Abstract
Indoleamine 2, 3-dioxygenases (IDO1 and IDO2) and tryptophan 2, 3-dioxygenase (TDO) are tryptophan catabolic enzymes that catalyze the conversion of tryptophan into kynurenine. The depletion of tryptophan and the increase in kynurenine exert important immunosuppressive functions by activating T regulatory cells and myeloid-derived suppressor cells, suppressing the functions of effector T and natural killer cells, and promoting neovascularization of solid tumors. Targeting IDO1 represents a therapeutic opportunity in cancer immunotherapy beyond checkpoint blockade or adoptive transfer of chimeric antigen receptor T cells. In this review, we discuss the function of the IDO1 pathway in tumor progression and immune surveillance. We highlight recent preclinical and clinical progress in targeting the IDO1 pathway in cancer therapeutics, including peptide vaccines, expression inhibitors, enzymatic inhibitors, and effector inhibitors.
Keywords: Indoleamine 2, 3-dioxygenases, IDO1, Immunosuppression, Immunotherapy, Clinical trial
Background
The tryptophan (Trp) catabolism pathway plays an important role in tumor cell evasion of the innate and adaptive immune systems [1, 2]. Trp is generally utilized in three major metabolic pathways: incorporation into proteins, production of serotonin, and breakdown into kynurenine (Kyn). Kyn is generated via two major routes: in peripheral tissues, controlled by the rate-limiting enzymes indoleamine 2, 3-dioxygenase 1 (IDO1) and indoleamine 2, 3-dioxygenase 2 (IDO2), and the hepatic route, in which tryptophan 2, 3-dioxygenase (TDO) is the rate-limiting enzyme [3] (Fig. 1). Discovered in the 1950s, IDO1 is the most fully characterized enzyme in the Kyn biosynthesis pathway. In healthy post-natal individuals, IDO1 facilitates tolerance by dampening the immune response, whereas during gestation, IDO1 helps protect the fetus from maternal T lymphocytes [4]. On the other hand, IDO1 is a crucial innate immunity regulator that acts by depleting Trp in both the inflammatory and tumor microenvironments [1, 5, 6]. IDO1 is involved in the suppression of effector T and NK cells and differentiation and activation of regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs) [7–9]. In addition, IDO1 plays a key role in promoting tumor neovascularization by modulating the expression of interferon-γ (IFN-γ) and interleukin-6 (IL-6) [10, 11]. In prostate cancer cells, IFN-γ and TNF-α-treatment induce IDO1 and IL-6 gene expression [12]. Furthermore, IDO1 is involved in the formation of resistance to immune checkpoint inhibitors [13], and the combination of an IDO1 inhibitor with checkpoint inhibitors represents an alternative strategy in cancer immunotherapy [14].
Fig. 1.
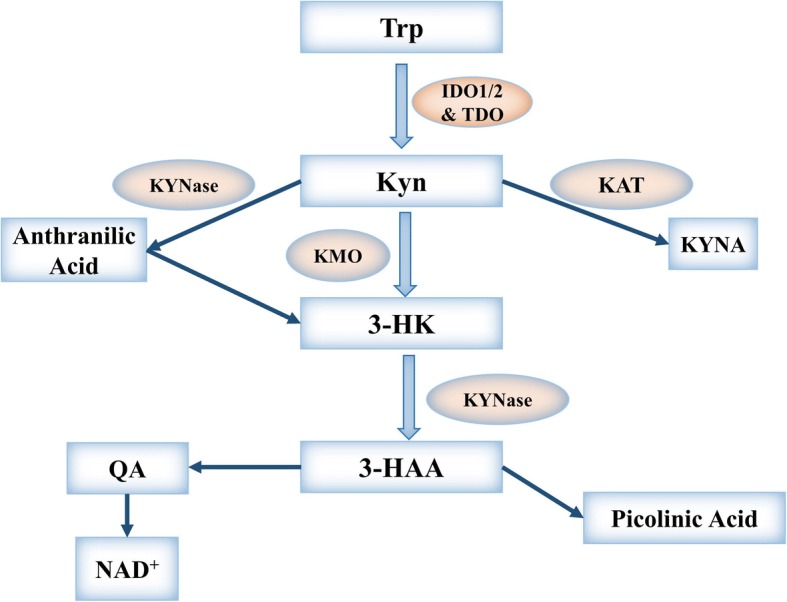
Overview of the IDO metabolic pathway. Approximately 95% of L-tryptophan (Trp) is catabolized into kynurenine (Kyn) through three rate-limiting enzymes: tryptophan 2,3-dioxygenase (TDO) in the liver and indoleamine 2, 3-dioxygenase 1/2 (IDO1/2) in peripheral tissues. Kyn is converted to 3-hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO), to anthranilic acid (AA) by kynureninase (KYNase), or to kynurenic acid (KYNA) by kynurenine aminotransferase (KAT). Next, catalyzed by KYNase, 3-HK is converted to 3-hydroxyanthranilic acid (3-HAA), which is further converted to quinolinic acid (QA), picolinic acid, nicotinamide adenine dinucleotide (NAD+), and other molecules
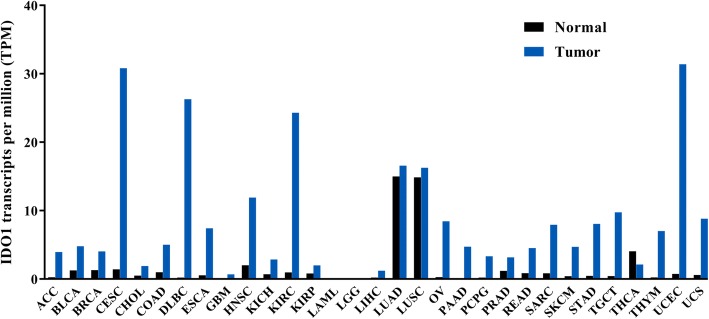
IDO1 is overexpressed in the vast majority of cancers (Fig. 2, data are summarized from The Cancer Genome Atlas, https://cancergenome.nih.gov/). Several strategies for targeting IDO1 have been assessed in multiple clinical trials and have produced encouraging results. Blockade of IDO1 activity decreased tumor proliferation in a T-lymphocyte-mediated manner and enhanced the efficacy of chemotherapy, radiotherapy, targeted therapy, and immunotherapy [15–19]. In this review, we will first discuss the complex role of IDO1 in regulating the innate and adaptive immune responses. We will then highlight the role of IDO1-related signaling pathways in the tumor microenvironment. Finally, we summarize the current preclinical and clinical studies of IDO-targeting interventions.
Fig. 2.
