Abstract
Background
Currently, no standard of care or therapies have been established for patients with advanced HCC. We evaluated the efficacy and safety of conventional transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin (cTACE) and TACE with doxorubicin-loaded drug eluting beads (DEB-TACE).
Methods
This retrospective study included 273 patients who received cTACE (n = 201) or DEB-TACE. Tumor response, survival, and adverse events were evaluated over a 5-year follow-up period.
Results
During 5-year follow-up, a greater percentage of patients treated with cTACE died than those treated with DEB-TACE (76.1% vs. 66.7%) (P = 0.045). At the last evaluation, all surviving patients had disease progression and no differences were seen between treatment groups. However, the time to disease progression differed between groups; median time to disease progression was 11.0 months for cTACE and 16.0 months for DEB-TACE (P = 0.019). The median survival time was 37 months in both treatment groups. No significant differences were observed between cTACE and DEB-TACE therapies in subgroups of patients with BCLC stage A or stage B + C either in survival time or time to disease progression (P values > 0.05). No significant differences were observed in survival status or disease progression between cTACE and DEB-TACE in patient subgroups with either tumor number > 5 or with the sum of the diameter of largest five HCC tumors being > 7 cm.
Conclusions
DEB-TACE demonstrates greater long-term benefits than cTACE in treating treatment-naïve patients with HCC. Results of this long-term study support the use of DEB-TACE in treating HCC.
Keywords: Drug-eluting bead transcatheter arterial chemoembolization, DEB-TACE, Hepatocellular carcinoma, Transcatheter arterial chemoembolization, TACE
Background
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide and the sixth most common cancer [1, 2]. Due to chronic liver infection resulting from the high incidence of hepatitis C infection and the large number of persons with metabolic syndrome, the incidence of HCC is anticipated to rise [1, 2]. A large percentage of HCC patients are diagnosed at the intermediate or advanced stage [3, 4]. Currently, no established standard of care or therapeutic possibilities exist for patients with advanced HCC. Only 30 to 40% patients with HCC are candidates for curative treatment such as liver transplant [3, 4]. Hence, most patients can only be treated with locoregional or palliative treatment [5].
Transcatheter arterial chemoembolization (TACE) is used as a palliative local therapeutic option for patients with nonresectable HCC who may be waiting for liver transplant or who are not candidates for liver transplantation. TACE is also used to decrease the tumor burden, allowing for tumor resection [6]. Conventional TACE (cTACE) involves the delivery of embolic material to the tumor plus chemotherapeutic agents such as doxorubicin, either dissolved or emulsified in lipiodol, which is noted for causing both ischemia and strong cytotoxic effects [2, 7]. Lipiodol is a lymphographic agent which is selectively deposited in HCC tumors by arterial infusion and permits the slow release of the chemotherapeutic agent into the tumor [8–10]. In randomized controlled trials, cTACE has demonstrated a survival benefit for HCC patients with nonresectable HCC [11–14] and is recommended as a standard treatment option for patients with Barcelona stage B (BCLC) (intermediate stage) HCC [15, 16]. However, with TACE, the tumor does not always retain the lipiodol, resulting in decreased effectiveness of therapy and risk of liver damage [17, 18].
Drug-eluting beads (DEB) have been introduced into TACE (DEB-TACE) to promote the controlled release of cytotoxic drugs for the treatment of HCC [19, 20]. The drug-eluting microspheres added to TACE result in the delivery of high concentration of chemotherapeutic drugs to the tumor. The use of DEB-TACE is associated with a better safety profile than cTACE; DEB-TACE has been observed to have a lower incidence of adverse events such as abdominal pain, fever, nausea and vomiting [21–24]. A number of clinical studies and meta-analyses have found that DEB-TACE was associated with a significant advantage in tumor response and survival compared with cTACE [21–24]. However, several recent studies failed to demonstrate the superiority of DEB-TACE over cTACE in treatment efficacy, although DEB-TACE was associated with a better safety profile than cTACE [25–28].
Although DEB-TACE has shown benefits relative to TACE in some randomized controlled studies, the method is still controversial in clinical practice. Early results of our study of DEB-TACE and cTACE published in 2015 [29] showed that, at a mean follow-up of 15 months, DEB-TACE was associated with a better safety profile and more patients achieved a complete response and fewer had disease progression than cTACE. The present retrospective study evaluated the long-term benefits of DEB-TACE and cTACE on disease progression and overall survival (OS) during 5-year follow up of patients with HCC.
Methods
This retrospective study recruited consecutive patients with HCC who were treated with TACE at the National Cheng-Kung University Hospital (Tainan, Taiwan) from November 2010 to November 2011. The retrospective analysis was approved by the Ethics Committee of the institutional review board, National Cheng Kung University Hospital (IRB No: A-ER-103-311). The study was performed in accordance with the Declaration of Helsinki and the protocol was reviewed and approved by the Institutional Review Board of the hospital. All patients provided signed informed consent.
