Abstract
Background:
The risk of Kaposi’s sarcoma–associated herpesvirus (KSHV) acquisition among children is increased by HIV infection. Antiretroviral therapy (ART) was recently made widely available to HIV-infected children in Zambia. However, the impact of early ART on KSHV transmission to HIV-infected children is unknown.
Methods:
We enrolled and followed a cohort of 287 HIV-exposed, KSHV-negative children under 12 months of age from Lusaka, Zambia, to identify KSHV seroconversion events. Potential factors associated with KSHV infection—with an emphasis on HIV, ART, and immunological measures—were assessed through structured questionnaires and blood analyses. Incidence rate, Kaplan-Meier, and multivariable Cox regression models were used to assess differences in time to event (KSHV seroconversion) between groups. All statistical tests were two-sided.
Results:
During follow-up, 151 (52.6%) children underwent KSHV seroconversion. Based on 3552 months of follow-up, we observed similar KSHV incidence rates between HIV-infected and uninfected children. Among HIV-infected children, ART-naïve children had statistically significantly increased risk of KSHV acquisition (adjusted hazard ratio [AHR] = 5.04, 95% confidence interval [CI] = 2.36 to 10.80, P < .001). Time-updated CD4+ T-cell percentage was also statistically significantly associated with risk of KSHV acquisition (AHR = 0.82, 95% CI = 0.74 to 0.92, P < .001), such that each 5% increase of CD4+ T-cells represented an 18% decrease in risk of acquiring KSHV.
Conclusions:
Our data suggest that early ART and prevention of immune suppression reduce the risk of KSHV acquisition among HIV-infected children in an area where both viruses are highly endemic. This study highlights the importance of programs in Africa to provide children with ART immediately after HIV infection is diagnosed.
Kaposi’s sarcoma (KS) is one of the most common malignancies in many countries of sub-Saharan Africa—where approximately 84% of global cases occur (1). An endemic form of KS was first identified in these countries in the 1960s, presenting in male adults but rarely in women and young children (2,3). Subsequently, an HIV-associated form of KS, known as epidemic or AIDS-KS, emerged in both adults and children in parallel with the HIV/AIDS epidemic (4). For example, in Zambia, KS accounted for approximately 25% of all pediatric malignancies by 1992, with a peak incidence between one and two years of age, making it the most common childhood cancer (5). The introduction of antiretroviral therapy (ART) has decreased the incidence of AIDS, and consequently epidemic KS, in resource-rich and -limited countries alike (6). Nevertheless, ART coverage in resource-limited countries of sub-Saharan Africa remains low, at approximately 37% (7). Hence, KS continues to be a substantial source of morbidity and mortality among HIV-infected children and adults in this region (1,8–10).
All forms of KS, along with the lymphoproliferative malignancies primary effusion lymphoma and multicentric Castleman’s disease, are etiologically linked with Kaposi’s sarcoma–associated herpesvirus (KSHV; or human herpesvirus-8 [HHV-8]) (11–13). Global KSHV seroprevalence is uneven; it is low in the United States and Western Europe, moderate in the Mediterranean, and high in sub-Saharan Africa (14–16). Concordantly, in a previous prospective cohort study, we observed that KSHV infection is common among Zambian children, as approximately 40% of children acquired KSHV by four years of age (17). KSHV DNA is frequently detected in saliva of infected individuals—implicating salivary exchange as the major route of transmission to children (18,19). Indeed, we recently reported that specific child feeding behaviors are associated with early childhood infection (20). Counseling and educating caregivers regarding KSHV transmission and feeding habit changes may therefore reduce early childhood infection. However, in the absence of a KSHV vaccine, counseling alone cannot be anticipated to eliminate viral transmission and the risk of developing KS. This underscores the urgent need to prevent transmission of KSHV in the pediatric population, thereby reducing the burden of KS in both children and adults.
We previously observed that children infected with HIV had a five-fold higher risk of acquiring KSHV compared with HIV uninfected children (17). However, this cohort study was conducted before the widespread use of ART in Zambia. We hypothesized that HIV-induced immunosuppression predisposed children to infection by KSHV; thus prevention of CD4+ T-cell depletion and subsequent immune suppression by early ART should reduce KSHV incidence. The Zambian government recently increased ART availability and, in accordance with the World Health Organization (WHO) recommendations, the current policy is to provide HIV-infected children with ART after confirmatory diagnosis regardless of CD4+ T-cell status (21,22). Additionally, the University Teaching Hospital (UTH) in Lusaka was one of the first hospitals to successfully implement a routine HIV counseling and testing program for children (23). These programs supported investigating the relationship between immune suppression and KSHV transmission. In the present study, we have evaluated the impact of ART on KSHV acquisition in a prospective cohort of young Zambian children.