The IDO1 gene transcripts across tumor samples and paired normal tissues. Data are summarized from The Cancer Genome Atlas, https://cancergenome.nih.gov/. The Y axis denotes the number of IDO1 transcripts per million of total RNA reads. Abbreviations: ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCS, uterine carcinosarcoma; UCEC, uterine corpus endometrial carcinoma
IDO regulatory and effector signaling pathways
Multiple upstream pathways regulate IDO1 expression and function, including the Janus kinase–signal transducer and activator of transcription (JAK–STAT), RAS–protein kinase C (RAS–PKC), nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB), and Kit signaling pathways [20–22]. Downstream of IDO1 are three effector pathways that transduce the effects of IDO1 activity: general control over nonderepressible 2 (GCN2) is activated, mammalian target of rapamycin (mTOR) is inhibited, which is related to Trp deprivation, and the aryl hydrocarbon receptor (AhR) pathway is activated with Kyn as an endogenous AhR ligand [23–25]. These regulatory and effector pathways mediate immunosuppression and neovascularization in the tumor microenvironment.
Upstream regulators
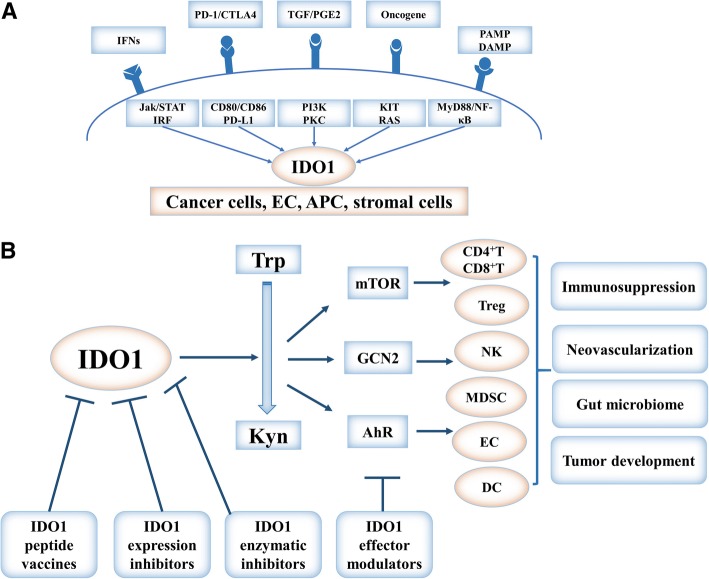
IDO1 is not expressed in most tissues in adult humans under physiological conditions but is constitutively expressed in many types of cancer cells, stromal cells, and immune cells in the tumor microenvironment (Fig. 3a). IDO1 is activated by diverse inflammatory molecules, such as IFN-γ, tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and prostaglandin E2 (PGE2), through canonical and non-canonical NF-κB and JAK–STAT pathways [21, 23, 25–27]. Constitutive IDO1 expression in human cancers is driven by cyclooxygenase 2 (COX-2) and PGE2 via the PKC and PI3K pathways [28]. Moreover, autocrine TGF-β sustains the activation of IDO1 in a tolerogenic subpopulation of CD8+ dendritic cells (DCs), while exogenous TGF-β converts immunogenic CD8− DCs into tolerogenic cells in conjunction with induction of IDO1 [29]. IDO1 is under genetic control of the cancer-suppression gene bridging integrator 1 (Bin1), and Bin1 knockout results in tumor growth and immune suppression in mice by upregulating STAT1- and NF-κB-dependent expression of IDO1 [20]. Ras and Kit also upregulate IDO1 expression in cancer cells [22, 27]. Importantly, several immune checkpoints (e.g., PD-1 and CTLA4) on the T cell surface modulate IDO1 expression in antigen-presenting cells. Recently, Wang et al. [30] demonstrated that the IL-6-inducible proto-oncogene protein intestine-specific homeobox (ISX) gene induces IDO1 and TDO expression, which increases Kyn and AhR and thereby promotes the tumorigenic potential and immunosuppression of hepatocellular carcinoma cells expressing CD86 and PD-L1. Others report that hypoxia enhances IDO1 production in monocyte-derived dendritic cells, yet the underlying mechanisms remain elusive [31].
Fig. 3.
Regulation, function, and targeting of IDO1 in cancer. a The upstream regulators of IDO1. IDO1 is expressed in cancer cells, endothelial cells, antigen-presenting cells, and stromal cells. IDO1 expression is regulated by IFNs, PD-1, oncogene activation (KIT or RAS), PAMPs, and DAMPs through relevant signaling pathways like IFN-γ/JAK/STAT, PI3K/PKC, and NF-κB. b The downstream effectors of IDO1 and IDO1 targeting. Three effector pathways (mTOR, GCN2, and AhR) mediate the effects of IDO1 activities in various types of cells in regard to immunosuppression, neovascularization, interactions with the gut microbiome, and the tumor microenvironment. Four strategies have been developed to target the IDO1 pathway in preclinical and clinical studies. IDO1, indoleamine 2, 3-dioxygenase 1; Trp, tryptophan; Kyn, kynurenine; IFN, interferon; PD-1, programmed death receptor 1; PAMP, pathogen-associated molecular pattern; DAMP, damage-associated molecular pattern; GCN2, general control over nonderepressible 2; mTOR, mammalian target of rapamycin; AhR, aryl hydrocarbon receptor; NK, natural killer cell; Treg, regulatory T cell; MDSC, myeloid-derived suppressor cell; EC, endothelial cell; DC, dendritic cell
Downstream effectors
IDO1 transduces signaling through three major effectors: GCN2, mTOR, and AhR. The depletion of Trp by IDO1 leads to the accumulation of uncharged Trp–tRNA, which binds and activates GCN2, a stress-response kinase. Activated GCN2 phosphorylates and inhibits eukaryotic initiation factor 2α kinase, resulting in attenuation of RNA transcription and protein translation. Specific to T effector cells, GCN2 activation mediated by Trp deprivation leads to cell cycle arrest and/or apoptosis [25, 32]. Another signaling molecule inhibited by Trp deprivation is the mammalian target of rapamycin (mTOR) [23]. IDO1-mediated suppression through mTORC1 triggers autophagy, leading to anergy in T cells in the tumor microenvironment [23, 33]. Most relevant publications report that mTOR inhibition suppresses effector T cell function and promotes Treg cell function although others dispute these findings [34], and the precise role of IDO1 in mTOR signaling and immune regulation continues to be debated. Both GCN2 kinase activation and mTOR inhibition are immunosuppressive. During CD4+ T-cell differentiation, GCN2 kinase activation suppresses only Th2 differentiation, whereas mTOR inhibition induces Treg cell differentiation and suppresses differentiation of the Th1, Th2, and Th17 lineages [35]. These findings suggest that Trp deprivation-mediated GCN2 kinase activation and mTOR inhibition have differential impacts on the function of distinct CD4+ T cell populations. AhR is a cytosolic ligand-activated transcription factor involved in embryogenesis, adaptive immunity, mucosal barrier function, and malignancy. Its most potent ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), activates AhR signaling to upregulate IDO1 expression [36]. A breakthrough was reported in 2011 in which Kyn was found to be an endogenous ligand of AhR [37]. The binding of Kyn to AhR promotes naive CD4+ T cell differentiation into Treg cells, which contributes to an immunosuppressive tumor microenvironment [38].