Study patients
The study design and protocol were as described previously in Liu et al. [29]. Eligible patients were ≥ 18 years of age with a diagnosis of HCC, had a at least one tumor that had not been treated previously and was > 1 mm in diameter, had Barcelona Clinic Liver Cancer (BCLC) criteria A or B, and had an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1. Included patients were also required to have a serum creatinine of < 1.2 mg/dL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels < 100 IU/L, and total bilirubin < 3 mg/dL. Patients were excluded if the tumor had invaded the portal vein, hepatic vein, and/or biliary duct, if the tumor had an extrahepatic arterial supply, or if the patient was diagnosed with atypical HCC such as, for example, infiltrative.
Treatment
A multidisciplinary team determined the treatment for a given patient. All patients were treated with a single cycle of TACE. Study patients either received cTACE with a gelatin sponge or with Embosphere microspheres (Biosphere, Roissy, France), or chemoembolization with doxorubicin-containing DEB (DC Bead, Biocompatibles, Farnham, United Kingdom). Prior to treatment, the attending physician described to the patient the tumor response and complication rates for each treatment method, as determined by the published literature. Subsequently, the patient decided which method should be used. No other simultaneous or combined treatment was permitted during the cTACE or DEB-TACE treatment period.
On the day of treatment, the patient underwent a complete diagnostic angiographic evaluation of the hepatic artery, superior mesenteric artery, and celiac trunk so as to evaluate the vascular anatomy and portal flow [30]. The segmental and subsegmental arteries feeding the tumor were subsequently catheterized using super-selective angiography with a microcatheter. The right hepatic artery was used in patients whose right hepatic artery came from the superior mesenteric artery. Care was taken to avoid embolization of the cystic and falciform arteries. The phrenic artery was investigated if it was determined to be supplying the tumor.
Patients in the cTACE group were injected with the doxorubicin/lipiodol mixture consisting of 50 mg of doxorubicin mixed with 10 mL of lipiodol. The mixture was injected into a segmental or subsegmental artery, followed by an injection of 500 to 700 μm gelatin sponge (Spongostan standard, Johnson & Johnson, Gargrave, Skipton, United Kingdom), or 100 to 300 μm Embosphere microspheres. The amount of lipiodol/doxorubicin injected was determined by the tumor size [31]. The DEB-TACE group was injected with 2 mL of 300 to500 μm DEB combined with 70 mg of doxorubicin [32]. An additional volume(s) of DEB was injected if “near stasis” was not obtained after the first injection until “near stasis” was achieved. The amount of beads injected was based upon the manufacturer’s instructions. The study used 300 to 500 μm beads because the 100 to 300 μm beads were not yet approved in Taiwan at the time the study started.
Follow-up and evaluation of treatment response
Tumor status was evaluated every 3 to 4 months according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [33]. If the evaluation suggested partial response or stable disease, the patients continued to be followed every 3 to 4 months for up to and over 5 years. Partial response was defined as ≥30% reduction in the sum of the diameter of the visible target lesions compared with baseline. If the assessment indicated progressive disease, patients were treated according to the BCLC guidelines and disease status [34]. Progressive disease was defined using the mRECIST criteria; i.e., ≥20% increase in the sum of the diameters of the visible target lesions compared with the smallest measurements observed from the start of therapy. Stable disease was defined as cases in which the tumor evaluation did not meet the criteria of partial response or progressive disease [33]. Two experienced radiologists evaluated the images, and any discrepancies were resolved by consensus.
Safety was evaluated throughout the study.
Statistical analysis
Clinical data are summarized as mean ± standard deviation (SD) and n (%) for continuous and categorical variables by treatment. Differences between treatments were compared using Mann-Whitney U test for continuous variables and Chi-square test / or Fisher’s exact test for categorical variables and for survival time and progression time. Pearson Chi-square test / or Fisher’s exact test were used to evaluate survival and progression status between treatments. The overall survival time and progression time by treatment were graphed using Kaplan-Meier curve and the estimated survival time was presented as median with 95% confidence intervals (95% CI). The log-rank test was applied to compare the difference in OS time between treatments. Results are represented as a P value. All statistical assessments were two-tailed and considered significant at P < 0.05. All statistical analyses were carried out using IBM SPSS statistical software version 22 for Windows (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY.).