Methods
Study Setting and Cohort
The current study is part of an ongoing observational cohort following children born to HIV-infected mothers to investigate the effect of ART on KSHV. Screening and enrollment was conducted between December 2009 and June 2012 at the UTH in Lusaka, Zambia. Local community workers recruited and informed potential participants about the study aims (24). Interested mothers who visited the study clinic and provided written informed consent to participate were given monetary compensation for transportation costs. Mothers were also counseled about HIV and KSHV infections and ways to prevent transmission. Eligibility criteria for enrollment was as follows: 1) child was less than 12 months of age; 2) child was negative for KSHV antibodies in plasma and KSHV DNA in the oral cavity; 3) child HIV status was confirmed; and 4) mother was HIV infected. All eligible mother-child pairs returned within a week for enrollment and were scheduled for follow-up every three months for up to 48 months after enrollment. This study was approved by the Institutional Review Board of the University of Nebraska and the University of Zambia Biomedical Research Ethics Committee.
Data Collection and Laboratory Testing
Data and Biological Specimen Collection
Trained interviewers conducted intake interviews with each mother using structured questionnaires at all study visits. The self-reported questionnaires collected data pertaining to socio-demographics, medical history, laboratory results, and ART status, adherence, and regimen. Blood samples and oral swabs were collected from all children during a free-of-charge medical examination at each study visit. Plasma and oral swabs were shipped to the University of Nebraska-Lincoln for KSHV antibody and DNA detection, respectively.
HIV Diagnosis and Blood Testing
HIV diagnosis was conducted at the UTH laboratory in Lusaka. Because all children were under 12 months of age, HIV testing was performed using DNA polymerase chain reaction (PCR) of dried blood spots. Confirmation of mother HIV status was performed using two rapid tests—Abbott RealTime HIV-1 Qualitative (Abbott Laboratories, Des Plaines, IL) and Unigold Recombigen HIV-1/2 (Trinity Biotech, Bray, Ireland)—according to manufacturer’s protocols. Whole blood samples of HIV-infected children were analyzed at the UTH clinic to determine the percentage of total T-cells (CD3+) that were CD4+ or CD8+ using a FacsCount Cell Analyzer (BD Biosciences, San Jose, CA) according to the manufacturer’s protocol. Blood chemistry and full blood count were also conducted for each child.
KSHV Detection
Plasma samples were tested for KSHV antibodies using a monoclonal antibody–enhanced immunofluorescence assay (mIFA) previously standardized in our laboratory (20). Briefly, plasma samples were diluted 1:40 in phosphate-buffered saline (PBS) and incubated on stimulated and fixed BC3 cells. Mouse monoclonal antihuman IgG (CRL-1786; American Type Culture Collection, Manassas, VA) was used as a secondary antibody and DyLight 488-conjugated donkey antimouse IgG (Jackson ImmunoResearch, West Grove, PA) as the tertiary antibody. A plasma sample was considered KSHV positive if two readers independently determined the sample to be positive on two separate mIFAs. Oral swabs collected at screening were tested for the presence of KSHV DNA by PCR and Southern blot analysis, as described previously (19,25), to verify KSHV-negative status at study entry.
Statistical Analysis
Categorical data were summarized by count and percentages using Chi-square test to determine statistical significance, and continuous variables were summarized by median and interquartile range (IQR) using Wilcoxon rank-sum test for statistical significance. Independent variables analyzed for all children, and HIV-infected children only, are detailed in Tables 1 and 2. Generalized symptoms include any of the following: fever, sore throat, rash, diarrhea, vomiting, mouth sores, sneezing, or cough. Missing CD4% or CD8% data were imputed using that child’s median value from other visits. Additional independent variables considered for HIV-infected children taking ART were age at ART initiation (in months) and ART regimen (three nucleoside reverse transcriptase inhibitors [NRTIs], two NRTIs and one non-nucleoside reverse transcriptase inhibitors [NNRTIs], or two NRTIs and one protease inhibitor [PI]). Logistic regression was used to measure associations between KSHV seroconversion and generalized symptoms or full blood count data at either the KSHV seroconversion visit or study censor date.
Table 1.
