analyses were performed for OS and DFS, and the significance of differences between the groups was determined using the log-rank test. A Cox proportional hazards model was used to calculate hazard ratios for OS and 95% confidence intervals, adjusting for prognostic variables. A P-value of <0.05 was considered to indicate statistically significant differences.
All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (12).
Results
Patient characteristics
A total of 114 patients aged 28–84 years were included in this study, including 80 patients in the elderly group and 34 in the younger group. The patient characteristics are summarized in Table I. There was a significant difference in the distribution of histological types between the elderly and younger patients (P=0.006), with clear cell carcinoma being the most common histological type in younger patients (33.8%), vs. serous carcinoma in elderly patients (44.1%). More advanced cancers (stage III and V) were observed among elderly patients, whereas earlier-stage cancers (stage I) were observed among younger patients, although there was no significant difference in stage distribution between the groups. However, there was a significant difference between the two groups in terms of residual tumor after PDS: 56.2% of younger patients had no residual tumor, compared with only 32.4% of elderly patients (P=0.03). There were no statistically significant differences between the two groups in terms of the other variables.
Table I.
Patient characteristics.
| Groups | ||||
|---|---|---|---|---|
| Characteristics | <65 years n=80, n (%) | ≥65 years n=34, n (%) | P-value | |
| Age, years | Median (Q1, Q3) | 51 (47, 58) | 71 (68,73) | <0.001 |
| BMI, kg/m2 | Median (Q1, Q3) | 22.7 (19.9, 24.9) | 22.2 (20.2, 24.2) | 0.57 |
| PS | 0 | 61 (76.2) | 26 (76.5) | 0.63 |
| 1 | 17 (21.2) | 6 (17.6) | ||
| 2 | 2 (2.5) | 2 (5.9) | ||
| Histological type | Serous | 16 (20.0) | 15 (44.1) | 0.006 |
| Mucinous | 15 (18.8) | 5 (14.7) | ||
| Clear cell | 27 (33.8) | 2 (5.9) | ||
| Endometrioid | 17 (21.2) | 7 (20.6) | ||
| Other | 5 (6.2) | 5 (14.7) | ||
| Stage | I | 31 (38.8) | 5 (14.7) | 0.08 |
| II | 7 (8.8) | 5 (14.7) | ||
| III | 35 (43.8) | 19 (55.9) | ||
| IV | 7 (8.8) | 5 (14.7) | ||
| Nodal status | pN0 | 42 (52.5) | 14 (41.2) | 0.12 |
| pN1 | 18 (22.5) | 5 (14.7) | ||
| pNx | 20 (25.0) | 15 (44.1) | ||
| Albumin, g/dl | Median (Q1, Q3) | 3.9 (3.5, 4.2) | 3.9 (3.3, 4.1) | 0.71 |
| Hemoglobin, g/dl | Median (Q1, Q3) | 12.0 (10.6, 13.1) | 11.9 (11.0, 12.9) | 0.78 |
| CA-125, U/ml | Median (Q1, Q3) | 328.5 (76.5, 1,780.5) | 589.0 (99.5, 3,178.3) | 0.27 |
| NAC | No | 75 (93.8) | 28 (82.4) | 0.12 |
| Yes | 5 (6.2) | 6 (17.6) | ||
| Surgery | No | 1 (1.2) | 1 (2.9) | 1 |
| Yes | 79 (98.8) | 33 (97.1) | ||
| Intraoperative blood loss, ml | Median (Q1, Q3) | 985 (560, 2,160) | 1,060 (515, 1,920) | 0.63 |
| Operative time, min | Median (Q1, Q3) | 246 (195, 299) | 204 (169, 247) | 0.06 |
| Residual tumor | No | 45 (56.2) | 11 (32.4) | 0.03 |
| Yes | 35 (43.8) | 23 (67.6) | ||
| SPTa | No | 34 (42.5) | 17 (50.0) | 0.60 |
| Yes | 46 (57.5) | 17 (50.0) | ||
| Recurrence status | No | 41 (51.2) | 13 (38.2) | 0.29 |
| Yes | 39 (48.8) | 21 (61.8) | ||
| SDS | No | 75 (93.8) | 33 (97.1) | 0.79 |
| Yes | 5 (6.2) | 1 (2.9) | ||
SPT was defined as completing at least 6 cycles of adjuvant chemotherapy after surgery in principle. Bold print indicates statistically significant P-values. Q, quartile; BMI, body-mass index; NAC, neoadjuvant chemotherapy; SPT, standard primary therapy; SDS, secondary debulking surgery.