Results
Baseline demographics and disease characteristics
A total of 273 patients (187 males / 86 females) with a mean age of 64.4 years were included in the study (Table 1). Of these, 201 patients were treated with cTACE and 72 with DEB-TACE. The baseline clinical characteristics were similar between treatment groups except for age, CLIP stage, and BCLC stage; patients in the cTACE group were older (65.3 vs. 61.7 years of age; P = 0.009), a greater percentage of patients had CLIP stage 0 or 1 cancer (81.6% vs. 66.7%; P = 0.018)) and BCLC A stage disease (35.7% vs. 22.2%; P = 0.040) than patients in the DEB-TACE group. Across groups, 63% of patients had no prior treatment, about 50% had unilobar disease, and the majority had HCV or HBV infections. About 80% of patients had Child-Pugh stage 5 disease and approximately 99% had an ECOG status of 0. The mean largest tumor size was 3.64 cm and most patients (72.2%) had ≤5 tumors.
Table 1.
Patients’ clinical characteristics by cTACE and DEB-TACE groups. (N = 273)
| Variables | Total (N = 273) | cTACE (n = 201) | DEB-TACE (n = 72) | p-value |
|---|---|---|---|---|
| Age, years | 64.4 ± 10.7 | 65.3 ± 10.7 | 61.7 ± 10.3 | 0.009 |
| Sex | ||||
| Female | 86 (31.5) | 63 (31.3) | 23 (31.9) | 0.925 |
| Male | 187 (68.5) | 138 (68.7) | 49 (68.1) | |
| Previous treatment | ||||
| None | 172 (63.0) | 126 (62.7) | 46 (63.9) | 0.166 |
| Operation | 53 (19.4) | 35 (17.4) | 18 (25.0) | |
| Locoregional treatment | 41 (15.0) | 33 (16.4) | 8 (11.1) | |
| Operation + locoregional | 7 (2.6) | 7 (3.5) | 0 (0) | |
| Uni-/Bi-lobar | ||||
| Unilobar | 136 (49.8) | 106 (52.7) | 30 (41.7) | 0.107 |
| Bilobar | 137 (50.2) | 95 (47.3) | 42 (58.3) | |
| HBV/HCV | ||||
| Non-B or C | 22 (8.1) | 18 (9.0) | 4 (5.6) | 0.225 |
| HBV | 127 (46.5) | 87 (43.3) | 40 (55.6) | |
| HCV | 108 (39.6) | 82 (40.8) | 26 (36.1) | |
| HBV + HCV | 16 (5.9) | 14 (7.0) | 2 (2.8) | |
| GOT (IU/L) | 70.63 ± 48.72 | 69.88 ± 50.4 | 70.63 ± 48.72 | 0.331 |
| GOT | ||||
| Not normal | 156 (57.1) | 112 (55.7) | 44 (61.1) | 0.428 |
| Normal | 117 (42.9) | 89 (44.3) | 28 (38.9) | |
| GPT (IU/L) | 72.08 ± 80.27 | 69.97 ± 81.77 | 72.08 ± 80.27 | 0.109 |
| GPT | ||||
| Not normal | 132 (48.4) | 91 (45.3) | 41 (56.9) | 0.089 |
| Normal | 141 (51.6) | 110 (54.7) | 31 (43.1) | |
| Bilirubin (mg/dL) | 0.76 ± 0.44 | 0.73 ± 0.42 | 0.76 ± 0.44 | 0.087 |
| Bilirubin | ||||
| Not normal | 22 (8.1) | 13 (6.5) | 9 (12.5) | 0.107 |
| Normal | 251 (91.9) | 188 (93.5) | 63 (87.5) | |
| AFP (ng/mL) | 875.28 ± 4510.43 | 766.21 ± 4607.3 | 875.28 ± 4510.43 | 0.497 |
| AFP | ||||
| Negative | 224 (82.1) | 169 (84.1) | 55 (76.4) | 0.145 |
| Positive | 49 (17.9) | 32 (15.9) | 17 (23.6) | |
| Albumin (g/dL) | 4 ± 0.49 | 3.98 ± 0.48 | 4 ± 0.49 | 0.312 |
| Albumin | ||||
| Not normal | 41 (15.0) | 29 (14.4) | 12 (16.7) | 0.648 |
| Normal | 232 (85.0) | 172 (85.6) | 60 (83.3) | |
| Ascites | ||||
| None | 257 (94.1) | 191 (95.0) | 66 (91.7) | 0.432 |
| Mild | 15 (5.5) | 9 (4.5) | 6 (8.3) | |
| Moderate | 1 (0.4) | 1 (0.5) | 0 (0) | |
| Child-Pugh stage | ||||
| 5 | 219 (80.2) | 161 (80.1) | 58 (80.6) | 0.883 |
| 6 | 44 (16.1) | 33 (16.4) | 11 (15.3) | |
| 7 | 9 (3.3) | 6 (3.0) | 3 (4.1) | |
| 8 | 1 (0.4) | 1 (0.5) | 0 (0) | |
| ECOG stage | ||||
| 0 | 271 (99.3) | 199 (99.0) | 72 (100) | 1.000 |
| 1 | 2 (0.7) | 2 (1.0) | 0 (0) | |
| CLIP stage | ||||
| 0 | 40 (14.7) | 32 (15.9) | 8 (11.1) | 0.018 |
| 1 | 172 (63.0) | 132 (65.7) | 40 (55.6) | |
| 2 | 49 (17.9) | 29 (14.4) | 20 (27.8) | |
| 3 | 10 (3.7) | 8 (4.0) | 2 (2.8) | |
| 4 | 2 (0.7) | 0 (0) | 2 (2.8) | |
| Okuda stage | ||||
| 0 | 250 (91.6) | 185 (92.0) | 65 (90.3) | 0.471 |
| 1 | 20 (7.3) | 13 (6.5) | 7 (9.7) | |
| 2 | 3 (1.1) | 3 (1.5) | 0 (0) | |
| BCLC stage | ||||