Role of the IDO1 pathway in cancers
IDO1 is expressed by various cancer and cancer-associated cells in the tumor microenvironment, including DCs, endothelial cells, tumor-associated macrophages, tumor-associated fibroblasts, mesenchymal stromal cells (MSCs), and MDSCs [2, 39]. Most cancer types have high IDO1 expression (Fig. 2), which is correlated with poor survival and prognosis [40–42]. Besides its role in immunosuppression, IDO1 also contributes to cancer development by promoting inflammatory neovascularization [27], interacting with checkpoint inhibitors, and modulating gut microbiota [43].
IDO1 ablates T effector cells and promotes the induction of Treg cells and MDSCs in the tumor microenvironment
Increased IDO1 and accumulating Trp metabolites prevent the activation of CD8+ and CD4+ effector T cells, inhibit NK cell function, stimulate the activation of Treg cells, and promote the expansion and activation of DCs and MDSCs [25, 44–46]. Treg cells are a major suppressive cell type in the tumor microenvironment, as their recruitment is induced by high IDO1 levels and correlates with poor prognosis in several tumor types [47]. Furthermore, IDO1 presence leads to activation of the phosphatase and tensin homolog (PTEN) pathway in Treg cells to maintain their immunosuppressive phenotype in vitro [48] .
IDO1 is also essential for the recruitment and sustenance of MDSCs through a Treg-dependent mechanism [49]. MDSCs can suppress antitumor immune responses through STAT3-dependent IDO1 expression in breast cancer [50]. Increased STAT3 activation in MDSCs was found to be correlated with activation of the non-canonical NF-κB pathway, in which RelB–p52 dimers directly bind to the IDO1 promoter, leading to IDO1 expression in MDSCs [51]. In a Kras mutant lung cancer model, Ido1-deficient mice had impaired MDSC function [27].
Cancer-associated fibroblasts recruit and educate DCs to become IDO1-producing regulatory DCs through IL-6-mediated STAT3 activation [52]. Mesenchymal stem/stromal cells in the tumor microenvironment promote tumor growth through IDO1-mediated immune suppression [53]. Expression of IDO1 in tumor endothelial cells is negatively associated with long-term survival in patients with renal cell carcinoma [54]. In addition, the IDO1 expression level in tumor endothelial cells is associated with the tumor’s response to checkpoint inhibitor treatment in metastatic renal cell carcinoma [55].
IDO1 and immune checkpoint inhibitors
Antibodies targeting cytotoxic T cell antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1) have been approved by the Food and Drug Administration (FDA) for multiple cancers [56, 57]. Recent research further revealed that IDO1 is closely linked to both CTLA-4 and PD-1–PD-L1 via complex pathways. Treg cell-expressed CTLA-4 induced IDO1 expression in DCs [46], and PD-1/PTEN signaling in IDO1-activated Treg cells was required to maintain immune suppression of the Tregs [58]. IDO1 expression in DCs is induced by the interaction of PD-1 with PD-L1 on the surface of mast cells and/or with PD-L2 on the surface of DCs [59]. Moreover, IDO1 participates in the intracellular signaling events responsible for long-term tolerance by DCs [29]. Based on its upstream effects on DCs, this IDO1 activity is non-redundant with that of the more distal T cell checkpoints, and combined therapy with IDO1 inhibitors and CTLA-4 or PD-1 inhibitors should confer additional benefit.
IDO1 has also been reported to participate in a resistance mechanism to checkpoint inhibitors, and the combination of CTLA-4 blockade and an IDO1 inhibitor resulted in more effective antitumor immunity in a melanoma mouse model [13]. In a phase 2 trial, excessive infiltration of IDO1+ macrophages and a significant increase in the kynurenine-to-Trp ratio were observed in the majority of patients who had poor response to metronomic cyclophosphamide and pembrolizumab combination treatment [60]. In addition, Liu and colleagues identified a Kyn–AhR pathway-dependent mechanism that promoted tumor-repopulating cell immune escape by increasing PD-1 binding to CD8+ T cells. Local IFN-γ produced by tumor-infiltrating CD8+ T cells stimulates tumor-repopulating cell release at high levels of Kyn, which then activates AhR on the T cell surface to promote PD-1 upregulation. Thus, targeting the Kyn–AhR pathway (such as by inhibiting IDO1 in tumor-repopulating cell or AhR in CD8+ T cells) may enhance the efficacy of adoptive T cell therapy. Taken together, these results suggest that combination strategies targeting multiple immune checkpoints might be the preferred weapon of the future.
Tumor neovascularization
Neovascularization, characterized by excessive and disorganized growth of blood vessels, is critical for tumor development, progression, and metastasis. In mice, Ido1 deficiency inhibited lung tumor growth and improved animal survival with reduced density of the underlying pulmonary blood vessels [27]. Recent work also showed a new role for IDO1 in supporting pathologic neovascularization [11]. In 4T1 breast cancer pulmonary metastases and oxygen-induced retinopathy mouse models, IDO1 ablation was sufficient to reduce pathological neovascularization in both lung metastases and reinopathy. IL-6 is regarded as a pro-angiogenic cytokine potentiated by IDO1, and IL-6 deletion results in IFN-γ-dependent reduction in neovascularization and increases resistance to metastasis [11]. Administration of the IDO1 inhibitor epacodostat (INCB024360) in mice with tumors or vascularized metastases significantly reduced neovascularization [11]. High IDO1 expression positively correlates with microvessel density and poor prognosis in breast cancer patients [61]. In summary, IDO1-mediated effects on neovascular development and established neovascular networks broaden the potential effectiveness of IDO1 inhibitors in clinical trials and in practice.
IDO1 and the gut microbiome
The gut microbiome has been found to regulate cancer initiation, progression, and response to therapies [62]. Preclinical mouse models suggest that resistance in melanoma patients to anti-PD-1 immunotherapy can be attributed to abnormal gut microbiome composition [63]. Bacteria, including Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium, are more abundant in treatment responders, whereas the efficacy of immune checkpoint blockade therapies is diminished with administration of antibiotics [64–66]. As an essential nutrient in mammals, Trp and its IDO1-catalyzed endogenous metabolites play a key role in the gut microbiota and immune homeostasis. Recent discoveries have underscored the modulation of host immune systems by changes in the microbiota that affect Trp metabolism [43]. Activation of toll-like receptors by microbial components has been identified as a key factor in initiating Kyn metabolism and gut microbial homeostasis, as reduced toll-like receptor stimulation in germ-free mice resulted in decreased Trp metabolism [53, 67].
IDO1 inhibitors in preclinical development and clinical trials
Based on the important role of IDO1 in cancer immune tolerance and development, targeting IDO1 is becoming an attractive approach in cancer therapeutic development (Fig. 3b). Unlike cell-surface checkpoint receptor molecules that can be effectively targeted by antibody-based therapeutics, IDO1 and its downstream effector molecules are intracellular targets that are still best addressed by small molecule drugs. An increasing number of IDO1 inhibitors are being tested in preclinical development or clinical trials [15, 16] (Table 1).
