Abstract
Background
Aerobactin is a critical factor for the hypervirulent Klebsiella pneumoniae (hvKp), but data for the aerobactin-positive genotype of hvKp in elderly persons with ventilator-associated pneumonia (VAP) is limited. The purpose of this study is to understand the risk factors and characteristics of the hvKp genotype for elderly patients with VAP.
Methods
A retrospective study of 73 elderly patients with Kp was conducted from November 2008 to December 2017 in two tertiary hospitals. The clinical and microbiological data, including inflammatory reaction, nutritional status, antimicrobial susceptibility testing, string test, extended-spectrum-β-lactamase (ESBL) production, virulence-associated gene (capsular serotype-specific gene and rmpA/A2,magA,aerobaction) and multilocus sequence typing, of the hvKp group defined as aerobactin positive were compared with those of classic Kp strains.
Results
Of 73 Kp isolates, 46.6% were hvKp. ST23 is highly prevalent in two hospitals but is not highly associated with hvKp in different hospitals. Additionally, ST23, ST37 and ST2906 are more likely to induce lethal VAP. Most hvKp strains are sensitive to common antibiotics, but the number of multidrug-resistant (MDR) hvKp is increasing. Importantly, 38.2% of hvKp isolates produced ESBLs. Hypermucoviscosity and virulence-associated genes (K1,magA and rmpA/A2) were highly clustered in the hvKp group (P < 0.001). Cancer (P = 0.004), digestive disease (P = 0.038) and surgery (P = 0.023) within 1 month are strongly associated with the VAP-hvKp group. The incidence of septic shock (P = 0.016) and Sequential Organ Failure Assessment (SOFA) scores (P < 0.001) are significantly higher in the hvKp group. Multivariate analysis indicated that cancer (odds ratio [OR] = 5.365) is an independent risk factor for VAP-hvKp infection.
Conclusions
The morbidity for elderly patients with VAP due to hvKp is high. MDR-HvKp is emerging, which is a great challenge for clinical practice.
Keywords: Klebsiella pneumoniae, Hypervirulent, Aerobactin, Risk factor, ESBL-hvKp, Ventilator-associated pneumonia
Background
Ventilator-associated pneumonia (VAP) is the most frequent life-threatening nosocomial infection in critically ill patients [1, 2]. Klebsiella pneumoniae (Kp) is one of the most common gram-negative bacteria causing hospital infections, especially various notoriously fatal infections. Kp includes two distinct groups: hypervirulent (hvKp) and classical (cKp). One of the characteristics of cKp is acquiring an antibiotic-resistant gene. The other type is hvKp, which is traditionally defined as hypermucoviscosity by string test, inducing aggressive invasive community infection, bloodstream infection, and pyogenic liver abscesses (PLA) for immunocompetent ambulatory younger adults with no underlying diseases [3–6]. However, the definition of hvKp by string test is controversial [7, 8]. Previous studies showed that certain hypermucoviscous K. pneumonia (hmvKp) strains are not closely related to high virulence in in vitro and in vivo models [7, 8]. Thus, to differentiate hvKp from cKp by the hypermucoviscosity phenotype alone may be inappropriate [9, 10]. Recently, aerobactin, a dominant component of the siderophore system, has been established as a critical virulence factor for the hvKp genotype [3, 10, 11]. A previous multi-centre study in China targeting middle-aged patients, illustrated the clinical and molecular characteristics of hvKp (defined as aerobactin positive) infection [10]. However, the data for the hospital infection in the elderly with hvKp VAP, who commonly suffer from various underlying disease and nutritional complications, is rare.
Although most previous studies demonstrated that hvKp is sensitive to most antibiotics, which is prominently different from cKp strains, the multi-drug resistance (MDR) and extended-spectrum-β-lactamase (ESBL)-producing hvKp, especially those resistant to colistin and carbapenems, are emerging in China [12–14]. However, there are not enough referable data for the elderly with VAP due to antimicrobial-resistant hvKp.
To date, no data about the clinical and microbiological characteristics of VAP caused by the hvKp genotype in the elderly have been demonstrated. The aim of this study was to further investigate the clinical characteristics of elderly patients with hvKp infection and microbiological features and the epidemiology of multi-centre hvKp. Therefore, we conducted a retrospective study in two tertiary hospitals focusing on the genotype of hvKp (defined as aerobactin positive) in China.