| A | 87 (32) | 71 (35.7) | 16 (22.2) | 0.040 |
| B + C | 184 (67.9) | 128 (64.3) | 56 (77.8) | |
| Largest target (cm) | 3.64 ± 2.59 | 3.47 ± 2.32 | 4.12 ± 3.20 | 0.175 |
| Tumor numbers | 3.5 ± 1.9 | 3.4 ± 1.9 | 3.6 ± 2.0 | 0.690 |
| Tumor numbers ≤5 | 197 (72.2) | 148 (73.6) | 49 (68.1) | 0.365 |
| Tumor numbers > 5 | 76 (27.8) | 53 (26.4) | 23 (31.9) | |
| Sum of the largest five hepatocellular carcinoma diameter (cm) | 6.64 ± 2.33 | 6.63 ± 2.26 | 6.66 ± 2.53 | 0.908 |
Aspartate aminotransferase (GOT), Alanine aminotransferase (GPT)
Data are summarized as mean ± SD and n (%) for continuous and categorical variables by treatment. Differences between treatments were compared using Mann-Whitney U test for continuous variables and Chi-square test / or Fisher’s exact test for categorical variables
Bold p-values indicate statistical significance (p < 0.05)
Treatment response
Clinical characteristics after treatment differed between cTACE and DEB-TACE patients (Table 2). Mean aspartate aminotransferase (GOT), alanine aminotransferase (GPT) and bilirubin were significantly different between the two groups. DEB-TACE patients had lower mean GOT (73.31 ± 45.50), and higher percentages had normal GOT values (36.1%). The same was also true for bilirubin levels; DEB-TACE patients had lower mean (0.98 ± 0.57) and higher percentages with normal bilirubin values (80.6%). Compared to cTACE patients, DEB-TACE patients also had significantly lower mean GPT (94.17 ± 101.44), but the percentage of patients with normal values was similar between the two groups. No significant differences were found in the percentage of patients with cholangitis between the cTACE and DEB-TACE groups. (Table 2).
Table 2.
Patients’ clinical characteristics after treatment with cTACE and DEB-TACE. (N = 273)
| Variables | cTACE (n = 201) | DEB-TACE (n = 72) | p-value |
|---|---|---|---|
| GOT (IU/L) | 156.76 ± 176.58 | 73.31 ± 45.50 | < 0.001 |
| GOT | |||
| Not normal | 153 (76.1) | 46 (63.9) | 0.045 |
| Normal | 48 (23.9) | 26 (36.1) | |
| GPT (IU/L) | 170.91 ± 266.13 | 94.17 ± 101.44 | 0.014 |
| GPT | |||
| Not normal | 139 (69.2) | 47 (65.3) | 0.545 |
| Normal | 62 (30.8) | 25 (34.7) | |
| Bilirubin (mg/dL) | 1.38 ± 0.99 | 0.98 ± 0.57 | 0.002 |
| Bilirubin | |||
| Not normal | 75 (37.3) | 14 (19.4) | 0.006 |
| Normal | 126 (62.7) | 58 (80.6) | |
| Albumin (g/dL) | 3.81 ± 0.43 | 3.87 ± 0.42 | 0.325 |
| Albumin | |||
| Not normal | 37 (18.4) | 12 (16.7) | 0.741 |
| Normal | 164 (81.6) | 60 (83.3) | |
| Cholangitis | |||
| No | 193 (96) | 70 (97.2) | 0.641 |
| Yes | 8 (4) | 2 (2.8) | |
Aspartate aminotransferase (GOT), Alanine aminotransferase (GPT)
Data are summarized as mean ± SD and n (%) for continuous and categorical variables by treatment. Differences between treatments were compared using Mann-Whitney U test for continuous variables and Chi-square test / or Fisher’s exact test for categorical variables
All serious AE were classified as cholangitis, none as biloma
Bold p-values indicate statistical significance (p < 0.05)
Survival time for the overall population was median 37 months (95% CI = 32.2–41.8 months) for cTACE treatment and a similar median 37 months (95%CI = 23.5–50.5 months) for the DEB-TACE treatment group (P = 0.091) (Table 3 and Fig. 1a). Over the 5-year follow-up period, a greater percentage of patients treated with cTACE died than those treated with DEB-TACE (76.1% vs. 66.7%, respectively) (P = 0.045). All patients who were still alive at the last evaluation (15 patients died during the 5-year follow-up) had disease progression (Table 3). However, the time to disease progression differed between groups; median time to disease progression was 11.0 months for cTACE and 16.0 months for DEB-TACE (P = 0.019). (Table 3 and Fig. 1b).