Table 1.
IDO1 inhibitors in clinical trials
| Drug | Strategies | Tumor type | Phase | Clinical efficacy | Safety (% patients) | Trial number | Status |
|---|---|---|---|---|---|---|---|
| Indoximod | Single agent | Metastatic or refractory solid tumors | I | NR | NR | NCT00739609 | Terminated |
| Metastatic or refractory solid tumors | I | ORR 10% (5/48) | Fatigue (56.3%), anemia (37.5%), anorexia (37.5%) dyspnea (35.4%) cough (33.3%) nausea (29.2%) | NCT00567931 | Completed | ||
| Docetaxel | Metastatic solid tumors | I | 4/22PRs, 9/22 SD, and 9/22 PD | Fatigue (58.6%) anemia (51.7%) hyperglycemia (48.3%), infection (44.8%), nausea (41.4%) | NCT01191216 | Completed | |
| Temozolomide/bevacizumab | Primary malignant brain tumors | I/II | NR | NR | NCT02052648 | Recruiting | |
| Temozolomide | Progressive primary malignant brain tumors | I | NCT02502708 | Recruiting | |||
| Docetaxel/paclitaxel | Metastatic breast cancer | II | NCT01792050 | Unknown | |||
| Nab-Paclitaxel/gemcitabine | Metastatic pancreatic cancer | I/II | ORR 11/30 (37%) | 1/30 (colitis) | NCT02077881 | Recruiting | |
| Idarubicin/cytarabine | Acute myeloid leukemia | I/II | NCT02835729 | Recruiting | |||
| Adenovirus-p53 transduced dendritic cell (DC) vaccine | Metastatic breast cancer | I/II | Chemosensitization effect, median PFS 13.3 weeks and median OS 20.71 weeks. 9/22 patients benefitted from chemotherapy after vaccination. | Most common grade 1–2 (fatigue, anemia, transient lymphopenia, nausea, anorexia) | NCT01042535 | Completed | |
| Sipuleucel-T | Refractory metastatic prostate cancer | II | NR | NR | NCT01560923 | Active, not recruiting | |
| Tergenpumatucel-L/docetaxel | Advanced previously treated non-small cell lung cancer | I/II | NCT02460367 | Unknown | |||
| Ipilimumab/nivolumab/pembrolizumab | Metastatic melanoma | II/ III | NCT03301636 | Recruiting | |||
| Epacadostat | Single agent | Advanced malignancy | I/II | NR | Fatigue, nausea, decreased appetite, vomiting, constipation, abdominal pain, diarrhea, dyspnea, back pain, cough | NCT01195311 | Completed |
| Solid tumor | I | NCT03471286 | Not yet recruiting | ||||
| Myelodysplastic syndromes | II | NCT01822691 | Completed | ||||
| Fludarabine/cyclophosphamide/NK cells/IL-2 | Recurrent ovarian, fallopian tube, and primary peritoneal cancer | I | NCT02118285 | Completed | |||
| Tamoxifen | Ovarian cancer genitourinary tumors | II | Median PFS, 3.75 months | Fatigue (36.4%) rash (18.2%) pruritus (9.1%) |
NCT01685255 | Terminated | |
| Azacitidine + pembrolizumab | Advanced solid tumors | I/II | NR | NR | NCT02959437 | Recruiting | |
| MELITAC 12.1 peptide vaccine | Stage III-IV melanoma | II | NR | NR | NCT01961115 | Active, not recruiting | |
| DPX-survivac/cyclophosphamide | Ovarian cancer | I | NR | NR | NCT02785250 | Recruiting | |
| ALVAC(2)-NY-ESO-1 (M)/TRICOM vaccine | Ovarian, fallopian tube, or primary peritoneal cancer | I/II | NR | NR | NCT01982487 | Withdrawn | |
| DEC-205/NY-ESO-1 fusion protein CDX-1401/poly ICLC | Ovarian, fallopian tube, or primary peritoneal cancer | I/II | NR | NR | NCT02166905 | Recruiting | |
| CRS-207/pembrolizumab | Metastatic pancreas cancer, platinum-resistant ovarian, fallopian, or peritoneal cancer | II,I/II | NR | NR | NCT03006302 | Recruiting active, not recruiting | |
| NCT02575807 | |||||||
| Atezolizumab | Non-small cell lung cancer and urothelial carcinoma | I | NR | NR | NCT02298153 | Terminated | |
| Durvalumab | Advanced solid tumor | I/II | NR | NR | NCT02318277 | Recruiting | |
| Ipilimumab | Melanoma | I/II | NCT01604889 | Terminated | |||
| Nivolumab/ipilimumab/lirilumab | Solid tumors | I/II | NCT03347123 | Recruiting | |||
| Nivolumab/chemotherapy | Advanced cancers | I/II | ORR 75%(melanoma) 11% (ovarian) 4% (colorectal) | Rash (10% and 12% in epacadostat 100 and 300 mg subgroups) | NCT02327078 | Recruiting | |
| Pembrolizumab/chemotherapy | Advanced solid tumors | I, I/II | NR | NR | NCT02862457 | Recruiting | |
| NCT03085914 | Recruiting | ||||||
| pembrolizumab | Solid tumors, thymic carcinoma, sarcoma, junction or gastric cancer, lung cancer, urothelial cancer, metastatic melanoma, and others. | I, I/II, III | Melanoma (ORR 57% and DCR 86%), Renal cell carcinoma (ORR 40% and DCR 80%) | Fatigue, diarrhea, rash, arthralgia, and nausea | NCT02178722 | Recruiting | |
| NCT02364076 | Recruiting | ||||||
| NCT03414229 | Recruiting | ||||||
| NCT03196232 | Recruiting | ||||||
| NCT03322540 | Recruiting | ||||||
| NCT03361865 | Recruiting | ||||||
| Pembrolizumab | Melanoma | III | NCT02752074 | Halted | |||
| Navoximod | Single agent | Advanced solid tumors | I | NR | NR | NCT02048709 | Completed |
| Atezolizumab | Solid tumors | I | NCT02471846 | Active, not recruiting | |||
| PF-06840003 | Single agent | Malignant gliomas | I | NCT02764151 | Active, not recruiting | ||
| BMS-986205 | Nivolumab | Melanoma advanced cancers | III, I/II | NR | 3/42 patients with grade 3 autoimmune hepatitis | NCT03329846 | Recruiting |
| NCT03192943 | Recruiting | ||||||
| Nivolumab/ipilimumab | Advanced cancer melanoma non-small cell lung cancer | I/II | ORR 32%(bladder cancer) 14%(cervical cancer) PD-L1 > 1%: 46% (bladder cancer)and 25% (cervical cancer) |
Fatigue (18.2% nausea (18.2%) decreased appetite (13.6%) vomiting (6.8%) | NCT02658890 | Recruiting | |
| Nivolumab/ipilimumab/relatlimab | Advanced gastric cancer, advanced renal cell carcinoma, advanced cancer | II, I/II | NR | NR | NCT02935634 | Recruiting | |
| NCT02996110 | Recruiting | ||||||
| NCT03459222 | Not yet recruiting | ||||||
| NCT03335540 | Recruiting | ||||||
| Nivolumab/cetuximab/chemotherapy | Head and neck cancer | III | NR | NR | NCT03386838 | Halted | |
| Nivolumab/chemotherapy | Lung cancer | III | NCT03417037 | Halted |
The effector inhibitor indoximod