Survival comparison
There was no significant difference in OS between the elderly and younger groups (median OS, 48 months vs. not reached, respectively; P=0.501, Fig. 1). The 5-year OS in elderly patients was 48.3% compared with 57.6% in younger patients. The median DFS was lower in elderly patients compared with that in younger patients, but the difference was not statistically significant (19.5 vs. 35.0 months, respectively; P=0.107, data not shown).
Figure 1.
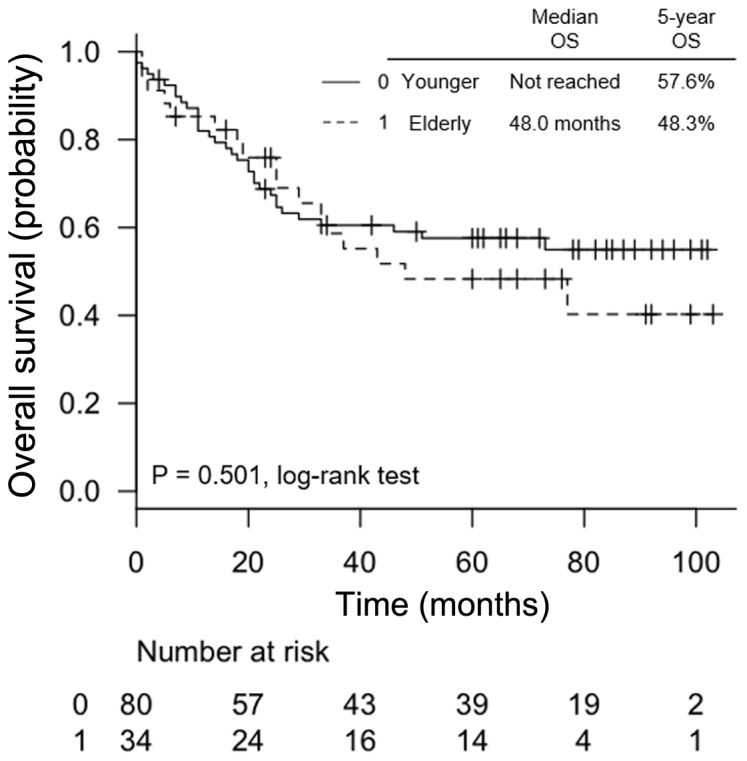
Kaplan-Meier curves of overall survival (OS) according to age (0: <65 years, 1: ≥65 years).
Uni- and multivariate analysis
The univariate Cox proportional hazards model of OS for all patients identified PS, stage, residual tumor and SPT as significant prognostic factors (P<0.0001, P<0.0001, P<0.0001 and P=0.001, respectively), while stage and SPT were independent prognostic factors according to multivariate analysis (P=0.0006 and P<0.0001, respectively, Table II). In younger patients, PS, stage, residual tumor and SPT were significant prognostic factors in univariate analysis (P=0.02, P<0.0001, P<0.0001 and P=0.04, respectively), whereas stage and SPT were independent prognostic factors in multivariate analysis, similar to the results for the overall study population (P=0.0007 and P=0.0006, respectively, Table III). However, in elderly patients, only PS and SPT were found to be significant prognostic factors in univariate analysis (P=0.0006 and P<0.0001, respectively), and only PS was an independent prognostic factor in multivariate analysis (P=0.02, Table IV).
Table II.
Univariate and multivariate analysis of OS for all patients (n=114).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age, years | ||||
| <65 vs. ≥65 | 1.21 (0.68–2.19) | 0.51 | 0.59 (0.31–1.12) | 0.11 |
| PS | ||||
| 0 vs. 1,2 | 3.12 (1.76–5.51) | <0.0001 | 1.53 (0.84–2.79) | 0.16 |
| Stagea | ||||
| 1,2,3,4 | ||||
| 2.46 (1.73–3.50) | <0.0001 | 2.19 (1.41–3.43) | 0.0006 | |
| Residual tumor | ||||
| No vs. yes | 4.30 (2.24–8.25) | <0.0001 | 1.79 (0.77–4.18) | 0.18 |
| SPTb | ||||
| No vs. yes | 0.40 (0.23–0.70) | 0.001 | 0.30 (0.16–0.54) | <0.0001 |
Stage is an ordinal variable.