Table 3.
Survival times and progression times between cTACE and DEB-TACE treatments
| Variables | cTACE | DEB-TACE | p-value |
|---|---|---|---|
| Total | (n = 201) | (n = 72) | |
| Survival status | 0.045 | ||
| Died within ≤5 years follow-up | 153 (76.1) | 48 (66.7) | |
| Died after more than 5 years follow-up | 10 (5.0) | 1 (1.4) | |
| Survived until last follow-up | 38 (18.9) | 23 (31.9) | |
| Progression status | 0.218 | ||
| Progression | 192 (95.5) | 66 (91.7) | |
| Loss of follow-up/censored | 9 (4.5) | 6 (8.3) | |
| Survival time, months | 37 (32.2, 41.8) | 37 (23.5, 50.5) | 0.091 |
| Progression time, months | 11.0 (9.6, 12.4) | 16.0 (13.1, 18.9) | 0.019 |
| BCLC stage = A | (n = 73) | (n = 16) | |
| Survival status | 0.083 | ||
| Died within ≤5 years follow-up | 57 (78.1) | 9 (56.3) | |
| Died after more than 5 years follow-up | 3 (4.1) | 0 (0) | |
| Survived until last follow-up | 13 (17.8) | 7 (43.8) | |
| Progression status | 0.219 | ||
| Progression | 70 (95.9) | 14 (87.5) | |
| Loss of follow-up/censored | 3 (4.1) | 2 (12.5) | |
| Survival time, months | 42 (36.1, 47.9) | 45 (0, 90.1) | 0.149 |
| Progression time, months | 12 (10.5, 13.5) | 19 (17.8, 20.2) | 0.217 |
| BCLC stage = B + C | (n = 128) | (n = 56) | |
| Survival status | 0.270 | ||
| Died within ≤5 years follow-up | 96 (75) | 39 (69.6) | |
| Died after more than 5 years follow-up | 7 (5.5) | 1 (1.8) | |
| Survived until last follow-up | 25 (19.5) | 16 (28.6) | |
| Progression status | 0.495 | ||
| Progression | 122 (95.3) | 52 (92.9) | |
| Loss of follow-up/censored | 6 (4.7) | 4 (7.1) | |
| Survival time, months | 33 (27.1, 38.9) | 36 (21.3, 50.7) | 0.191 |
| Progression time, months | 10 (8.1, 11.9) | 15 (12.4, 17.7) | 0.032 |
Survival and progression status are summarized as n (%) by treatment; Survival time- and progression time-related data are summarized as median (95%CI) by treatment
Differences between treatments were compared using Pearson Chi-square test / or Fisher’s exact test for survival and progression status and Log-rank test for survival time or progression time
Bold p-values indicate statistical significance (p < 0.05)
Fig. 1.
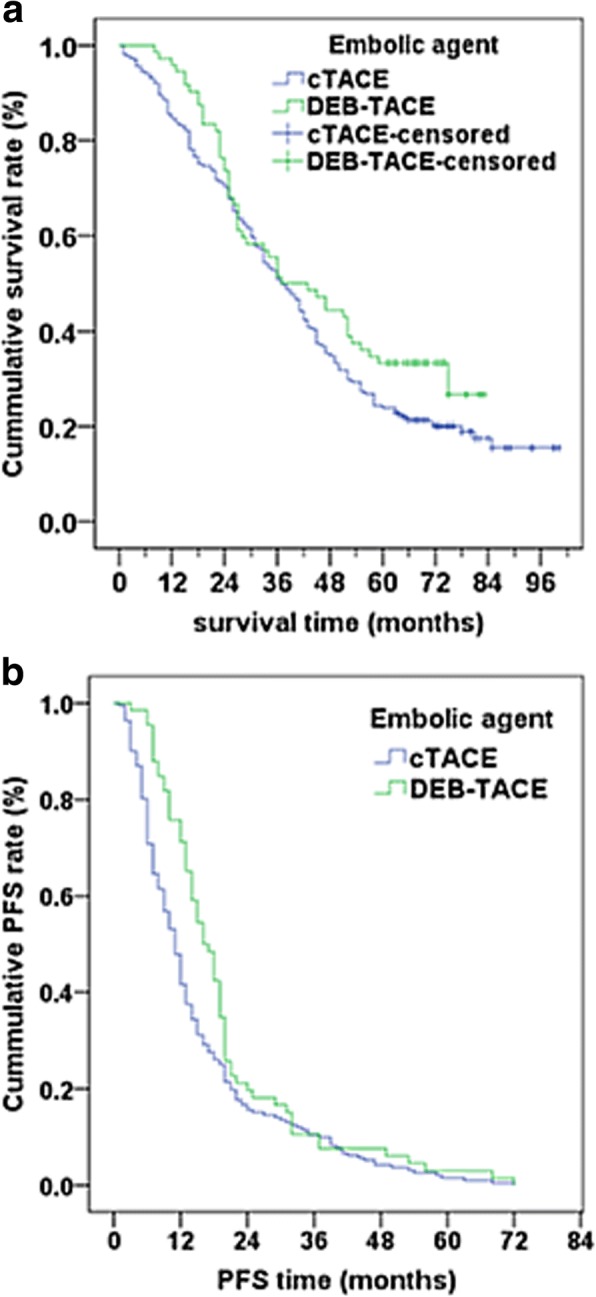
Kaplan-Meier curve of overall survival time (a) and PFS time (b) by treatments. a The estimated median overall survival time was derived as 37 months (95%CI = 32.2–41.8 months) for cTACE and 37 months (95%CI = 23.5–50.5 months) for DEB-TACE. The log-rank test p-value = 0.091. b The estimated median PFS time was derived as 11 months (95%CI = 9.6–12.4 months) for cTACE and 16 months (95%CI = 13.1–18.9 months) for DEB-TACE. The log-rank test p-value = 0.019
Survival time and time to disease progression between treatments was also evaluated by BCLC stage, and by patients with > 5 tumors or whose sum of the five largest tumors was > 7 cm in diameter. No significant differences were observed between cTACE and DEB-TACE therapies in the subgroups of patients with BCLC stage A or stage B + C, either in survival time or progression time (P values > 0.05) (Table 3). In addition, no significant differences were observed in survival status or disease progression between cTACE and DEB-TACE in the subgroups of patients with either tumor number > 5 or the sum of the diameter of the five largest HCC tumors was > 7 cm. (Table 4).