Methods
Patients
A retrospective study was conducted on 73 Kp culture-positive patients diagnosed from November 2008 to December 2017 at two tertiary hospitals: Beijing Tsinghua Changgung Hospital and Chinese PLA General Hospital. These two hospitals are located in Beijing, and the capacity of the hospitals is more than 800 beds. Patients currently receiving mechanical ventilation were included in this study. The definition of VAP is according to the 2016 ATS guideline [15]. Standard bronchoalveolar lavage and aspiration were achieved. The clinical characteristics, including underlying disease, infection type, nutritional status, mortality in 30 days, and sequential organ failure assessment (SOFA) were collected. The primary outcome of this study was to investigate the 30-day mortality of hvKp infection in the elderly with VAP compared with that of the cKp group. Additionally, the white blood cell count (WBC) and neutrophil percentage (NEU%) were applied as primary inflammatory factors. Total protein (TP) and albumin (ALB) were used to evaluate the nutrition status. The main inclusion criteria were 1) the definition of the elderly as 65 years old or older (≥65 year) and 2) at least one K. pneumoniae-positive culture. The exclusion criteria was 1) insufficient clinical data or bacterial strain sample storage and 2) cases of co-infection.
Clinical Kp strains
All isolates were stored at − 80 °C and identified by the API 20 NE system and the VITEK II system. Further species identification was confirmed by 16S rRNA gene sequencing. HvKp is defined as aerobactin positive. The hypermucoviscous phenotype was detected by the string test as described previously [16].
Antimicrobial susceptibility testing and phenotypic detection of ESBLs
Antimicrobial susceptibility testing was performed, and the results were interpreted according to 2017 Clinical and Laboratory Standards Institute (CLSI) guidelines. The antibiotics include amikacin, gentamicin, tobramycin, Sulbactam/sulbactam, aztreonam, cefazolin, cefepime, ceftriaxone, ceftazidime, ciprofloxacin, levofloxacin, piperacillin/tazobactam, and trimethoprim/sulfamethoxazole. ESBL was detected by an agar dilution test using ceftazidime and cefotaxime combined with clavulanate according to the CLSI guideline [10]. The definition of an MDR strain is resistance to three or more different antimicrobial categories, as described previously [17]. The carbapenem-resistant (CR) phenotype is defined as resistant to both imipenem and meropenem.
Detection of virulence-associated gene
Genomic DNA of all Kp isolates was extracted. RmpA, rmpA2, magA, aerobactin and the capsular serotype-specific (cps) genes (K1, K2, K5, K20, K54, and K57) were detected by polymerase chain reaction (PCR) as described previously [10, 18–20]. The primers are listed in Table 1.
Table 1.
Primers
| Name | sequence |
|---|---|
| rmpA | |
| Forward | 5-ACTGGGCTACCTCTGCTTCA-3 |
| Reverse | 5-CTTGCATGAGCCATCTTTCA-3 |
| rmpA2 | |
| Forward | 5-CTTTATGTGCAATAAG-GATGTT-3 |
| Reverse | 5-CCTCCTGGAGAGTAAGCATT-3 |
| magA | |
| Forward | 5-GGTGCTCTTTACATCATTGC-3 |
| Reverse | 5-GCAATGGCCATTTGCGTTAG-3 |
| aerobactin | |
| Forward | 5-GCATAGGCGGATACGAACAT-3 |
| Reverse | 5-CACAGGGCAATTGCTTACCT-3 |
| K1 | |
| Forward | 5-GTAGGTATTGCAAGCCATGC-3 |
| Reverse | 5-GCCCAGGTTAATGAATCCGT-3 |