Indoximod (1-methyl-D-tryptophan, 1MT, NLG-8189) is the most-studied IDO1 inhibitor and has been granted orphan-drug designation by the US FDA for the indication of stage IIb to stage IV melanoma. Recent results suggest that indoximod is not only a valid inhibitor of IDO1 enzymatic activity but also acts as a high-potency Trp mimetic in reversing mTORC1 inhibition [23]. mTORC1 is a central integrator of cell growth signals that monitors levels of essential amino acids that are needed to activate cell growth [68]. Clinical results indicated that indoximod exerted little antitumor efficacy as a single agent, but efficacy was markedly enhanced when it was combined with other therapies, such as PD-1 checkpoint inhibitors (in advanced melanoma), cancer vaccines (in metastatic prostate cancer), and chemotherapy (in pancreatic cancer and acute myeloid leukemia). A phase 1 dose-escalation trial aiming to evaluate the safety, dosing, pharmacokinetics, and immunologic effects of indoximod found indoximod to be safe at doses up to 2000 mg orally twice daily [69]. Another phase 1 dose-escalation trial designed to study co-administration of docetaxel and indoximod reported that this combination is well tolerated, with no increase in expected toxicities in patients with metastatic solid tumors [70]. Administering indoximod with the PD-1 antibody pembrolizumab (Keytruda) led to a 61% overall response rate, including 10 complete responses (20%) and 21 partial responses (41%), in patients with advanced melanoma. The median progression-free survival under combinatorial treatment was 12.9 months, with a 1-year rate of 56%, suggesting a synergistic antitumor therapeutic effect [71].
IDO1 enzymatic inhibitors
Epacadostat (INCB024360)
Epacadostat is a Trp competitive inhibitor that has been widely investigated in clinical trials. With an IC50 of 72 nM, it has a > 1000-fold selectivity for the IDO1 enzyme relative to IDO2 or TDO [72]. In vitro studies found that epacadostat promoted T cell and NK cell proliferation, increased the number of CD86high DCs, and reduced Treg cells [73], and its administration to tumor-bearing syngeneic mice inhibited plasma and tumor Kyn levels by approximately 90% and reduced tumor growth in immunocompetent but not immunocompromised mice.
In a phase 1 clinical study, epacadostat was well tolerated at doses of 100 mg twice daily. No objective responses were detected although stable disease was observed in 7 of 52 patients over 16 weeks of observation [74]. A randomized phase 2 study compared epacadostat with tamoxifen treatment in 42 patients with biochemically recurrent epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer, found that epacadostat was well tolerated but its efficacy was no better than tamoxifen [75]. Based on encouraging results from its combination with immune checkpoint inhibitors, epacadostat was propelled into three clinical trials [76]. In melanoma, anti-PD-1 antibody combinations (either pembrolizumab or nivolumab) showed rates of overall response and disease control similar to those produced by the approved combination of anti-PD-1 and anti-CTLA-4 antibodies (ipilimumab) without the significant side effects of the latter. In head and neck cancer, an interim report on 38 patients who were previously treated suggested that epacadostat increased rates of overall response and disease control without any notable increase in side effects when administered with an anti-PD-1 antibody. Epacadostat is currently being tested in clinical trials in 14 tumor types (including the above cancers) with co-administration of anti-PD-1 antibodies (nivolumab or pembrolizumab) or anti-PD-L1 antibodies (atezolizumab and durvalumab).
Navoximod (NLG-919, GDC-0919)
Navoximod was initially developed as an orally bioavailable IDO1 and TDO inhibitor with a superior pharmacokinetic and toxicity profile based on 4-phenylimidazole, a compound that binds the heme iron at the IDO1-active site [77]. In preclinical studies, navoximod inhibited IDO1-induced T cell suppression and restored robust T cell responses (EC50 = 80 nM). Kyn levels in plasma were reduced by approximately 50% in mice treated with navoximod [78]. In a syngeneic murine B16-F10 melanoma model, navoximod potentiated the antitumor efficacy of paclitaxel without increasing side effects [79]. A combination of navoximod, anti-PD1/PD-L1/PD-L2 antibodies, and indoximod with chemotherapy and a glycoprotein 100 (gp100) peptide vaccine achieved a significant synergistic antitumor effect [80]. In a phase 1b clinical trial, the combination of navoximod and atezolizumab was well tolerated [81]; preliminary efficacy data from 45 patients with over 1 on-treatment tumor assessments included 4 patients (9%) with partial response and 11 (24%) patients with stable disease.
BMS-986205
BMS-986205 is an irreversible IDO1 inhibitor. In preclinical studies, BMS-986205 was found to specifically target and bind to IDO1 but not IDO2 or TDO [15]. By inhibiting IDO1 and decreasing Kyn levels in tumor cells, BMS-986205 reversed immunosuppression in cancer patients. In a phase 1/2a study, BMS-986205 was administered alone or in combination with nivolumab in multiple advanced malignancies. It was well tolerated, with no grade 3 events in BMS-986205 monotherapy and no grade 4 or 5 events in the combination group. In a dose escalation and expansion study (NCT02658890), an encouraging response was observed for BMS-986205 plus nivolumab. The maximum tolerated dose of BMS-986205 in combination with nivolumab was 200 mg, and the recommended dose for further study was 100 mg. Objective response rates in the bladder and cervical cancer cohorts were 32% and 14%, respectively, which improved to 46% and 25%, respectively, in tumors where over 1% of tumor cells expressed PD-L1. An increase in tumor-infiltrating CD8+ T cells and a decrease in Kyn were also observed [82]. Based on this potent effect and encouraging results overall, about 10 ongoing trials are investigating the effect of BMS-986205 combined with nivolumab as compared with nivolumab alone in patients with advanced melanoma, non-small cell lung cancer, head and neck cancer, advanced gastric cancer, and other types of cancer.