Demographic characteristics in a longitudinal cohort of 287 children, by child HIV status, from Lusaka, Zambia, 2009–2012
| Characteristic* | Whole cohort | Children without HIV | Children with HIV | P† |
|---|---|---|---|---|
| (N = 287) No. (%) | (n = 196) No. (%) | (n = 91) No. (%) | ||
| Age at enrollment, mo (range) | 7 (5–11) | 6 (4–9) | 10 (6–12) | <.001 |
| Sex | ||||
| Female | 136 (47.4) | 91 (46.4) | 45 (49.5) | .63 |
| Male | 151 (52.6) | 105 (53.6) | 46 (50.5) | |
| Household members, no. | ||||
| ≤3 | 47 (16.4) | 29 (14.8) | 18 (19.8) | .48 |
| 4–5 | 148 (51.5) | 101 (51.5) | 47 (51.6) | |
| ≥6 | 92 (32.1) | 66 (33.7) | 26 (28.6) | |
| Monthly household income | ||||
| ≤$30 | 72 (25.1) | 38 (19.4) | 34 (37.4) | <.001 |
| >$30 | 202 (70.4) | 152 (77.5) | 50 (54.9) | |
| Unknown | 13 (4.5) | 6 (3.1) | 7 (7.7) | |
| Mother employment | ||||
| Unemployed | 193 (67.2) | 124 (63.3) | 69 (75.8) | .04 |
| Employed | 94 (32.8) | 72 (36.7) | 22 (24.2) | |
| Mother education | ||||
| 0–7 y | 172 (59.9) | 117 (59.7) | 55 (60.4) | .90 |
| ≥8 y | 115 (40.1) | 79 (40.3) | 36 (39.6) | |
| Mother breastfeeding‡ | ||||
| Never | 14 (4.9) | 11 (5.6) | 3 (3.3) | .56 |
| Ever | 273 (95.1) | 185 (94.4) | 88 (96.7) | |
| Months of breastfeeding (range) | 7.8 (5.2–11.8) | 6.8 (5.0–10.1) | 11 (6.3–14.1) | <.001 |
| Mother ART | ||||
| Never | 126 (43.9) | 62 (31.6) | 64 (70.3) | <.001 |
| Ever | 161 (56.1) | 134 (68.4) | 27 (29.7) | |
| Mother start ARTठ| ||||
| Before pregnancy | 95 (33.1) | 87 (44.4) | 8 (8.8) | <.001 |
| During pregnancy | 34 (11.8) | 31 (15.8) | 3 (3.3) | |
| After pregnancy | 32 (11.1) | 16 (8.2) | 16 (17.6) | |
| Months mother on ART§ (range) | 17 (8–36) | 24 (9–48) | 7 (4–10) | <.001 |
* Continuous variables are represented as median (interquartile range) and categorical variables as count (percentages). ART = antiretroviral therapy; HIV = human immunodeficiency virus.
† Wilcoxon rank-sum test was used for continuous data and chi-square test for categorical data, except where noted. All tests were two-sided.
‡ Fisher’s exact test used. All tests were two-sided.
§ n = 161 (only mothers with history of ART).
Table 2.
Child clinical characteristics at time of enrollment in a longitudinal cohort of 287 children, by child HIV status, from Lusaka, Zambia, 2009–2012
| Characteristic* | Whole cohort | Children without HIV | Children with HIV | P† |
|---|---|---|---|---|
| (N = 287) No. (%) | (n = 196) No. (%) | (n = 91) No. (%) | ||
| Height, cm (range) | 63 (60–69) | 63 (60–69) | 63.5 (60–69) | .86 |
| Weight, kg (range) | 7 (6.0–8.1) | 7 (6.0–8.2) | 6.9 (5.9–7.8) | .55 |
| Developmentally appropriate‡ | ||||
| No | 5 (1.7) | 3 (1.5) | 2 (2.2) | .65 |
| Yes | 282 (98.3) | 193 (98.5) | 89 (97.8) | |
| Generalized symptoms§ | ||||
| No | 239 (83.3) | 164 (83.7) | 75 (82.4) | .79 |
| Yes | 48 (16.7) | 32 (16.3) | 16 (17.6) | |
| Alanine aminotransferase, U/L (range) | 14.3 (10.4–21.1) | 13.4 (9.5–19.5) | 15.1 (10.7–21.6) | .14 |
| Aspartate aminotransferase, U/L (range) | 40.3 (32.7–52.4) | 41.1 (32.8–52.9) | 39.1 (31.6–49.5) | .55 |
| White blood cell, x109/L (range) | 10.1 (8.0–12.7) | 10.1 (8–12.4) | 10.1 (8.1–12.8) | .97 |
| Hemoglobin, g/dL (range) | 9.9 (9.1–10.9) | 9.9 (9.1–10.6) | 10.0 (9.1–11.5) | .13 |
| Hematocrit, % (range) | 30.8 (28.3–32.7) | 31.2 (28.8–32.7) | 30.0 (27.2–32.0) | .01 |
| Lymphocyte, % (range) | 64.2 (57.8–70.8) | 65.0 (57.8–71.1) | 63.1 (59.1–69.6) | .44 |
| Monocyte, % (range) | 8.4 (6.8–10.6) | 8.8 (7.0–10.9) | 7.9 (6.6–9.4) | .005 |
| Child ART | ||||
| No | - - | - - | 17 (18.7) | |
| Yes | - - | - - | 74 (81.3) | - |
| CD4, % (range) | - - | - - | 40.0 (31.0–57.0) | - |
| CD8, % (range) | - - | - - | 56.0 (47.0–57.0) | - |
| CD4:CD8 ratio (range) | - - | - - | 0.74 (0.50–1.02) | - |
| Immunosuppression statusǁ | ||||
| Not clinically significant | - - | - - | 62 (68.1) | |
| Mild | - - | - - | 18 (19.8) | |
| Advanced | - - | - - | 11 (12.1) | - |
* Continuous variables are represented as median (interquartile range) and categorical variables as count (percentages). ART = antiretroviral therapy; HIV = human immunodeficiency virus.