Table 4.
Survival times and progression times between cTACE and DEB-TACE treatments for patients with tumor number > 5 or sum of the diameter of the largest five HCC tumors was > 7 cm
| Variables | cTACE | DEB-TACE | p-value |
|---|---|---|---|
| (n = 78) | (n = 33) | ||
| Survival status | 0.610 | ||
| Died within ≤5 years follow-up | 62 (79.5) | 26 (78.8) | |
| Died after more than 5 years follow-up | 3 (3.8) | 0 (0) | |
| Survived until last follow-up | 13 (16.7) | 7 (21.2) | |
| Progression status | 0.360 | ||
| Progression | 75 (96.2) | 30 (90.9) | |
| Loss of follow-up/censored | 3 (3.8) | 3 (9.1) | |
| Survival time, months | 31 (26.7, 35.3) | 27 (22.3, 31.7) | 0.789 |
| Progression time, months | 9 (6.7, 11.3) | 12 (8.0, 16.0) | 0.492 |
Survival and progression status are summarized as n (%) by treatment; Survival time- and progression time-related data are summarized as median (95%CI) by treatment
Differences between treatments were compared using Pearson Chi-square test / or Fisher’s exact test for survival and progression status and Log-rank test for survival time or progression time
Discussion
This retrospective, observational study compared clinical outcomes of treatment-naive HCC patients who underwent cTACE (conventional TACE) or DEB-TACE (drug-eluting bead TACE). The earlier published findings from the 15-month follow-up of this study population showed that DEB-TACE treatment resulted in a higher percentage of patients with complete response and a lower percentage with disease progression than patients receiving cTACE treatment [29]. In addition, DEB-TACE was associated with a better safety profile than cTACE [29]. The data presented here are those of the same study after 5-years of follow-up. Five-years after treatment, both treatments were associated with a survival time of 37 months. However, over the 5-year follow-up period, a greater percentage of patients treated with cTACE died (76.1%) than those treated with DEB-TACE (66.7%) (P = 0.045). After five-years, all surviving patients had disease progression. However, the time to disease progression differed between groups; cTACE was associated with a shorter median time to disease progression (11 months) than DEB-TACE (16.0 months) (P = 0.019). Subgroup analysis indicated that survival and disease progression were similar for both treatments in patients with BLCC stage A or stage B + C, and in patients with > 5 tumors, or if the diameter of the patient’s five largest tumors was > 7 cm. The findings of the long-term study suggest that DEB-TACE demonstrates long-term benefits in treating treatment-naive HCC patients.
Only a limited number of studies have directly compared the efficacy and safety of DEB-TACE and cTACE in treating HCC. A number of systematic reviews and meta-analyses have evaluated the effectiveness of cTACE and DEB-TACE in treating HCC [21–24].