PF-06840003 and other inhibitors
PF-06840003 is an orally bioavailable, highly selective IDO1 inhibitor that can cross the blood–brain barrier. In vitro, it reverses IDO1-induced T-cell anergy. In preclinical syngeneic mouse tumor models, PF-06840003 reduced Kyn levels in mice by > 80% and enhanced the antitumor efficacy of anti-PD-1 or anti-PD-L1 antibodies. This compound has favorable human pharmacokinetic characteristics, with a prolonged half-life that enables single-dose daily administration. More importantly, its central nerve system (CNS) penetration properties allow its application in brain metastases [83]. An ongoing multi-center clinical trial aims to assess the safety, pharmacokinetic, and pharmacodynamic activity of PF-06840003 in malignant glioma and to validate its CNS penetration and effectiveness in combination with other drugs. Recently, BGB-5777, a potent CNS-penetrating IDO1 inhibitor, when combined with nivolumab and radiation therapy, achieved a durable survival benefit in patients with advanced glioblastoma [17]. A few other IDO1 inhibitors are in preclinical development, including Trp analogs, imidazoles, phenyl benzenesulfonylhydrazides, N-hydroxyamidines, and tryptanthrin derivatives [84]. These compounds offer novel scaffolds for central nerve system (CNS) optimization, providing abundant possibilities for developing highly specific IDO1 inhibitors.
IDO1 peptide vaccines
Previous studies have found that IDO1 peptides elicit specific CD8+ T cells that recognize and kill IDO1-expressing tumor cells and DCs and simultaneously enhance other T-cell responses [85]. In a phase 1 trial to evaluate the efficacy and safety of IDO1 vaccines, 15 patients with advanced NSCLC were injected with an IDO1-derived human leukocyte antigen A2-restricted epitope. The median overall survival was 25.9 months with no grade 3 or 4 toxicities. Furthermore, all treated patients had a significantly reduced Treg cell population after the sixth dose of vaccine [86]. Based on these promising results and distinct mechanisms of action, an additional phase 1 trial combining IDO1 vaccines with CTLA-4 inhibitor ipilimumab (Yervoy) for stage III or IV melanoma patients and a phase 1/2 clinical trial that tests a combination treatment with a PD-1 monoclonal antibody (nivolumab) and a PD-L1–IDO1 peptide vaccine, have been initiated. Other potential strategies, such as combining IDO1 inhibitors with IDO1 vaccines may also produce synergistic anti-tumor effects [87].
IDO1 expression inhibitors
Gene expression is often repressed by microRNAs. Our group analyzed IDO1 downregulation by microRNA-153 (miR-153) in colon cancer cells and the association of IDO1 and miR-153 expression with colorectal patient survival [18]. We found that IDO1 is highly expressed in colorectal tumors and is inversely associated with patient survival. miR-153 directly inhibits IDO1 expression by targeting its 3′ untranslated region in colon cancer cells, yet miR-153 overexpression does not affect colon cancer cell survival, apoptosis, or colony formation. When colon cancer cells are targeted by chimeric antigen receptor (CAR) T cells, miR-153 overexpression within tumor cells significantly enhances T cell killing in vitro and suppresses xenograft tumor growth in mice. These findings indicate that miR-153 is a tumor-suppressive miRNA that enhances CAR T cell immunotherapy and supports the combinatorial use of IDO1 inhibitors and CAR T cells in treating solid tumors [18].
Conclusions
IDO1 has diverse biological roles in immune suppression and tumor progression, rendering it an attractive target in cancer therapeutic development. IDO1 inhibitors may serve as an “immunometabolic” adjuvant to enhance systemic immune responses and turn immunologically “cold” tumors “hot.” Targeting IDO1 represents a therapeutic opportunity in cancer immunotherapy beyond checkpoint blockade [88–90] or adoptive transfer of CAR T cells [91–95]. Although IDO1 inhibitor monotherapies have shown disappointing efficacy, combinations of IDO1 inhibitors with conventional treatments show satisfactory efficacy in several trials. We note that IDO1 inhibitors suffered major setbacks in recent clinical trials. For the pivotal phase 3 ECHO-301/KEYNOTE-252 clinical trial (NCT02752074) that evaluated epacadostat in combination with pembrolizumab in patients with unresectable or metastatic melanoma, the study did not meet the primary endpoint of improving progression-free survival in the overall population compared with pembrolizumab monotherapy [96]. Two phase 3 trials (NCT03386838 and NCT03417037) evaluating the IDO1 inhibitor BMS-986205 in combination with nivolumab were also halted after re-evaluation. These negative results underscore that much about IDO1’s role in immune suppression remains unresolved. Further studies on the basic biology of IDO1/2 and TDO are essential to guide clinicians in identifying drug combinations that are more effective and selecting patients who are most likely to benefit from them. IDO1’s role in neovascular development suggests that when clinical results of IDO1 inhibitors are assessed, the impact on tumor vasculature should be examined. Targeting IDO2 and TDO in addition to IDO1 may open new windows for cancer immunotherapy.
Acknowledgements
The authors are grateful to Dr. Cassandra Talerico for editing the manuscript and providing critical comments.
Funding
This study was supported in part by an NIH R01 grant (CA177810 to YL); Natural Science Foundation of Guangdong Province grants (no. 2014A030313505 to ML, no. 2015A030315372 and no. 2017A030311004 to XM); and Science and Technology Program of Guangdong Province grants (no. 2015A020212034 to ML and no. 2014A050503062 to XM).
Availability of data and materials
All data and materials supporting the conclusions of this study have been included within the article.
Abbreviations
- AhR
Aryl hydrocarbon receptor
- CAF
Cancer-associated fibroblast
- CAR
Chimeric antigen receptor
- CNS
Central nerve system
- CTLA-4
Cytotoxic T cell antigen 4
- DAMP
Damage-associated molecular pattern
- DC
Dendritic cell
- DCR
Disease control rate
- GCN2
General control over nonderepressible 2
- IDO
Indoleamine 2, 3-dioxygenase
- IFN-γ
Interferon-γ
- JAK
Janus kinase
- Kyn
Kynurenine
- MDSC
Myeloid-derived suppressor cell
- MSC
Mesenchymal stromal cell
- mTOR
Mammalian target of rapamycin
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NR
Not reported
- ORR
Objective response rate
- OS
Overall survival
- PAMP
Pathogen-associated molecular pattern
- PD
Progressive disease
- PD-1
Programmed death receptor 1
- PD-L1
Programmed death receptor ligand 1
- PFS
Progression-free survival
- PGE2
Prostaglandin E2
- PKC
Protein kinase C
- PR
Partial response
- SD
Stable disease
- STAT
Signal transducer and activator of transcription
- TAM
Tumor-associated macrophage
- TDO
Tryptophan 2,3-dioxygenase
- TGF-β
Transforming growth factor β
- TNF-α
Tumor necrosis factor α
- Treg
Regulatory T cell
- Trp
Tryptophan
Authors’ contributions
ML and YL designed the study and wrote the manuscript. All authors contributed to data analyses and interpretation, and read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Liu, Email: mingliu128@hotmail.com.
Yong Li, Email: liy2@ccf.org.
