Abstract
Background:
The purpose of this study was to assess the diagnostic role of preoperative hematological parameters, especially neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) in germ cell testicular malignancies and their prediagnostic role in staging of testicular cancer.
Materials and Methods:
In this cross-sectional retrospective study, we analyzed 39 patients who underwent radical orchiectomy due to a testicular cancer (Group 1) and 82 patients on whom varicocelectomy procedure was performed as control group (Group 2) between January 2006 and January 2016 in our clinic. Evaluation of the preoperative hematological parameters in both groups and also the subgroups in malignancy group according to histopathological stages was conducted in this study.
Results:
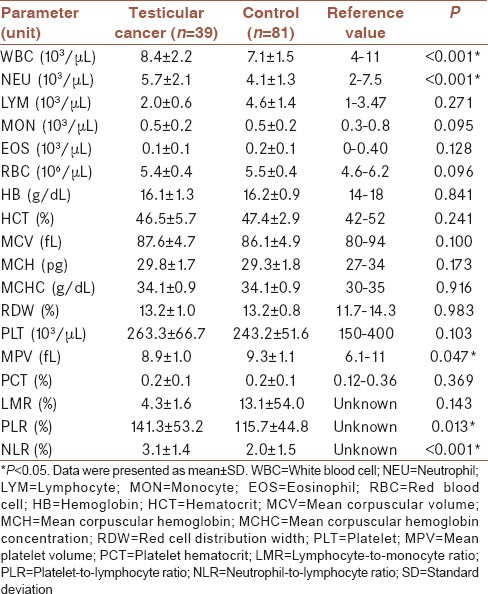
When the hematological parameters were compared, a statistically significant difference was found between the two groups in terms of neutrophil counts, NLR, PLR, and MPV. NLR and PLR were significantly higher and MPV was significantly lower in testicular cancer group compared to the control group. NLR was 3.1 ± 1.4 and 2.0 ± 1.5, PLR was 141.3 ± 53.2 and 115.7 ± 44.8, and MPV was 8.9 ± 1.0 and 9.3 ± 1.1 for testicular cancer and control groups, respectively (P < 0.05). Furthermore, differences were observed between only mean corpuscular volume, mean corpuscular hemoglobin, and MPV (P < 0.05) in different stages of malignancy.
Conclusion:
In accordance with these findings, NLR, PLR, and MPV may be helpful for prediagnosis of testicular malignancies. Hematological parameters will become important in the preoperative assessment for those patients.
Keywords: Hematological parameters, neutrophil-to-lymphocyte ratio, testicular cancer
INTRODUCTION
Studies have shown that inflammatory response is closely correlated with tumorigenesis and progression of the tumors.[1] The interactions between the tumor and inflammation occur through complex and various mechanisms. Inflammation has a significant role in every stage of carcinogenesis including the onset of tumor, angiogenesis, apoptosis inhibition, and tumor metastasis.[2]
Changes in the systemic inflammatory response can be reflected by measuring the hematological parameters. Blood-based indexes such as Modified Glasgow Prognostic Score, neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP) are examined for their capability of prognosis of various malignancies.[3,4] It is shown that increased preoperative NLR is correlated with poor prognosis in patients with various malignancies including urothelial cancers.[5] A great majority of the studies have reported that the increase in NLR is related to poor clinical results in many malignant tumors.[6] It is shown that NLR can be used as an indicator of systemic inflammatory response in not only several tumor types but also other inflammatory situations and various diseases.[6,7] It is reported that NLR, which is found at higher levels within the scope of preoperative assessments, can be an indicator related to poor prognosis factor and pathological staging in urologic tumors,[8,9] as well as gastric cancer,[10] hepatocellular carcinoma,[3] breast cancer,[11] and pancreatic cancer.[12]
In malignant tumors, platelets (PLTs) take place in the inflammatory process and may cause tumor progression and angiogenesis.[13] A meta-analysis demonstrated that platelet-to-lymphocyte ratio (PLR) could act as a significant biomarker in the prognosis of various cancers.[14] Even though the diagnostic role of mean platelet volume (MPV) is reported in various malignant tumors such as ovary,[15] pancreas,[16] and colon[17] cancers, the diagnostic and prognostic role of MPV cannot be precisely revealed for testicular tumors.
Although testicular tumors constitute only approximately 1% of all the solid tumors in men, it is the most common solid malignancy affecting men between the ages of 15 and 35.[18] Testicular tumors are gathered in two main categories as germ cell tumors with a prevalence of 95% and sex cord-stromal tumors with a prevalence of 5%.
The purpose of this study was to assess whether preoperative hematological parameters have a predictive role in the diagnosis of testicular cancer and the determination of its stages.
MATERIALS AND METHODS
This cross-sectional retrospective study included 39 patients who underwent radical orchiectomy due to testicular cancer and 82 patients as the control group who underwent varicocelectomy between January 2006 and January 2016 in a urology service in a tertiary care center. Inclusion criteria were the determination of germ cell testicular malignancies in the pathology results for the patient group and the presence of varicocelectomy operation for control group. Exclusion criteria were the presence of nongerm cell testicular malignancies in testicular cancer group and the presence of an active infection process in both groups. Hematological parameters were evaluated by peripheral blood samples taken during preoperative period. These hematological parameters were as follows: white blood cell (WBC), neutrophil (NEU), lymphocyte (LYM), monocyte, eosinophil, red blood cell, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration, red cell distribution width, PLT, platelet hematocrit, and MPV.