| K2 | |
| Forward | 5-GGAGCCATTTGAATTCGGTG-3 |
| Reverse | 5-TCCCTAGCACTGGCTTAAGT-3 |
| K5 | |
| Forward | 5-GCCACCTCTAAGCATATAGC-3 |
| Reverse | 5-CGCACCAGTAATTCCAACAG-3 |
| K20 | |
| Forward | 5-CCGATTCGGTCAACTAGCTT-3 |
| Reverse | 5-GCACCTCTATGAACTTTCAG-3 |
| K54 | |
| Forward | 5-CATTAGCTCAGTGGTTGGCT-3 |
| Reverse | 5-GCTTGACAAACACCATAGCAG-3 |
| K57 | |
| Forward | 5-CGACAAATCTCTCCTGACGA-3 |
| Reverse | 5-CGCGACAAACATAACACTCG-3 |
| rpoB | |
| Forward | 5-GGCGAAATGGCWGAGAACCA-3 |
| Reverse | 5-GAGTCTTCGAAGTTGTAACC-3 |
| gapA | |
| Forward | 5-TGAAATATGACTCCACTCACGG-3 |
| Reverse | 5-CTTCAGAAGCGGCTTTGATGGCTT-3 |
| mdh | |
| Forward | 5-TGAAATATGACTCCACTCACGG-3 |
| Reverse | 5-CTTCAGAAGCGGCTTTGATGGCTT-3 |
| pgi | |
| Forward | 5-GAGAAAAACCTGCCTGTACTGCTGGC-3 |
| Reverse | 5-CGCGCCACGCTTTATAGCGGTTAAT-3 |
| phoE | |
| Forward | 5-ACCTACCGCAACACCGACTTCTTCGG-3 |
| Reverse | 5-TGATCAGAACTGGTAGGTGAT-3 |
| infB | |
| Forward | 5-CTCGCTGCTGGACTATATTCG-3 |
| Reverse | 5-CGCTTTCAGCTCAAGAACTTC-3 |
| tonB | |
| Forward | 5-CTTTATACCTCGGTACATCAGGTT-3 |
| Reverse | 5-ATTCGCCGGCTGRGCRGAGAG-3 |
Multilocus sequence typing (MLST) for Kp
Seven housekeeping genes (gapA, mdh, phoE, tonB, infB, pgi and rpoB) were detected by PCR according to the MLST website (http://bigsdb.pasteur.fr/klebsiella/ klebsiella.html) (Table 1). Allelic profiling and sequence types (STs) were also performed using the above website. Moreover, to further distinguish the relationship among different STs, phylogenetic analysis of the seven spliced housekeeping genes for these isolates that contributed to mortality was performed by the neighbour-joining method (MEGA 6.0). The common STs, including ST1, ST11, ST37, ST258 and ST412, were used as a reference.
Statistical analysis
SPSS software (version 20.0) was employed for data analysis. Measurement data was assessed as the means ± standard deviation (SD). The count data were analysed as percentages. Continuous variables were analysed by Student’s t-tests and the Wilcoxon rank-sum tests. Categorical variables were analysed by the χ2 or Fisher’s exact tests. Univariate logistic regression analysis was performed for the risk factors. A multivariable logistic regression analysis was conducted for independent risk factors (the variables with P<0.05 were included). All tests were 2-tailed. A p-value<0.05 was considered significant.
Results
Clinical characteristics
Seventy-three Kp culture-positive patients were diagnosed at the two hospitals from November 2008 to December 2017 (Table 2). Thirty-four (46.6%) strains were hvKp, and 37 (50.7%) were hmvKp. Most of the patients (67, 91.8%) presented with sepsis, and 24 patients (32.9%) were diagnosed with septic shock. Sixty-six (90.4%) were males, and the mean age of patients in this study was 84.96 ± 8.33 years. Patients with cancer (38.2% versus 7.7%; P = 0.004), surgery history within 1 month (23.5% versus 5.1%; P = 0.023) and digestive diseases (29.4% versus 10.3%; P = 0.046) were more likely to be infected with hvKp. Although host responsibility (WBC and NEU%) and nutritional status (TP and ALB) were not significantly different at the primary endpoint (30-day mortality), the SOFA scores of the patients with hvKp were notably higher at 30-day mortality (8.94 ± 3.03 vs 6.62 ± 2.09, P = 0.000) (Table 2).
Table 2.