References
- 1.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63(7):721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy - challenges and opportunities. Trends Pharmacol Sci. 2018;39(3):307–325. doi: 10.1016/j.tips.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond) 2015;129(7):601–672. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida R, Urade Y, Tokuda M, Hayaishi O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci USA. 1979;76(8):4084–4086. doi: 10.1073/pnas.76.8.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164(7):3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 9.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mondal A, Smith C, DuHadaway JB, Sutanto-Ward E, Prendergast GC, Bravo-Nuevo A, Muller AJ. IDO1 is an integral mediator of inflammatory neovascularization. EBioMedicine. 2016;14:74–82. doi: 10.1016/j.ebiom.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banzola I, Mengus C, Wyler S, Hudolin T, Manzella G, Chiarugi A, Boldorini R, Sais G, Schmidli TS, Chiffi G, et al. Expression of indoleamine 2,3-dioxygenase induced by IFN-gamma and TNF-alpha as potential biomarker of prostate cancer progression. Front Immunol. 2018;9:1051. doi: 10.3389/fimmu.2018.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210(7):1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017;77(24):6795–6811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohrig UF, Majjigapu SR, Vogel P, Zoete V, Michielin O. Challenges in the discovery of indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors. J Med Chem. 2015;58(24):9421–9437. doi: 10.1021/acs.jmedchem.5b00326. [DOI] [PubMed] [Google Scholar]
- 17.Ladomersky E, Zhai L, Lenzen A, Lauing KL, Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F, et al. IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin Cancer Res. 2018; [DOI] [PMC free article] [PubMed]
- 18.Huang Q, Xia J, Wang L, Wang X, Ma X, Deng Q, Lu Y, Kumar M, Zhou Z, Li L, et al. miR-153 suppresses IDO1 expression and enhances CAR T cell immunotherapy. J Hematol Oncol. 2018;11(1):58. doi: 10.1186/s13045-018-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G, Heslop HE, Brenner MK, Rooney CM, Ramos CA. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125(25):3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11(3):312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 21.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105(44):17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1(9):1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Harden JL, Anderson CD, Egilmez NK. Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J Immunol. 2016;197(3):962–970. doi: 10.4049/jimmunol.1502615. [DOI] [PubMed] [Google Scholar]
- 25.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Prendergast GC, Mondal A, Dey S, Laury-Kleintop LD, Muller AJ. Inflammatory reprogramming with IDO1 inhibitors: turning immunologically unresponsive ‘cold’ tumors ‘hot’. Trends Cancer. 2018;4(1):38–58. doi: 10.1016/j.trecan.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2(8):722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen E, Van den Eynde BJ. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res. 2017;5(8):695–709. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 29.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 30.Wang LT, Chiou SS, Chai CY, Hsi E, Yokoyama KK, Wang SN, Huang SK, Hsu SH. Intestine-specific homeobox gene ISX integrates IL6 signaling, tryptophan catabolism, and immune suppression. Cancer Res. 2017;77(15):4065–4077. doi: 10.1158/0008-5472.CAN-17-0090. [DOI] [PubMed] [Google Scholar]
- 31.Song X, Zhang Y, Zhang L, Song W, Shi L. Hypoxia enhances indoleamine 2,3-dioxygenase production in dendritic cells. Oncotarget. 2018;9(14):11572–11580. doi: 10.18632/oncotarget.24098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravishankar B, Liu H, Shinde R, Chaudhary K, Xiao W, Bradley J, Koritzinsky M, Madaio MP, McGaha TL. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci USA. 2015;112(34):10774–10779. doi: 10.1073/pnas.1504276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci USA. 2012;109(35):14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 2015;36(1):13–20. doi: 10.1016/j.it.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Tsogka K, Sounidaki M, Stefanidis I. Differential effects of the two amino acid sensing systems, the GCN2 kinase and the mTOR complex 1, on primary human alloreactive CD4(+) T-cells. Int J Mol Med. 2016;37(5):1412–1420. doi: 10.3892/ijmm.2016.2547. [DOI] [PubMed] [Google Scholar]
- 36.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375(3):331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 38.Grohmann U, Puccetti P. The coevolution of IDO1 and AhR in the emergence of regulatory T-cells in mammals. Front Immunol. 2015;6:58. doi: 10.3389/fimmu.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17(22):6985–6991. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 40.Ferdinande L, Decaestecker C, Verset L, Mathieu A, Moles Lopez X, Negulescu AM, Van Maerken T, Salmon I, Cuvelier CA, Demetter P. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106(1):141–147. doi: 10.1038/bjc.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornyak L, Dobos N, Koncz G, Karanyi Z, Pall D, Szabo Z, Halmos G, Szekvolgyi L. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol. 2018;9:151. doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vigneron N, van Baren N, Van den Eynde BJ. Expression profile of the human IDO1 protein, a cancer drug target involved in tumoral immune resistance. Oncoimmunology. 2015;4(5):e1003012. doi: 10.1080/2162402X.2014.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 45.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196(4):459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 47.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munn DH, Sharma MD, Johnson TS, Rodriguez P. IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment. Cancer Immunol Immunother. 2017;66(8):1049–1058. doi: 10.1007/s00262-017-2010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015;13(2):412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, Ren X. Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014;193(5):2574–2586. doi: 10.4049/jimmunol.1400833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y, Shi Y. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74(5):1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, Castro M, Kammerer R, Takikawa O, Hatz RA, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. 2007;13(23):6993–7002. doi: 10.1158/1078-0432.CCR-07-0942. [DOI] [PubMed] [Google Scholar]
- 55.Seeber A, Klinglmair G, Fritz J, Steinkohl F, Zimmer KC, Aigner F, Horninger W, Gastl G, Zelger B, Brunner A, et al. High IDO-1 expression in tumor endothelial cells is associated with response to immunotherapy in metastatic renal cell carcinoma. Cancer Sci. 2018;109(5):1583–1591. doi: 10.1111/cas.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel SA, Minn AJ. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48(3):417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe AH, Francisco LM, et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015;1(10):e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodrigues CP, Ferreira AC, Pinho MP, de Moraes CJ, Bergami-Santos PC, Barbuto JA. Tolerogenic IDO(+) dendritic cells are induced by PD-1-expressing mast cells. Front Immunol. 2016;7:9. doi: 10.3389/fimmu.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S, Grellety T, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol. 2018;4(1):93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei L, Zhu S, Li M, Li F, Wei F, Liu J, Ren X. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol. 2018;9:724. doi: 10.3389/fimmu.2018.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.York A. Microbiome: Gut microbiota sways response to cancer immunotherapy. Nat Rev Microbiol. 2018;16(3):121. doi: 10.1038/nrmicro.2018.12. [DOI] [PubMed] [Google Scholar]