† Wilcoxon rank-sum test was used for continuous data and chi-square test for categorical data, except where noted. All tests were two-sided.
‡ Fisher’s exact test used. All tests were two-sided.
§ Includes fever, sore throat, rash, diarrhea, vomiting, mouth sores, sneezing, or cough.
ǁ Based on CD4% according to age appropriate World Health Organization categories (29).
All time-to-event data were right-censored at 24 months of follow-up. Crude KSHV incidence rate per 100 child-months was calculated for all variables. Cumulative incidence curves were generated depicting the probability of KSHV infection as a function of age for the total cohort, HIV-uninfected vs HIV-infected children, and HIV-uninfected vs HIV-infected children stratified by ART status. Univariate Cox proportional hazards modeling was used to assess the risk of KSHV infection for all variables, including those associated with caregivers, for the HIV-infected children and children taking ART. The proportional hazards assumption was verified using the empirical score process. Variables that were statistically significant in the univariate analysis were then considered for multivariable Cox proportional hazards models using forward stepwise selection. Percentages of CD4+ and CD8+ T-cells collected at each study visit were analyzed as time-updated data. Adjusted hazard ratios (AHRs), along with corresponding 95% confidence intervals (CIs), are reported. All statistical tests were two-sided, and P values of less than or equal to .05 were considered statistically significant. Analyses were performed using statistical packages SAS (v9.3; SAS Institute, Cary, NC) and SPSS (v22; IBM, Armonk, NY).
Results
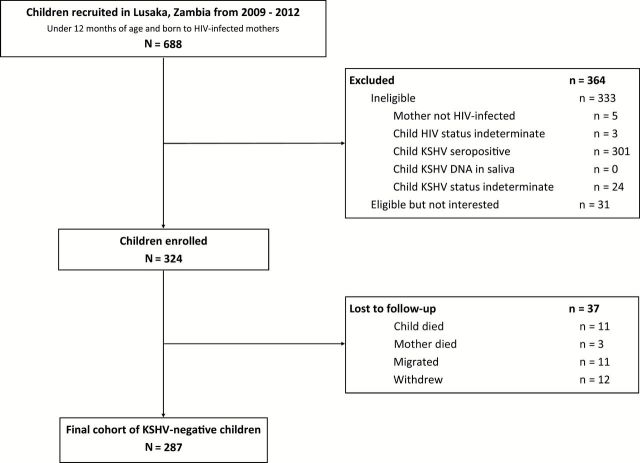
Initially, 688 children from Lusaka, Zambia, were screened for HIV and KSHV infections. A total of 324 eligible children were enrolled for follow-up, of which 287 returned for at least one follow-up visit (Figure 1). Reasons for exclusion and loss to follow-up are detailed in Figure 1.
Figure 1.
Flow chart summarizing the recruitment and enrollment procedures for the mother-child pair cohort in Lusaka, Zambia, 2009–2012. HIV = human immunodeficiency virus; KSHV = Kaposi’s sarcoma–associated herpesvirus.
Table 1 summarizes the demographics of the current study cohort (N = 287), including HIV-uninfected (n = 196) and infected (n = 91) children. We detected statistically significant differences in maternal characteristics and child age between HIV-uninfected and infected children (Table 1). Because the aim of our study was to evaluate the impact of ART on KSHV acquisition, we first investigated whether there were any differences in overall health status between HIV-infected and uninfected children at study enrollment. We found that all clinical characteristics were similar between HIV-infected and uninfected children, with the exception of small yet statistically significant differences in hematocrit and monocyte levels (Table 2).
During the follow-up period, 151 (52.6%) children underwent KSHV seroconversion. We did not detect any associations between KSHV seroconversion and recent generalized symptoms when analyzed individually or as a group (data not shown). Analysis of full blood count data revealed a statistically significant association between KSHV seroconversion and higher total lymphocyte percentage (median [IQR] = 61 (53–68) vs 55 (47–62), odds ratio [OR] = 1.05, 95% confidence interval [CI] = 1.03 to 1.08). Higher lymphocyte counts suggest that acute KSHV infection occurred recently, despite the lack of distinguishable clinical symptoms associated with KSHV seroconversion.