Clinical features of patients with VAP due to hvKp
| Characteristic | HvKp(34) | cKp(39) | P value |
|---|---|---|---|
| Basic demographics | |||
| Age | 83.06 ± 8.55 | 86.62 ± .87 | 0.068 |
| Male | 31 (91.2%) | 35 (89.7%) | 1.000 |
| Underlying diseases | |||
| Pulmonary disease | 31 (91.2%) | 32 (82.1%) | 0.321 |
| Diabetes | 19 (55.9%) | 18 (46.2%) | 0.407 |
| Cardiovascular disease | 12 (35.3%) | 17 (43.6%) | 0.470 |
| Cerebrovascular disease | 11 (32.4%) | 15 (38.5%) | 0.587 |
| Cancer | 13 (38.2%) | 3 (7.7%) | 0.004 |
| Surgery within 1 mo | 8 (23.5%) | 2 (5.1%) | 0.023 |
| Digestive disease | 10 (29.4%) | 4 (10.3%) | 0.038 |
| Infection type | |||
| Sepsis | 30 (88.2%) | 37 (94.9%) | 0.408 |
| Septic shock | 16 (47.1%) | 8 (20.5%) | 0.016 |
| Host responsibility | |||
| WBC | 12.57 ± 4.47 | 11.68 ± 4.36 | 0.397 |
| NEU% | 78.99 ± 9.54 | 77.13 ± 7.14 | 0.344 |
| Nutrition status | |||
| TP | 63.38 ± 6.34 | 61.88 ± 6.02 | 0.304 |
| ALB | 33.33 ± 3.75 | 32.51 ± 3.39 | 0.326 |
| SOFA score | 8.94 ± 3.03 | 6.62 ± 2.09 | 0.000 |
| Infection occurred in ICU | 11 (32.4%) | 10 (25.6%) | 0.527 |
| Mortality in 30 days | 14 (41.2%) | 14 (35.9%) | 0.644 |
TP total protein, ALB albumin, WBC white blood cell count, NEU% neutrophils percentage
Genetic and phenotype characteristics: hvKp vs cKp
It is noted that hypermucoviscosity was strongly clustered in the hvKp group (P < 0.001). Our results showed that K1, rmpA, rmpA2 and magA were highly clustered in hvKp (P < 0.001), but K2, K5, K20, K54, and K57 were not associated with hvKp (P = 0.073, 0.213, 1.000, 0.096 and 0.849, respectively). Additionally, there is no isolate in the cKp group with K5 or K54 (Table 3).
Table 3.
Microbiological features of patients with VAP due to hvKp
| Characteristic | HvKp(34) | cKp(39) | P value |
|---|---|---|---|
| K serotype | |||
| K1 | 17 (50.0%) | 1 (2.6%) | 0.000 |
| K2 | 7 (2.9%) | 2 (5.1%) | 0.073 |
| K5 | 2 (5.9%) | 0 (0%) | 0.213 |
| K20 | 2 (5.9%) | 2 (5.1%) | 1.000 |
| K54 | 3 (8.8%) | 0 (0%) | 0.096 |
| K57 | 4 (11.8%) | 3 (7.7%) | 0.849 |
| rmpA | 27 (79.4%) | 4 (10.3%) | 0.000 |
| rmpA2 | 28 (82.4%) | 5 (12.8%) | 0.000 |
| magA | 30 (88.2%) | 17 (43.6%) | 0.000 |
| Hypermucoviscosity | 29 (85.3%) | 8 (20.5%) | 0.000 |
Antimicrobial resistance analysis and detection rate of ESBL-producing Kp isolates
All Kp strains were resistant to Sulbactam. Most of the hvKp isolates represented a higher antimicrobial sensitivity rate to most of the antibiotics than cKp, except cefepime, ceftriaxone, ciprofloxacin, levofloxacin, imipenem, meropenem, and amikacin (Table 4). Although the detection rate of MDR (41.2% vs 74.4%, P = 0.001) and ESBLs (38.2% vs 69.2%, P = 0.001) was significantly lower than cKp, it is noted that 41.2% (14/34) of hvKp isolates were MDR and 38.2% (13/34) of hvKp isolates were ESBL-producing. Moreover, 8 isolates were identified as CR-hvKp.
Table 4.
Antibiotics resistance: hvKp vs cKp
| Antibiotic agent | HvKp(34) | cKp(39) | P value |
|---|---|---|---|
| MDR | 14 (41.2%) | 29 (74.4%) | 0.004 |
| ESBLs | 13 (38.2%) | 27 (69.2%) | 0.008 |
| Ampicillin | 34 (100%) | 39 (100%) | NA |
| Amikacin | 6 (17.6%) | 12 (30.8%) | 0.194 |
| Gentamicin | 8 (23.5%) | 21 (53.8%) | 0.008 |
| Ampicillin/Sulbactam | 13 (38.2%) | 27 (69.2%) | 0.008 |
| Aztreonam | 10 (29.4%) | 21 (53.8%) | 0.035 |
| Cefazolin | 14 (41.2%) | 28 (71.8%) | 0.008 |
| Cefotetan | 8 (23.5%) | 16 (41.0%) | 0.112 |
| Cefepime | 9 (26.5%) | 18 (46.2%) | 0.082 |
| Ceftriaxone | 13 (38.2%) | 23 (59.0%) | 0.077 |
| Ceftazidime | 10 (29.4%) | 23 (59.0%) | 0.011 |
| Ciprofloxacin | 10 (29.4%) | 20 (51.3%) | 0.058 |
| Levofloxacin | 9 (26.5%) | 19 (48.7%) | 0.051 |
| Trimethoprim/Sulfamethoxazole | 7 (20.6%) | 22 (56.4%) | 0.002 |
| Piperacillin/Tazobactam | 8 (23.5%) | 18(46.2%) | 0.044 |
| Imipenem | 8 (23.5%) | 10(25.6%) | 0.835 |
| Meropenem | 9 (26.5%) | 10(25.6%) | 0.936 |
| Tobramycin | 10 (29.4%) | 21(53.8%) | 0.027 |
Risk factors for VAP-hvKp
Univariate regression analysis showed that cancer (odds ratio [OR] = 7.429), digestive diseases (OR = 3.646) and surgery history within 1 month (OR = 5.692) were risk factors for hvKp infection. Multivariate analysis revealed that cancer history (OR = 5.365) was an independent risk factor for hvKp infection (Table 5).