- 63.Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, Chu Q, Wu K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):47. doi: 10.1186/s13045-018-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 66.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018; [DOI] [PMC free article] [PubMed]
- 67.Pavlova T, Vidova V, Bienertova-Vasku J, Janku P, Almasi M, Klanova J, Spacil Z. Urinary intermediates of tryptophan as indicators of the gut microbial metabolism. Anal Chim Acta. 2017;987:72–80. doi: 10.1016/j.aca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 68.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 69.Soliman HH, Minton SE, Han HS, Ismail-Khan R, Neuger A, Khambati F, Noyes D, Lush R, Chiappori AA, Roberts JD, et al. A phase I study of indoximod in patients with advanced malignancies. Oncotarget. 2016;7(16):22928–22938. doi: 10.18632/oncotarget.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, Ismail-Khan R, Minton S, Vahanian NN, Link C, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5(18):8136–8146. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berrong Z, Mkrtichyan M, Ahmad S, Webb M, Mohamed E, Okoev G, Matevosyan A, Shrimali R, Eid RA, Hammond S, et al. Antigen-specific antitumor responses induced by OX40 agonist are enhanced by the IDO inhibitor indoximod. Cancer Immunol Res. 2018;6(2):201–208. doi: 10.1158/2326-6066.CIR-17-0223. [DOI] [PubMed] [Google Scholar]
- 72.Dhiman V, Giri KK, SP S, Zainuddin M, Rajagopal S, Mullangi R. Determination of epacadostat, a novel IDO1 inhibitor in mouse plasma by LC-MS/MS and its application to a pharmacokinetic study in mice. Biomed Chromatogr. 2017;31(2) 10.1002/bmc.3794. [DOI] [PubMed]
- 73.Jochems C, Fantini M, Fernando RI, Kwilas AR, Donahue RN, Lepone LM, Grenga I, Kim YS, Brechbiel MW, Gulley JL, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget. 2016;7(25):37762–37772. doi: 10.18632/oncotarget.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beatty GL, O'Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA, Newton RC, Schaub R, Maleski J, Leopold L, et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res. 2017;23(13):3269–3276. doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kristeleit R, Davidenko I, Shirinkin V, El-Khouly F, Bondarenko I, Goodheart MJ, Gorbunova V, Penning CA, Shi JG, Liu X, et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)-only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol. 2017;146(3):484–490. doi: 10.1016/j.ygyno.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Yue EW, Sparks R, Polam P, Modi D, Douty B, Wayland B, Glass B, Takvorian A, Glenn J, Zhu W, et al. INCB24360 (Epacadostat), a highly potent and selective indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor for immuno-oncology. ACS Med Chem Lett. 2017;8(5):486–491. doi: 10.1021/acsmedchemlett.6b00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan D, Lin YW, Tan X. Heme-containing enzymes and inhibitors for tryptophan metabolism. Metallomics. 2017;9(9):1230–1240. doi: 10.1039/C7MT00105C. [DOI] [PubMed] [Google Scholar]
- 78.Mautino MR, Jaipuri FA, Waldo J, Kumar S, Adams J, Van Allen C, Marcinowicz-Flick A, Munn D, Vahanian N, Link CJ. Abstract 491: NLG919, a novel indoleamine-2,3-dioxygenase (IDO)-pathway inhibitor drug candidate for cancer therapy. Cancer Res. 2013;73(8 Supplement):491. doi: 10.1158/1538-7445.AM2013-491. [DOI] [Google Scholar]
- 79.Meng X, Du G, Ye L, Sun S, Liu Q, Wang H, Wang W, Wu Z, Tian J. Combinatorial antitumor effects of indoleamine 2,3-dioxygenase inhibitor NLG919 and paclitaxel in a murine B16-F10 melanoma model. Int J Immunopathol Pharmacol. 2017;30(3):215–226. doi: 10.1177/0394632017714696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mautino MR, Link CJ, Vahanian NN, Adams JT, Allen CV, Sharma MD, Johnson TS, Munn D. Abstract 5023: Synergistic antitumor effects of combinatorial immune checkpoint inhibition with anti-PD-1/PD-L antibodies and the IDO pathway inhibitors NLG-919 and indoximod in the context of active immunotherapy. Cancer Res. 2014;74(19 Supplement):5023. doi: 10.1158/1538-7445.AM2014-5023. [DOI] [Google Scholar]
- 81.Burris HA, Gordon MS, Hellmann MD, LoRusso P, Emens LA, Hodi FS, Lieu CH, Infante JR, Tsai FY-C, Eder JP, et al. A phase Ib dose escalation study of combined inhibition of IDO1 (GDC-0919) and PD-L1 (atezolizumab) in patients with locally advanced or metastatic solid tumors. J Clin Oncol. 2017;35(15_suppl):105. [Google Scholar]
- 82.Siu LL, Gelmon K, Chu Q, Pachynski R, Alese O, Basciano P, Walker J, Mitra P, Zhu L, Phillips P, et al. Abstract CT116: BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res. 2017;77(13 Supplement):CT116. doi: 10.1158/1538-7445.AM2017-CT116. [DOI] [Google Scholar]
- 83.Tumang J, Gomes B, Wythes M, Crosignani S, Bingham P, Bottemanne P, Cannelle H, Cauwenberghs S, Chaplin J, Dalvie D, et al. Abstract 4863: PF-06840003: a highly selective IDO-1 inhibitor that shows good in vivo efficacy in combination with immune checkpoint inhibitors. Cancer Res. 2016;76(14 Supplement):4863. doi: 10.1158/1538-7445.AM2016-4863. [DOI] [Google Scholar]
- 84.Dounay AB, Tuttle JB, Verhoest PR. Challenges and opportunities in the discovery of new therapeutics targeting the kynurenine pathway. J Med Chem. 2015;58(22):8762–8782. doi: 10.1021/acs.jmedchem.5b00461. [DOI] [PubMed] [Google Scholar]
- 85.Andersen MH, Svane IM. Indoleamine 2,3-dioxygenase vaccination. Oncoimmunology. 2015;4(1):e983770. doi: 10.4161/2162402X.2014.983770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, Zeyher C, Gouttefangeas C, Thomsen BM, Holm B, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20(1):221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 87.Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. J Leukoc Biol. 2016;100(2):275–290. doi: 10.1189/jlb.5RI0116-013RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diggs LP, Hsueh EC. Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res. 2017;5:12. doi: 10.1186/s40364-017-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J Hematol Oncol. 2017;10(1):174. doi: 10.1186/s13045-017-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang X, Yang Y, Fan D, Xiong D. The development of bispecific antibodies and their applications in tumor immune escape. Exp Hematol Oncol. 2017;6:12. doi: 10.1186/s40164-017-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan M, Li M, Gao L, Geng S, Wang J, Wang Y, Yan Z, Yu L. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J Hematol Oncol. 2017;10(1):151. doi: 10.1186/s13045-017-0519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu B, Song Y, Liu D. Clinical trials of CAR-T cells in China. J Hematol Oncol. 2017;10(1):166. doi: 10.1186/s13045-017-0535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin L, Zhao R, Li P. Incorporation of functional elements enhances the antitumor capacity of CAR T cells. Exp Hematol Oncol. 2017;6:28. doi: 10.1186/s40164-017-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei G, Ding L, Wang J, Hu Y, Huang H. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol. 2017;6:10. doi: 10.1186/s40164-017-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mullard A. IDO takes a blow. Nature Rev Drug Disc. 2018;17(5):307. doi: 10.1038/nrd.2018.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials supporting the conclusions of this study have been included within the article.