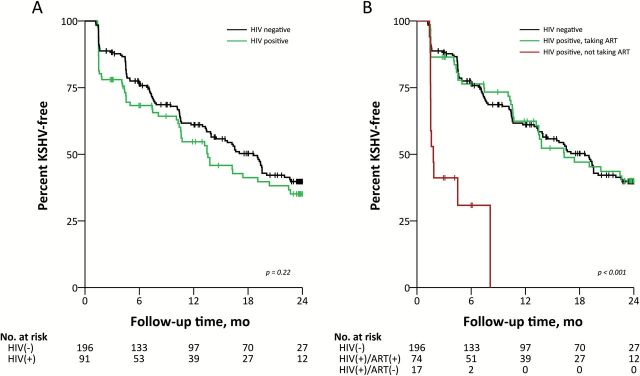
The entire cohort contributed 3552 months of follow-up, with a median follow-up time from enrollment to seroconversion or censor date of 10.5 months (IQR = 4.5–22.8). Overall, we calculated an incidence rate of 4.25 KSHV infections per 100 child-months (Table 3). We did not observe any socio-demographic, medical, or maternal characteristics to be statistically significantly associated with an increased rate of KSHV infection. We also determined that the rates of KSHV infection were similar in HIV-infected and uninfected children (Table 3 and Figure 2A). However, when HIV-infected children were stratified by ART status prior to KSHV seroconversion or censor date, ART-naïve children had a statistically significantly increased rate of KSHV acquisition compared with HIV-uninfected children (incidence rate ratio [IRR] = 5.97, 95% CI = 3.13 to 11.41). In contrast, the HIV-infected children taking ART acquired KSHV at a rate indistinguishable from HIV-uninfected controls (IRR = 0.99, 95% CI = 0.68 to 1.43) (Table 3 and Figure 2B). The cumulative probability of KSHV infection was also statistically significantly higher in ART-naïve HIV-infected children when age of the child was analyzed as the time-dependent variable (P < .001) (Supplementary Figure 1, available online).
Table 3.
Crude incidence rate of KSHV per 100 child-months since time of enrollment in a longitudinal cohort of 287 children from Lusaka, Zambia, 2009–2012*
| Characteristic | No. of children | KSHV-positive children | % | KSHV-free child-months | Incidence rate per 100 child-months | IRR (95% CI) |
|---|---|---|---|---|---|---|
| Total | 287 | 151 | 52.6 | 3552 | 4.25 | - - |
| Mother ART | ||||||
| Never | 126 | 71 | 56.3 | 1431 | 4.96 | Ref. |
| Ever | 161 | 80 | 49.7 | 2120 | 3.77 | 0.76 (0.55 to 1.05) |
| Child HIV status | ||||||
| Negative | 196 | 100 | 51.0 | 2512 | 3.98 | Ref. |
| Positive | 91 | 51 | 56.0 | 1040 | 4.90 | 1.23 (0.88 to 1.73) |
| Child HIV/ART status | ||||||
| HIV(-) | 196 | 100 | 51.0 | 2512 | 3.98 | Ref. |
| HIV(+)/ART(+) | 74 | 39 | 52.7 | 989.6 | 3.94 | 0.99 (0.68 to 1.43) |
| HIV(+)/ART(-) | 17 | 12 | 70.6 | 50.47 | 23.78 | 5.97 (3.13 to 11.41) |
* ART = antiretroviral therapy; CI = confidence interval; HIV = human immunodeficiency virus; IRR = incidence rate ratio; KSHV = Kaposi’s sarcoma–associated herpesvirus.
Figure 2.
Kaplan-Meier plots estimating the probability of remaining Kaposi’s sarcoma–associated herpesvirus–free since enrollment in a longitudinal cohort of 287 children from Lusaka, Zambia, 2009–2012. A) Probabilities stratified by child HIV status. B) Probabilities stratified by child HIV and antiretroviral status. Vertical lines indicate censoring. Log-rank test was used to calculate statistical significance. All tests were two-sided. ART = antiretroviral therapy; HIV = human immunodeficiency virus; KSHV = Kaposi’s sarcoma–associated herpesvirus.
Among HIV-infected children, baseline demographic, maternal, and clinical characteristics were similar between ART-treated and ART-naïve children, with the exception of age and age-related variables (Supplementary Table 1, available online). In multivariable Cox proportional hazard analysis among HIV-infected children, we found that the absence of ART statistically significantly increased the risk of KSHV acquisition (AHR = 5.04, 95% CI = 2.36 to 10.80, P < .001). Time-updated CD4+ T-cell percentage was also statistically significantly associated with risk of KSHV acquisition (AHR = 0.82, 95% CI = 0.74 to 0.92, P < .001), such that each 5% increase of CD4+ T-cells represented an 18% decrease in risk of acquiring KSHV (Table 4). In order to investigate for possible associations of KSHV infection with the age at which ART was initiated or the ART regimen received, we analyzed data from HIV-infected children actively taking ART. The distribution of specific ART regimens among the HIV-infected children are presented in Supplementary Figure 2 (available online), and we found no differences in KSHV acquisition by ART regimen. Similar to all children infected with HIV, however, we found that higher CD4% in children taking ART was associated with a decreased risk of KSHV infection (AHR = 0.83, 95% CI = 0.73 to 0.93, P = .002). The observed risks of KSHV infection were similar whether or not age and sex were included in the models (Table 4).
Table 4.