Table 5.
Risk factor for hvKp vs cKp
| Variable | Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value |
|---|---|---|---|---|
| Infection occurred in ICU | 1.387 (0.502–3.832) | 0.528 | ||
| Male | 1.181 (0.245–5.694) | 0.836 | ||
| Pulmonary diseases | 2.260 (0.536–9.539) | 0.267 | ||
| Diabetes | 1.478 (0.586–3.725) | 0.408 | ||
| Cardiovascular disease | 0.706 (0.274–1.818) | 0.471 | ||
| Cerebrovascular disease | 0.765 (0.291–2.010) | 0.587 | ||
| Cancer | 7.429 (1.895–29.114) | 0.004 | 5.365 (1.199–24.007) | 0.028 |
| Surgery within 1 mo | 5.692 (1.117–29.013) | 0.036 | ||
| Digestive diseases | 3.646 (1.023–12.990) | 0.046 | 3.713 (0.970–14.212) | 0.055 |
MLST genotypic analysis
New STs were not detected among the 73 Kp isolates. The most prevalent ST in this study was ST23 (n = 15; 20.5%), followed by ST412 (n = 5; 6.8%), ST17 (n = 4; 5.5%), ST37 (n = 3; 4.1%), and ST2906 (n = 3; 4.1%). The above STs accounted for 41.1% (30/73) of the total strains. Among the prevalent STs, 73.3% ST23 (11/15), 60.0% ST412 (3/5), and 100% ST17 (4/4) were hvKp. The detailed features of patients with prevalent hvKp are shown in Table 6. The CR-hvKp distributed in ST17 (n = 2), ST23 (n = 1), ST347 (n = 1), ST412 (n = 1), ST2874 (n = 1), and ST2905 (n = 1). The most common clone complex (CC) of the CR-hvKp was CC17 (N = 2). The phylogenetic tree demonstrated the relationship between the prevalent STs in our study and the common STs in the world (Fig. 1).
Table 6.
Features of patients with prevalent hvKp(n ≥ 3)
| ST2906(3) | ST37(3) | ST17(4) | ST412(5) | ST23(15) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na = 0 | Nb = 3 | Na = 2 | Nb = 1 | Na = 2 | Nb = 2 | Na = 2 | Nb = 3 | Na = 10 | Nb = 5 | |
| K serotype | ||||||||||
| K1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 9 | 0 |
| K2 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 |
| K5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| K20 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| K54 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| K57 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 |
| rmpA | 0 | 0 | 1 | 0 | 2 | 2 | 2 | 0 | 10 | 0 |
| rmpA2 | 0 | 0 | 1 | 0 | 2 | 2 | 2 | 1 | 9 | 0 |
| magA | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 1 | 9 | 1 |
| Hmv | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 | 9 | 0 |
| hvKp (aerobactin) | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 1 | 10 | 1 |
| Sepsis Shock | 0 | 3 | 0 | 1 | 2 | 1 | 0 | 1 | 8 | 1 |
| Died in 30 Days | 0 | 3 | 2 | 1 | 2 | 0 | 0 | 0 | 8 | 1 |
Hmv hypermucoviscosity, a and b different hospital
Fig. 1.