SPT was defined as completing at least 6 cycles of adjuvant chemotherapy after surgery in principle. Bold print indicates statistically significant P-values. HR, hazard ratio; 95% CI, 95% confidence interval; PS, performance status; SPT, standard primary therapy.
Table III.
Univariate and multivariate analysis of OS for younger patients (n=80).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| PS | ||||
| 0 vs. 1,2 | 2.39 (1.17–4.87) | 0.02 | – | – |
| Stagea | ||||
| 1,2,3,4 | 3.11 (1.97–4.92) | <0.0001 | 2.83 (1.55–5.16) | 0.0007 |
| Residual tumor | ||||
| No vs. yes | 7.31 (3.14–17.02) | <0.0001 | 2.12 (0.71–6.29) | 0.18 |
| SPTb | ||||
| No vs. yes | 0.49 (0.24–0.96) | 0.04 | 0.28 (0.14–0.58) | 0.0006 |
Stage is an ordinal variable.
SPT was defined as completing at least 6 cycles of adjuvant chemotherapy after surgery in principle. Bold print indicates statistically significant P-values. HR, hazard ratio; 95% CI, 95% confidence interval; PS, performance status; SPT, standard primary therapy.
Table IV.
Univariate and multivariate analysis of OS for elderly patients (n=34).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| PS | ||||
| 0 vs. 1, 2 | 5.86 (2.14–16.03) | 0.0006 | 2.83 (1.25–11.75) | 0.02 |
| Stagea | ||||
| 1, 2, 3, 4 | 1.55 (0.83–2.89) | 0.17 | – | – |
| Residual tumor | ||||
| No vs. yes | 1.21 (0.43–3.45) | 0.72 | – | – |
| SPTb | ||||
| No vs. yes | 4.30 (2.24–8.25) | <0.0001 | 0.43 (0.13–1.39) | 0.16 |
Stage is an ordinal variable.
SPT was defined as completing at least 6 cycles of adjuvant chemotherapy after surgery in principle. Bold print indicates statistically significant P-values. HR, hazard ratio; 95% CI, 95% confidence interval; PS, performance status; SPT, standard primary therapy.
Elderly patients with a PS of 0 had a 5-year OS rate of 65.2%, which was comparable to that of younger patients. By contrast, the OS in elderly patients with a PS of 1 and 2 was very poor, with a 5-year OS rate of 0%, which was significantly poorer compared with that in younger patients (P=0.0003, Fig. 2).
Figure 2.
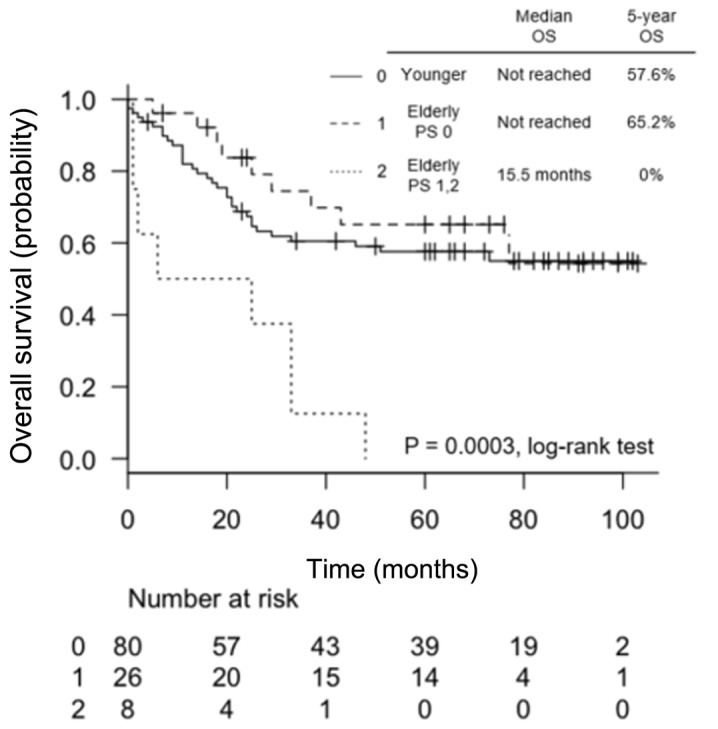
Kaplan-Meier curves of overall survival (OS) for elderly patients according to performance status (PS) compared with younger patients (0: <65 years, 1: ≥65 years with PS 0 and 2: ≥65 years with PS 1 and 2).