Multivariable Cox regression models for risk of KSHV acquisition since time of enrollment among 91 HIV-infected children in a longitudinal cohort of 287 children from Lusaka, Zambia, 2009–2012
| Characteristic | Model 1* | Model 2* | ||
|---|---|---|---|---|
| HR (95% CI) | P† | HR (95% CI) | P† | |
| HIV+ children | ||||
| Absence of ART | 5.04 (2.36 to 10.80) | <.001 | 4.34 (1.94 to 9.70) | <.001 |
| CD4% (5% intervals) | 0.82 (0.74 to 0.92) | <.001 | 0.83 (0.74 to 0.92) | <.001 |
| Age at enrollment, mo | - - | - | 0.96 (0.88 to 1.05) | .37 |
| Male sex | - - | - | 1.33 (0.76 to 2.34) | .31 |
| ART+ children | ||||
| CD4% (5% intervals) | 0.83 (0.73 to 0.93) | .002 | 0.85 (0.75 to 0.96) | .008 |
| Age at enrollment, mo | - - | - | 0.98 (0.88 to 1.08) | .63 |
| Male sex | - - | - | 1.72 (0.88 to 3.32) | .11 |
* Model 1 was adjusted for antiretroviral therapy (ART) status and CD4% among HIV+ children and CD4% alone among ART+ children. Model 2 was also adjusted for age and sex. ART = antiretroviral therapy; CI = confidence interval; HIV = human immunodeficiency virus; HR = hazard ratio; KSHV = Kaposi’s sarcoma–associated herpesvirus.
† Chi-square test was used to calculate P values. All tests were two-sided.
Discussion
This is the first study to investigate the effect of ART on KSHV incidence in HIV-infected children. Several cross-sectional studies conducted in sub-Saharan Africa have demonstrated higher KSHV seroprevalence among HIV-infected children compared with HIV-uninfected children (26–28). Our previous longitudinal cohort study in Zambia further established that HIV-infected children were five-fold more likely to acquire KSHV (17). However, these studies were conducted prior to the introduction of ART in sub-Saharan Africa. In the present study, we observed similar rates of KSHV acquisition between HIV-infected and uninfected children—likely because of the implementation of effective ART among HIV-infected children in Zambia. Consistent with this concept and our previous findings, we also observed that HIV-infected ART-naïve children were five-fold more likely to acquire KSHV compared with HIV-infected children taking ART.
ART success is routinely assessed by CD4+ T-cell levels. Since CD4 count varies in young children, because of normal infant lymphocytosis or factors such as malnutrition and infections, we used CD4 percentage as a more reliable measure for immune status (29). Although very few HIV-infected children in our cohort were classified with advanced immunosuppression by WHO CD4% categories, we did detect statistically significantly lower risk of KSHV infection among children with higher CD4+ T-cell percentages. This data supports the hypothesis that the risk of acquiring KSHV among HIV-infected children is related to CD4+ T-cell depletion and subsequent HIV-induced immune dysfunction. The specific immune dysfunction that would confer susceptibility to KSHV infection is unknown. Activated B-cells support KSHV infection and replication, whereas resting B-cells do not (30); therefore, B-cell hyperactivity, functional reduction of cytotoxic T-lymphocytes, and the pro-inflammatory state triggered by HIV may create an environment conducive for KSHV infection. Initiation of ART reduced immune activation in a different cohort of HIV-infected Zambian children (31). Furthermore, early ART statistically significantly reduced all-cause mortality and HIV disease progression in the Children with HIV Early Antiretroviral (CHER) trial (32). Coupled with the present data, it is likely that ART initiation within weeks of HIV infection, as was the case in our cohort, may protect and maintain normal immune functions, thereby reducing KSHV incidence and ultimately the burden of KS.
Alternatively, ART might be directly inhibiting KSHV acquisition. A recent study revealed that nelfinavir inhibited KSHV replication in vitro, but all other antiretrovirals tested—including multiple NRTIs, NNRTIs, and PIs—had no effect (33). Nelfinavir was not prescribed to any children in the present cohort; nevertheless, ART was still associated with lower risk of KSHV acquisition. Consequently, whether any ART components can directly prevent new KSHV infection or establishment of latency is unclear. Longitudinal follow-up of children after recent KSHV acquisition will allow us to delineate the impact of ART on KSHV latency and replication during the early stages of infection in HIV-infected children.
Our cohort study has several strengths, which include prospective design, regular and frequent follow-up visits, and a large number of HIV-infected children with access to ART. However, there are some limitations. Many children were not enrolled in the study because of evidence of prior KSHV seroconversion; therefore, the incidence measures described in this report are likely to underrepresent the true incidence among HIV-exposed children. Additionally, our mIFA is unable to distinguish between a primary or secondary seroconversion, as it detects IgG antibodies but not IgM. However, we have several other reasons to be confident that the seroconversion event represents primary KSHV acquisition: We enrolled very young children, KSHV was not detected in saliva of children at enrollment, and we observed an association between KSHV seroconversion and higher lymphocyte percentage. A final limitation was the low number of ART-naïve children in our cohort. This was expected, because ART was offered to all HIV-infected children as part of the study—consistent with strong ethical practices. Nevertheless, there were still a number of caregivers who decided not to start ART for their children, regardless of being counseled about the importance of early treatment. Although many factors related to the mother were not associated with KSHV acquisition, it is possible that these caregivers may have performed behaviors that we did not assess, such as specific feeding habits, further increasing risk of KSHV transmission. Despite these limitations, we still observed a large and statistically significant increase in risk of KSHV acquisition among ART-naïve children. Our findings are directly applicable to HIV-infected children in Zambia, a resource-limited country with high KSHV/HIV prevalence and early childhood acquisition. Our findings may also be applicable to other countries in the region, but not in other settings where KSHV/HIV prevalence or ART coverage differs substantially. Treatment with ART is not directly applicable to HIV-uninfected children at risk for KSHV infection, although reducing the overall number of KSHV infections and transmission sources may in turn reduce the risk of KSHV transmission to HIV-uninfected children.