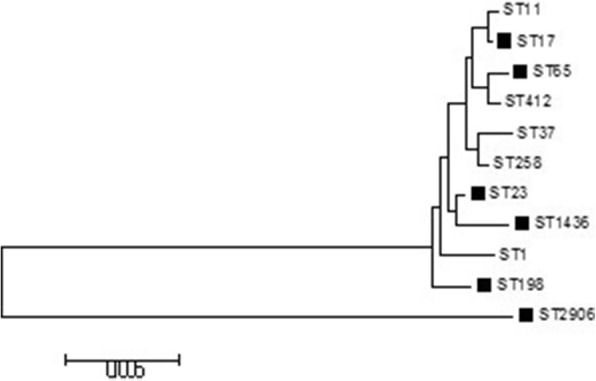
Neighbour-joining dendrogram of concatenated sequences of seven housekeeping genes from the MLST database for prevalent STs in our study and the common STs in the world. (Black solid box represents death in our study)
Discussion
To our knowledge, this is the largest multi-centre study focusing on elderly patients with VAP due to hvKp in China. Nearly half (46.6%, 34/73) of the hvKp-induced VAP in the elderly patients occurred among mechanically ventilated patients. The hvKp group showed a higher severity of disease than did the cKp group. The primary virulence factors, such as K1, rmpA/A2, and magA, were highly clustered in the hvKp group. ST23 was the most prevalent ST in two hospitals. However, the ST23 isolated in different hospitals was not highly associated with the aerobactin genetic background or hypermucoviscous phenotype, and it was concluded that just relying on STs to identify hvKp may be not appropriate. In our study, 50.7% of K. pneumonia isolates were identified as hypermucoviscous by the string test experiment, which is higher than that in a previous retrospective study conducted in a single centre in China, with a prevalence of 33% [16]. Thus, plasmid type, biofilm production, serotypes and the ability to induce inflammatory factors may be needed to further define hvKp. The prevalence of hvKp may be incorrectly estimated because of the lack of definite and objective diagnostic methods.
Aerobactin was considered a potential virulence trait for the hvKp genotype in in vitro and in vivo models [3]. Importantly, previous studies showed that the hvKp genotype (aerobactin-positive) often induced more serious invasive infection [10, 11, 21]. Previous studies reported that the detection rate of hvKp ranged from 4.5 to 37.8% in various clinical specimens [7, 10, 16, 19, 22]. In this study, 34 isolates (46.6%) were defined as hvKp due to aerobactin-positivity, which is the highest incidence for hospital-acquired infection (HAI) caused by the hvKp genotype. It is noted that the detection of the hvKp genotype in this study was also higher than that in previous multicentre studies(37.8%) based on adults [10] and a single centre retrospective study (28.6%) focusing on VAP [23]. It is concluded that hvKp may emerge as a major pathotype for elderly with VAP in the two hospitals.
Additionally, capsular serotype-specific genes are essential factors for K. pneumoniae. To date, various types of K-antigen have been reported [20, 24, 25]. In Asia, K1 and K2 are the most important elements, inducing severe infections. However, they are not the unique trait for hvKp [26–29]. Previous studies demonstrated that K1 distribution in hvKp ranged from 23 to 98% in various specimens in various populations [29–33]. The detection rate of K2 in hvKp ranged from 10 to 46% [16, 31, 33]. Moreover, a previous single centre study focusing on VAP showed that K1 and K2 among hvKp accounted for 42.9 and 21.4%, respectively [23]. The above data implied that K1 might be highly associated with hvKp compared with K2. In our study, half of the hvKp (50%) isolates were associated with K1, and K2 was detected in 7 hvKp isolates (2.9%), which was consistent with previous studies. In addition to K1 and K2, there were no significant difference between hvKp and cKp in K5, K20, K54, and K57 due to small specimens. Moreover, rmpA/rmpA2 and magA genes responsible for the hypermucoviscous phenotype were proposed as virulence factors in addition to major cps K1/K2 [5, 19, 30, 34]. In our study, 2 hvKp isolates inserted rmpA did not show hypermucoviscosity. It is implied that there might exist other regulatory mechanisms for hypermucoviscosity. Importantly, our results are consistent with previous study: most of the dominant virulence-associated factors (K1, rmpA/A2 and magA) and hypermucoviscosity are highly clustered in the hvKp group [10].