Discussion
The aim of this retrospective study was to evaluate the differences in patient characteristics, survival outcome, and prognostic factors between elderly and younger patients with FIGO stage I–IV ovarian cancer who received primary treatment at a single gynecological oncology institution in Japan. Although numerous studies have examined the association between age and prognosis in Europe and the United States, only few studies from Japan have been published to date. Given that diagnosis, treatment, environment and conditions differ among countries, regional differences should be considered when assessing prognostic factors.
Our data suggested that age, defined by a cut-off of 65 years, was not a prognostic factor for either OS or DFS. FIGO stage and SPT were prognostic factors in the overall patient population, but the prognostic factors differed between elderly and younger patients. Multivariate analysis identified FIGO stage and SPT as independent prognostic factors in younger patients, as well as for all patients, but only PS was an independent prognostic factor in elderly patients.
An association between age and prognosis has been reported in several studies. Trillsch et al evaluated 275 patients with FIGO stage II–IV EOC undergoing cytoreductive surgery and platinum-based chemotherapy, and found that age was a prognostic factor for OS, but not for PFS (7). Sabatier et al compared 109 elderly patients with 488 younger patients with histologically invasive EOC, and identified age as an independent prognostic factor for OS (8). There are several possible reasons why age was not found to be a significant prognostic factor in the present study. First, the results may have been affected by the cut-off value for elderly patients. Although a cut-off age of 65 years has commonly been used, the age criteria have differed among previous studies (6–8,13–17). Thus, the prognostic value of age may depend on the criteria for defining patients as ‘elderly’. Second, there was no significant difference in clinicopathological characteristics between the two groups, except for histological type, including body mass index, PS, FIGO stage, nodal status, albumin, hemoglobin and CA-125 levels. Third, there was no major difference in the implementation rate of SPT, defined as completion of at least six cycles of adjuvant chemotherapy after surgery, with 57.6% of younger patients and 50% of elderly patients treated with SPT. By contrast, several previous studies reported that elderly patients were less likely to receive standard treatments, such as debulking surgery and combination chemotherapy, due to more advanced disease and functional impairments (6–8,10). However, our data revealed similar implementation rates of SPT between the two groups. This fact may be based on the lack of a significant difference in patient characteristics between the two groups or the objective functional evaluation of the elderly patients at our facility, suggesting the importance of adapting standard therapy to elderly patients.
The elderly comprise a heterogeneous population. In the present study, elderly patients with a PS of 0 had an OS comparable to that of younger patients, while elderly patients with a PS of 1 and 2 had a dismal OS, with a 5-year OS rate of 0% (P=0.0003). The importance of PS in elderly patients is consistent with previous studies (7,8). We confirmed the backgrounds of the elderly patients according to PS, and found more advanced tumors in those with a PS of 1 and 2 compared with elderly patients with a PS of 0, although the difference was not statistically significant (P=0.09). Despite having more advanced tumors, only 12.5% of elderly patients with PS 1 and 2 completed six cycles of standard chemotherapy, compared with 61.5% of elderly patients with PS 0 (P=0.04).
Elderly patients with ovarian cancer may benefit from a multidisciplinary approach, including a comprehensive evaluation by a gynecologist, oncologist, nurse and pharmacist. Comprehensive geriatric assessments have been shown to be able to predict morbidity and mortality in elderly cancer patients (18). However, the high heterogeneity of elderly patients means that it is not feasible to make treatment decisions based on age alone, and a more objective assessment is required.
The present study had certain limitations. First, selection bias was unavoidable and inherent to the retrospective nature of this study. Second, the relatively small number of cases, particularly in the elderly group, meant that the statistical power may not have been sufficient to draw a definitive conclusion. Finally, other confounding factors may have been overlooked. However, this study also had certain strengths. As all patients with FIGO stage I–IV EOC within a certain period were enrolled, the results may strongly reflect the effect of age on prognosis in clinical practice compared with the selected patient cohort.
In conclusion, elderly and younger patients with EOC have different prognostic factors but similar prognoses. Elderly patients were as likely to receive standard primary therapy as younger patients, and age was not an independent significant prognostic factor. A key strategy for improving the prognosis of patients with EOC is to ensure the administration of at least six courses of standard adjuvant chemotherapy after PDS, in consideration of FIGO stage. Therefore, PS, rather than age, should be considered when adapting the optimal treatment to elderly patients with ovarian cancer based on objective assessment.