We did not detect associations between KSHV infection and the age of the child at ART initiation or type of ART regimen in Cox proportional hazard analysis. These data should not be interpreted that ART-related variables are unimportant for reducing KSHV incidence, but rather that our cohort was not designed to investigate these variables. Children in our cohort were recruited and followed at very young ages, therefore restricting the ART initiation age range that could be analyzed. Additionally, although three major ART regimens were represented in our cohort, over 80% of the children were taking a two-NRTI-and-one-NNRTI regimen. Numerous studies have demonstrated that ART can reduce progression of KS (34), and one suggests PI-based regimens may be more effective than others (35). Therefore, our observation that ART is also associated with a lower risk of acquiring KSHV warrants further research into whether particular ART regimens are more effective at preventing new KSHV infections.
Because a vaccine against KSHV is not available and efforts to develop one are limited (36), it is paramount to develop alternative strategies to prevent KSHV acquisition during early childhood. Our data suggest that ART and prevention of immune suppression play an important role in reducing the incidence and risk of KSHV acquisition among HIV-infected children in an area where both viruses are highly endemic. The WHO is currently advocating ART programs for HIV-infected pregnant women and their children to prevent new HIV infections and increase treatment retention (37). Our data highlight the importance of these efforts and additional programs to provide ART to children as soon as HIV infection is diagnosed, not only to reduce HIV-associated morbidity but also help prevent KSHV infection and reduce the burden of KS among HIV-infected children.
Funding
This work was supported by the US National Institutes of Health (NIH; grant numbers R01 CA75903, P30 GM103509, T32 AI060547 to CW) and the US Fogarty International Center (grant number D43 TW01492 to CW). LNO was supported in part by the NIH under a Ruth L. Kirschstein National Research Service Award from the National Institute of Allergy and Infectious Diseases and by a Maude Hammond Fling Fellowship from the University of Nebraska-Lincoln. CG was supported as a Fogarty Fellow.
Supplementary Material
The funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. We declare that we have no conflicts of interest.
We thank all children and caregivers for their participation in the study, as well as community health workers and laboratory staff at UTH in Lusaka for their contributions to recruitment, data management, and sample processing.
VM, CK, JW, CM, and CW conceived and designed the study. LNO, VM, and CG collected data, LNO and VM analyzed data and performed statistical analyses, and LNO wrote the first draft of the report. All authors interpreted the data, revised the drafts, and approved the final version.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Oettle AG. Geographical and racial differences in the frequency of Kaposi’s sarcoma as evidence of environmental or genetic causes. Acta - Unio Internationalis Contra Cancrum. 1962;18:330–363. [PubMed] [Google Scholar]
- 3. Cook-Mozaffari P, Newton R, Beral V, et al. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78(11):1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parkin DM, Sitas F, Chirenje M, et al. Part I: Cancer in Indigenous Africans—burden, distribution, and trends. Lancet Oncol. 2008;9(7):683–692. [DOI] [PubMed] [Google Scholar]
- 5. Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Child. 1995;73(2):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bohlius J, Valeri F, Maskew M, et al. Kaposi’s Sarcoma in HIV-infected patients in South Africa: Multicohort study in the antiretroviral therapy era. Int J Cancer. 2014;135(11):2644–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UNAIDS. HIV Fact Sheet. http://www.unaids.org/sites/default/files/media_asset/20140716_FactSheet_en.pdf. Accessed November 28, 2014.