Although there was no significant difference in sepsis incidence and mortality between the two VAP groups, the incidence of sepsis shock and SOFA score was higher than those in the cKp group. Thus, to further understand the risk factors for hvKp, timely and appropriately prevention is essential. Our results showed that elderly patients with cancer, digestive disease and surgery history within 1 month were more likely to be infected with hvKp. Moreover, cancer history was an independent risk factor for VAP-hvKp infection in the elderly, and cancer patients should be monitored more intensively to prevent infection due to immunocompromise, which may also be a critical factor for VAP-hvKp [10]. Previous multi-centre study of the hvKp genotype based on adults in China concluded that diabetes and cancer were independent risk factors for hvKp [10]. However, the incidence of diabetes (50.7%, 37/73) in our study is far more than previous multi-centre study focused on adults (17.0%, 39/230) [10]. This may be the characteristics of the elderly who often suffer from various underlying diseases. Additionally, a previous study concluded that major histocompatibility complex (MHC) variants, nutritional status, and the gut microbiota might be potential host factors for improving the understanding of the hypervirulence phenomenon [9]. Although the incidence of septic shock and SOFA score in our study were higher than those of the cKp group, there was no significant difference in the nutritional status (TP and ALB) and inflammatory reaction (WBC and NEU%), which was consistent with a previous study [23]. Thus, other inflammatory factors may be needed to screen as a marker for elderly patients with VAP-hvKp.
Previous studies revealed that most cKp and antimicrobial-resistant patterns were overlapping, which is not frequent in hvKp [10, 16]. In this study, most of the hvKp isolates were sensitive to most of the above antibiotics. However, in the hvKp group, the number of ESBL-hvKp (41.2%) and MDR-hvKp (38.2%) is significantly higher than that in previous studies [10, 23]. It is alarming that MDR-hvKp may prefer to induce VAP in the elderly. Moreover, eight CR-hvKp isolates were identified as another “superbug”. Taken together, these data revealed that MDR-hvKp is emerging among elderly patients with VAP, which needs to be confirmed by further investigations in larger populations. The CR-hvKp isolates were not detected in the nosocomial environment by routine nosocomial infection surveillance. Additionally, the composition of the gut microbiota was unclear because anal swabs were not applied for nosocomial infection surveillance. Previous study suggested that wards previously infected with CR-hvKp should be disinfected and left unoccupied for more than 2 weeks [12]. It may be a good choice to prevent fatal outbreaks of infection, especially in critically ill and immunocompromised elderly patients with mechanical ventilation.
The main limitation in our study is that it is a retrospective study from over 10 years. Although this study included two large tertiary hospitals, fewer patients with VAP-hvKp were included. However, this study is the largest cohort to investigate the clinical and microbiological characteristics of elderly patients with VAP-hvKp to now. Additionally, most of the key inflammatory factors, nutrition statue markers and environmental samples were not achieved. Moreover, the expression of aerobactin is unclear. Whole genome sequencing, transcriptomics and proteomics may be needed for further study to identify genetic expression.
Conclusions
HvKp is emerging as a common pathogen of VAP in the elderly in China. There is various epidemiology of VAP-hvKp in different hospitals. Although STs may not be associated with hvKp, ST23, ST37 and ST2906 are more likely to induce poor prognosis. Although the definition of hvKp is still controversial, the hvKp genotype is more likely to cause septic shock and a higher SOFA score. The emerging MDR-hvKp, especially CR-hvKp, will be a “superbug”, which is a great challenge for clinicians. It is essential to enhance clinical awareness and infection surveillance for various hvKp infections, especially in immunocompromised elderly patients.
Acknowledgements
We thank the team of curators at the Institut Pasteur MLST and whole genome MLST databases for curating the data and making them publicly available at http://bigsdb.pasteur.fr.
Funding
This work was supported by the China postdoctoral science foundation (grant number 2014 M562610); the Excellent Young Program of the Organization Department of Beijing Municipal Party Committee (grant number 2016000057592G258).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JG and CL were responsible for study design and article writing. CL and JG collected clinical data and performed PCR and statistical analyses. JG performed critical data review. All authors read and approved the final version of the manuscript.
Informed consent was not needed due to the retrospective nature of the study. The study was approved by the Chinese PLA General Hospital Ethics Committee, and the Guidelines for Human Experimentation (PR. China) were followed throughout.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chao Liu, Email: liuchao3619@163.com.
Jun Guo, Email: guojunhx301@163.com.