Acknowledgements
The authors would like to thank all those who contributed to this study, particularly the statisticians and colleagues of Osaka City University Graduate School of Medicine. We appreciate their help with data management and statistical support. We would also like to thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KY and TF contributed to the conception, design and conduction of the study and analysis and interpretation of the data. TS contributed to the conception of the study and interpretation of the data. RU, HM, TW, MK, RT, MK, YH, TI and TY contributed to data collection and the conduction of the study. All the authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
This study was performed according to the principles set out in the Declaration of Helsinki 1964 and all subsequent revisions, and was approved by the Institutional Review Board of Osaka City University Graduate School of Medicine (IRB approval no. 3779).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests to disclose.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Cancer information service, corp-author. http://ganjoho.jp/en/public/statistics/short_pred.html. [Jan 10;2018 ];Projected Cancer Statistics. 2017 [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Gynecologic Oncology Group: Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A gynecologic oncology group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Lück HJ, Meier W, Adams HP, Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 5.Bell J, Brady MF, Young RC, Lage J, Walker JL, Look KY, Rose GS, Spirtos NM. Gynecologic Oncology Group: Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: A gynecologic oncology group study. Gynecol Oncol. 2006;102:432–439. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Fourcadier E, Trétarre B, Gras-Aygon C, Ecarnot F, Daurès JP, Bessaoud F. Under-treatment of elderly patients with ovarian cancer: A population based study. BMC Cancer. 2015;15:937. doi: 10.1186/s12885-015-1947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trillsch F, Woelber L, Eulenburg C, Braicu I, Lambrechts S, Chekerov R, van Nieuwenhuysen E, Speiser P, Zeimet A, Castillo-Tong DC, et al. Treatment reality in elderly patients with advanced ovarian cancer: A prospective analysis of the OVCAD consortium. J Ovarian Res. 2013;6:42. doi: 10.1186/1757-2215-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabatier R, Calderon B, Jr, Lambaudie E, Chereau E, Provansal M, Cappiello MA, Viens P, Rousseau F. Prognostic factors for ovarian epithelial cancer in the elderly: A case-control study. Int J Gynecol Cancer. 2015;25:815–822. doi: 10.1097/IGC.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander ML. Prognostic factors in ovarian cancer. Semin Oncol. 1998;25:305–314. [PubMed] [Google Scholar]
- 10.Gibson SJ, Fleming GF, Temkin SM, Chase DM. The application and outcome of standard of care treatment in elderly women with ovarian cancer: A literature review over the last 10 years. Front Oncol. 2016;6:63. doi: 10.3389/fonc.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorio R, Roemer-Becuwe C, Hilpert F, Gibbs E, García Y, Kaern J, Huizing M, Witteveen P, Zagouri F, Coeffic D, et al. Safety and efficacy of single-agent bevacizumab-containing therapy in elderly patients with platinum-resistant recurrent ovarian cancer: Subgroup analysis of the randomised phase III AURELIA trial. Gynecol Oncol. 2017;144:65–71. doi: 10.1016/j.ygyno.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Woopen H, Inci G, Richter R, Ismaeel F, Sehouli J. Elderly ovarian cancer patients: An individual participant data meta-analysis of the North-Eastern German Society of Gynecological Oncology (NOGGO) Eur J Cancer. 2016;60:101–106. doi: 10.1016/j.ejca.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Tinquaut F, Freyer G, Chauvin F, Gane N, Pujade-Lauraine E, Falandry C. Prognostic factors for overall survival in elderly patients with advanced ovarian cancer treated with chemotherapy: Results of a pooled analysis of three GINECO phase II trials. Gynecol Oncol. 2016;143:22–26. doi: 10.1016/j.ygyno.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Joseph N, Clark RM, Dizon DS, Lee MS, Goodman A, Boruta D, Jr, Schorge JO, Del Carmen MG, Growdon WB. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol Oncol. 2015;137:401–405. doi: 10.1016/j.ygyno.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Deng F, Xu X, Lv M, Ren B, Wang Y, Guo W, Feng J, Chen X. Age is associated with prognosis in serous ovarian carcinoma. J Ovarian Res. 2017;10:36. doi: 10.1186/s13048-017-0331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