Similar to our findings, several of the meta-analyses found DEB-TACE to show treatment benefit and better safety profile compared with cTACE [23, 24]. A systematic review by Martin et al. [21] found DEB-TACE had a significant advantage compared with cTACE in objective response and had greater overall disease control in patients with advanced HCC (P values ≤0.038). Zhou et al. performed a meta-analysis which included nine studies and total of 830 patients [24]. The study found DEB-TACE significantly improved overall survival and increased objective response and disease control rates. Similarly, a meta-analysis performed by Huang et al. [23], which included seven clinical studies with 700 patients, found a significantly better objective response for DEB-TACE than cTACE (P = 0.004), with a relative risk difference of 0.15 (P = 0.0003). Those authors also found that patients with one- and two-year survival were significantly more with DEB-TACE than with cTACE (P values ≤0.007). However, Huang et al. found no difference between treatments for 6-month and 3-year survival (P values ≥0.11). A meta-analysis by Zou et al. [22] found DEB-TACE was associated with higher complete response (Odds ratio [OR], 1.35), overall survival rate (OR, 1.41), and survival time (WMD, 3.03). In contrast to the studies of Huang et al. and Zhou et al., Zou et al. found no difference between treatments for objective response. Those authors also found that the treatments had similar disease control rates. All four meta-analyses found that DEB-TACE was associated with fewer side effects compared with cTACE.
In contrast to the above results, a meta-analysis performed by Facciorusso et al. [25] found that DEB-TACE and cTACE had similar safety and effectiveness. Facciorusso et al. included 12 studies, four of which were randomized controlled trials. The meta-analysis included a total of 1449 patients. The observed 1-, 2- and 3-year survival times were similar between treatment groups (P values ≥0.06). Pooled data of objective response and incidence of adverse events also indicated no differences between the two therapies (P values ≥0.36). However, the study did show a non-significant trend of superiority for DEB-TACE for overall response rate (OR, 1.2), which was confirmed by subgroup and sensitivity analysis.
The difference in findings across the meta-analyses may, in part, reflect the small number of included studies and high heterogeneity in the studies overall. Moreover, the evaluation of response rate across the studies varied, with some of the included studies used EASL criteria and others using mRECIST criteria. In addition, length of follow-up was heterogeneous among the included studies and was found to be one of the main sources of heterogeneity. The present study supports the importance of follow-up in comparing studies. In an earlier phase of the present t study conducted at 15 months post-treatment, DEB-TACE treatment was associated with patients’ greater complete response and less disease progression than cTACE. However, at the 5-year follow-up, all surviving patients had disease progression but fewer patients treated with DEB-TACE had died.
The present study has several limitations, including that the study was retrospective in design and only included patients from a single institution. In addition, complete secondary treatment information was not available for the patients and only a small number of patients were treated with DEB-TACE (n = 73).
Conclusions
In conclusion, DEB-TACE shows greater long-term benefit compared with those of cTACE in treating treatment naïve patients with HCC. At 5 years after treatment, HCC patients receiving DEB-TACE have fewer deaths and a longer time to disease progression than patients receiving cTACE. DEB-TACE is also associated with fewer treatment-related adverse events than cTACE. Results of this long-term study support the use of DEB-TACE in treating HCC.
Acknowledgements
None.
Funding
None.
Availability of data and materials
Not applicable.
Abbreviations
- cTACE
Conventional transarterial chemoembolization
- DEB
With doxorubicin-loaded drug eluting beads
- ECOG
Eastern cooperative oncology group
- HCC
Hepatocellular carcinoma
- mRECIST
Modified response evaluation criteria in solid tumors
- OS
Overall survival
- TACE
Transarterial chemoembolization
- TACE
Transarterial chemoembolization
- BCLC
Barcelona clinic liver cancer
Authors’ contributions
YSL study concepts, study design, literature search, data acquisition, manuscript preparation; CYL study design, literature search, data acquisition, manuscript editing, data analysis, statistical analysis; MTC definition of intellectual content, data analysis, statistical analysis; YST statistical analysis; CKW data acquisition; MCO guarantor of integrity of the entire study, study concepts, study design, definition of intellectual content, manuscript editing, manuscript review. All authors read and approved the manuscript.
The study was performed in accordance with the Declaration of Helsinki and the protocol was reviewed and approved by the Institutional Review Board of the hospital. All patients provided signed informed consent.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi-Sheng Liu, Email: taicheng100704@yahoo.com.tw.
Chia-Ying Lin, Email: CYlin444@gmail.com.
Ming-Tsung Chuang, Email: u8501122@gmail.com.
Chia-Ying Lin, Email: gracelinchiaying@masn.com.
Yi-Shan Tsai, Email: crevice123@hotmail.com.