References
- 1.Timsit JF, Esaied W, Neuville M, Bouadma L, Mourvllier B. Update on ventilator-associated pneumonia. F1000Res. 2017;6:2061. doi: 10.12688/f1000research.12222.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50:1700582. [DOI] [PubMed]
- 3.Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun. 2015;83(8):3325–3333. doi: 10.1128/IAI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 6.Pomakova DK, Hsiao CB, Beanan JM, Olson R, MacDonald U, Keynan Y, Russo TA. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis. 2012;31(6):981–989. doi: 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zeng J, Liu W, Zhao F, Hu Z, Zhao C, Wang Q, Wang X, Chen H, Li H, et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71(5):553–560. doi: 10.1016/j.jinf.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Lin YC, Lu MC, Tang HL, Liu HC, Chen CH, Liu KS, Lin C, Chiou CS, Chiang MK, Chen CM, et al. Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain. BMC Microbiol. 2011;11:50. doi: 10.1186/1471-2180-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalan-Najera JC, Garza-Ramos U, Barrios-Camacho H. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence. 2017:1–13. [DOI] [PMC free article] [PubMed]
- 10.Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Wang R, Wang H. High prevalence of Hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115–6120. doi: 10.1128/AAC.01127-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo TA, Olson R, Macdonald U, Metzger D, Maltese LM, Drake EJ, Gulick AM. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun. 2014;82(6):2356–2367. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, Chan EW, Shu L, Yu J, Zhang R, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46 [DOI] [PubMed]
- 13.Gu DX, Huang YL, Ma JH, Zhou HW, Fang Y, Cai JC, Hu YY, Zhang R. Detection of Colistin resistance gene mcr-1 in Hypervirulent Klebsiella pneumoniae and Escherichia coli isolates from an infant with diarrhea in China. Antimicrob Agents Chemother. 2016;60(8):5099–5100. doi: 10.1128/AAC.00476-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Lin D, Chan EW, Gu D, Chen GX, Chen S. Emergence of Carbapenem-resistant serotype K1 Hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother. 2015;60(1):709–711. doi: 10.1128/AAC.02173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Sun G, Yu Y, Li N, Chen M, Jin R, Jiao Y, Wu H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58(2):225–232. doi: 10.1093/cid/cit675. [DOI] [PubMed] [Google Scholar]
- 17.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infec. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi MJ, Ko KS. Loss of hypermucoviscosity and increased fitness cost in colistin-resistant Klebsiella pneumoniae sequence type 23 strains. Antimicrob Agents Chemother. 2015;59(11):6763–6773. doi: 10.1128/AAC.00952-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42(10):1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 20.Cheng NC, Yu YC, Tai HC, Hsueh PR, Chang SC, Lai SY, Yi WC, Fang CT. Recent trend of necrotizing fasciitis in Taiwan: focus on monomicrobial Klebsiella pneumoniae necrotizing fasciitis. Clin Infect Dis. 2012;55(7):930–939. doi: 10.1093/cid/cis565. [DOI] [PubMed] [Google Scholar]
- 21.Nassif X, Sansonetti PJ. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun. 1986;54(3):603–608. doi: 10.1128/iai.54.3.603-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med. 2006;259(6):606–614. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 23.Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35(3):387–396. doi: 10.1007/s10096-015-2551-2. [DOI] [PubMed] [Google Scholar]
- 24.Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, Tsai FC, Keynan Y, Wang JT. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol. 2008;46(7):2231–2240. doi: 10.1128/JCM.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis. 2006;193(5):645–654. doi: 10.1086/499968. [DOI] [PubMed] [Google Scholar]
- 26.Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, Lin JC, Chen TL, Chang FY, Koh TH. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol. 2007;45(2):466–471. doi: 10.1128/JCM.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One. 2009;4(3):e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45(3):284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 29.Lin JC, Koh TH, Lee N, Fung CP, Chang FY, Tsai YK, Ip M, Siu LK. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathogens. 2014;6:21. doi: 10.1186/1757-4749-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199(5):697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YM, Li BB, Zhang YY, Zhang W, Shen H, Li H, Cao B. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother. 2014;58(9):5379–5385. doi: 10.1128/AAC.02523-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Compain F, Babosan A, Brisse S, Genel N, Audo J, Ailloud F, Kassis-Chikhani N, Arlet G, Decre D. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. 2014;52(12):4377–4380. doi: 10.1128/JCM.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SS, Chen YS, Tsai HC, Wann SR, Lin HH, Huang CK, Liu YC. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis. 2008;47(5):642–650. doi: 10.1086/590932. [DOI] [PubMed] [Google Scholar]
- 34.Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA. Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol. 2005;54(Pt 11):1111–1113. doi: 10.1099/jmm.0.46165-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