- 8. Tukei VJ, Kekitiinwa A, Beasley RP. Prevalence and outcome of HIV-associated malignancies among children. AIDS. 2011;25(14):1789–1793. [DOI] [PubMed] [Google Scholar]
- 9. Slone JS, Chunda-Liyoka C, Perez M, et al. Pediatric Malignancies, Treatment Outcomes and Abandonment of Pediatric Cancer Treatment in Zambia. PLoS ONE. 2014;9(2):e89102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davidson A, Wainwright RD, Stones DK, et al. Malignancies in South African children with HIV. J Pediatr Hematol Oncol. 2014;36(2):111–117. [DOI] [PubMed] [Google Scholar]
- 11. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869. [DOI] [PubMed] [Google Scholar]
- 12. Cesarman E, Chang Y, Moore PS, et al. Kaposi’s Sarcoma–Associated Herpesvirus-Like DNA Sequences in AIDS-Related Body-Cavity–Based Lymphomas. New Engl J Med. 1995;332(18):1186–1191. [DOI] [PubMed] [Google Scholar]
- 13. Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- 14. Dedicoat M, Newton R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br J Cancer. 2003;88(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao S-J, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925–928. [DOI] [PubMed] [Google Scholar]
- 16. Simpson GR, Schulz TF, Whitby D, et al. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348(9035):1133–1138. [DOI] [PubMed] [Google Scholar]
- 17. Minhas V, Crabtree KL, Chao A, et al. Early Childhood Infection by Human Herpesvirus 8 in Zambia and the Role of Human Immunodeficiency Virus Type 1 Coinfection in a Highly Endemic Area. Am J Epidemiol. 2008;168(3):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mbulaiteye SM, Pfeiffer RM, Engels EA, et al. Detection of Kaposi Sarcoma—Associated Herpesvirus DNA in Saliva and Buffy-Coat Samples from Children with Sickle Cell Disease in Uganda. J Infect Dis. 2004;190(8):1382–1386. [DOI] [PubMed] [Google Scholar]
- 19. Brayfield BP, Kankasa C, West JT, et al. Distribution of Kaposi Sarcoma-Associated Herpesvirus/Human Herpesvirus 8 in Maternal Saliva and Breast Milk in Zambia: Implications for Transmission. J Infect Dis. 2004;189(12):2260–2270. [DOI] [PubMed] [Google Scholar]
- 20. Crabtree KL, Wojcicki JM, Minhas V, et al. Risk Factors for Early Childhood Infection of Human Herpesvirus-8 in Zambian Children: The Role of Early Childhood Feeding Practices. Cancer Epidemiol Biomarkers Prev. 2014;23(2):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Republic of Zambia Ministry of Health. Guidlines for Antiretroviral Therapy for HIV in Infants and Children in Zambia. http://www.emtct-iatt.org/wp-content/uploads/2014/05/Zambia-pediatric-guidelines-2010.pdf. Accessed March 27, 2015.
- 22. WHO. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting. http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf. Accessed March 27, 2015.
- 23. Kankasa C, Carter RJ, Briggs N, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minhas V, Crabtree KL, Chao A, et al. The Zambia Children’s KS-HHV8 Study: Rationale, Study Design, and Study Methods. Am J Epidemiol. 2011;173(9):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olp LN, Shea DM, White MK, et al. Early childhood infection of Kaposi’s sarcoma-associated herpesvirus in Zambian households: A molecular analysis. Int J Cancer. 2013;132(5):1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wakeham K, Webb EL, Sebina I, et al. Risk Factors for Seropositivity to Kaposi Sarcoma-Associated Herpesvirus Among Children in Uganda. J Acquir Immune Defic Syndr. 2013;63(2):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minhas V, Brayfield BP, Crabtree KL, et al. Primary gamma-herpesviral infection in Zambian children. BMC Infect Dis. 2010;10(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dedicoat M, Newton R, Alkharsah KR, et al. Mother-to-Child Transmission of Human Herpesvirus-8 in South Africa. J Infect Dis. 2004;190(6):1068–1075. [DOI] [PubMed] [Google Scholar]
- 29. WHO. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillence. http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf. Accessed August 28, 2014.
- 30. Rappocciolo G, Hensler HR, Jais M, et al. Human Herpesvirus 8 Infects and Replicates in Primary Cultures of Activated B Lymphocytes through DC-SIGN. J Virol. 2008;82(10):4793–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rainwater-Lovett K, Nkamba H, Mubiana-Mbewe M, et al. Changes in Cellular Immune Activation and Memory T-Cell Subsets in HIV-Infected Zambian Children Receiving HAART. J Acquir Immune Defic Syndr. 2014;67(5):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382(9904):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gantt S, Carlsson J, Ikoma M, et al. The HIV Protease Inhibitor Nelfinavir Inhibits Kaposi’s Sarcoma-Associated Herpesvirus Replication In Vitro. Antimicrob Agents Chemother. 2011;55(6):2696–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krown SE. Highly Active Antiretroviral Therapy in AIDS-Associated Kaposi’s Sarcoma: Implications for the Design of Therapeutic Trials in Patients With Advanced, Symptomatic Kaposi’s Sarcoma. J Clin Oncol. 2004;22(3):399–402. [DOI] [PubMed] [Google Scholar]
- 35. Leitch H, Trudeau M, Routy JP. Effect of Protease Inhibitor-Based Highly Active Antiretroviral Therapy on Survival in HIV-Associated Advanced Kaposi’s Sarcoma Patients Treated with Chemotherapy. HIV Clin Trials. 2003;4(2):107–114. [DOI] [PubMed] [Google Scholar]
- 36. Wu TT, Qian J, Ang J, et al. Vaccine prospect of Kaposi sarcoma-associated herpesvirus. Curr Opin Virol. 2012;2(4):482–488. [DOI] [PubMed] [Google Scholar]
- 37. WHO. Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. http://whqlibdoc.who.int/hq/2012/WHO_HIV_2012.6_eng.pdf?ua=1. Accessed August 28, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.