Chien-Kuo Wang, Email: n625396@mail.hosp.ncku.edu.tw.
Ming-Ching Ou, Phone: (8866)2766108, Email: emilialiar@yahoo.com.tw.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute clinical trials planning meeting. J Clin Oncol. 2010;28(25):3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. American Association for the Study of liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The L. European Organisation For R. Treatment Of C EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 6.Gosalia AJ, Martin P, Jones PD. Advances and future directions in the treatment of hepatocellular carcinoma. Gastroenterol Hepatol. 2017;13(7):398–410. [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13(9 Pt 2):S211–S221. doi: 10.1016/S1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 8.Konno T, Maeda H, Iwai K, Maki S, Tashiro S, Uchida M, et al. Selective targeting of anti-cancer drug and simultaneous image enhancement in solid tumors by arterially administered lipid contrast medium. Cancer. 1984;54(11):2367–2374. doi: 10.1002/1097-0142(19841201)54:11<2367::AID-CNCR2820541111>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Konno T, Maeda H, Iwai K, Tashiro S, Maki S, Morinaga T, et al. Effect of arterial administration of high-molecular-weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol. 1983;19(8):1053–1065. doi: 10.1016/0277-5379(83)90028-7. [DOI] [PubMed] [Google Scholar]
- 10.Jinno K, Moriwaki S, Tanada M, Wada T, Mandai K, Okada Y. Clinicopathological study on combination therapy consisting of arterial infusion of lipiodol-dissolved SMANCS and transcatheter arterial embolization for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31(Suppl):S7–12. doi: 10.1007/BF00687097. [DOI] [PubMed] [Google Scholar]
- 11.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79(11):2087–2094. doi: 10.1002/(SICI)1097-0142(19970601)79:11<2087::AID-CNCR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 14.Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. doi: 10.1186/1471-230X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 16.Kang JY, Choi MS, Kim SJ, Kil JS, Lee JH, Koh KC, et al. Long-term outcome of preoperative transarterial chemoembolization and hepatic resection in patients with hepatocellular carcinoma. Korean J Hepatol. 2010;16(4):383–388. doi: 10.3350/kjhep.2010.16.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poyanli A, Rozanes I, Acunas B, Sencer S. Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol. 2001;42(6):602–607. doi: 10.1080/028418501127347278. [DOI] [PubMed] [Google Scholar]
- 18.Douhara A, Namisaki T, Moriya K, Kitade M, Kaji K, Kawaratani H, et al. Predisposing factors for hepatocellular carcinoma recurrence following initial remission after transcatheter arterial chemoembolization. Oncol Lett. 2017;14(3):3028–3034. doi: 10.3892/ol.2017.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(3):327–332. doi: 10.1016/j.jvir.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Martin R, Geller D, Espat J, Kooby D, Sellars M, Goldstein R, et al. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepato-Gastroenterology. 2012;59(113):255–260. doi: 10.5754/hge10240. [DOI] [PubMed] [Google Scholar]
- 22.Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17(8):510–517. doi: 10.1111/1751-2980.12380. [DOI] [PubMed] [Google Scholar]
- 23.Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(5):920–925. doi: 10.1111/jgh.12439. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Tang Z, Wang J, Lin P, Chen Z, Lv L, et al. Doxorubicin-eluting beads versus conventional transarterialchemoembolization for the treatment of hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Med. 2014;7(11):3892–3903. [PMC free article] [PubMed] [Google Scholar]
- 25.Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48(6):571–577. doi: 10.1016/j.dld.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee YK, Jung KS, Kim DY, Choi JY, Kim BK, Kim SU, et al. Conventional versus drug-eluting beads chemoembolization for hepatocellular carcinoma: emphasis on the impact of tumor size. J Gastroenterol Hepatol. 2017;32(2):487–496. doi: 10.1111/jgh.13501. [DOI] [PubMed] [Google Scholar]
- 27.Baur J, Ritter CO, Germer CT, Klein I, Kickuth R, Steger U. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med. 2016;8:69–74. doi: 10.2147/HMER.S105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arabi M, BenMousa A, Bzeizi K, Garad F, Ahmed I, Al-Otaibi M. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol. 2015;21(3):175–180. doi: 10.4103/1319-3767.157571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK, Tsai HM, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015;16(1):125–132. doi: 10.3348/kjr.2015.16.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224(2):542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 31.Idee JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013;88(3):530–549. doi: 10.1016/j.critrevonc.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Song MJ, Park CH, Kim JD, Kim HY, Bae SH, Choi JY, et al. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23(6):521–527. doi: 10.1097/MEG.0b013e328346d505. [DOI] [PubMed] [Google Scholar]
- 33.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graf D, Vallbohmer D, Knoefel WT, Kropil P, Antoch G, Sagir A, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25(5):430–437. doi: 10.1016/j.ejim.2014.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