Staging of the patients with testicular tumor was performed by examining the computed tomography and beta-human chorionic gonadotropin, alpha-fetoprotein, and lactate dehydrogenase (LDH) parameters as tumor markers. For this study, the approval of the University Ethics Committee for Non-invasive Clinical Trials was obtained (No: 2016-02/04). All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Analysis of variance was used on variables that fit parametric distribution for the comparison of variables having more than two categories, and similarly, Student's t-test was used on variables that fit parametric distribution for the comparison of variables with only two categories. Least square difference method was used as a post hoc test when a significant result was obtained from the analysis of variance. Confidence level of the tests was set as 95%.
RESULTS
Mean age of patients in cancer group and control group was 30.1 ± 7.0 and 28.5 ± 5.5 (mean ± standard deviation) years, respectively. There was no significant difference between their ages (P = 0.224). Among the testicular cancer patients, 25 were in Stage 1, 4 were in Stage 2, and 10 were in Stage 3. The mean ages and standard deviations according to stages were as follows: 32.0 ± 7.2, 26.8 ± 2.5, and 26.4 ± 5.9 in Stages 1, 2, and 3, respectively (P = 0.055; >0.05). WBC, NEU, PLR, and NLR values were significantly higher in patients with testicular cancer; however, MPV was significantly lower (P < 0.05). WBC was 8.4 ± 2.2 and 7.1 ± 1.5, NEU was 5.7 ± 2.1 and 4.1 ± 1.3, NLR was 3.1 ± 1.4 and 2.0 ± 1.5, PLR was 141.3 ± 53.2 and 115.7 ± 44.8, and MPV was 8.9 ± 1.0 and 9.3 ± 1.1 for testicular cancer and control groups, respectively (P = <0.001, <0.001, <0.001, <0.013, and < 0.047, respectively, <0.05). There was no significant difference between the groups in terms of other hematological parameters. Hematological parameters of the groups were shown in Table 1.
Table 1.
Hematological parameters of groups and Student's t-test results
Cutoff values and receiver operating characteristic analysis of NEU, NLR, PLR, and MPV according to cancer group and control group are shown in Table 2 and Figure 1.
Table 2.
Cutoff values of neutrophil, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume
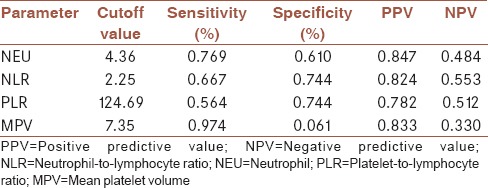
Figure 1.
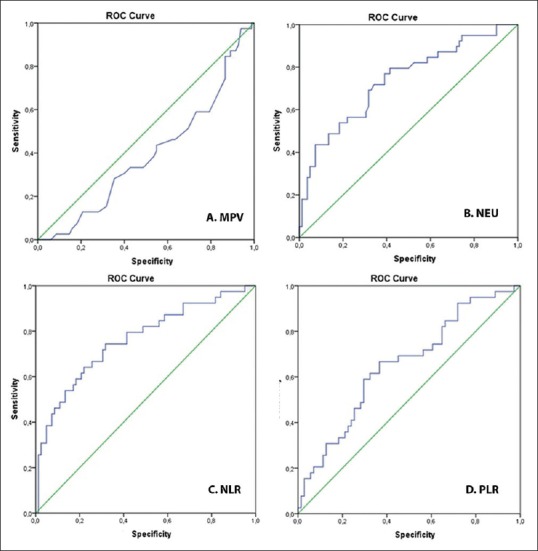
Receiver operating characteristic analysis of neutrophil, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume
Differences between hematological parameters of patients with testicular cancer according to the stages were examined, and differences were observed between MCV, MCH, and MPV (P < 0.05). MCV was significantly higher in Stage 1 compared to Stage 2 or 3 (P = 0.035 and P = 0.025, respectively). MCH was significantly higher in Stage 1 compared to Stage 3 (P = 0.022). MPV was significant lower in Stage 1 compared to Stage 3 (P = 0.016). Hematological parameters of patients with testicular cancer according to the stages were displayed in Table 3.
Table 3.
Hematological parameters of patients with testicular cancer according to the stages and F-test results
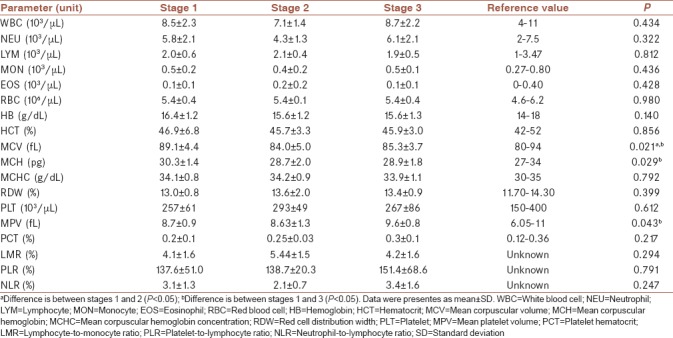
Even though NLR and PLR were significantly higher in patients with testicular cancer; when this discrepancy was examined, no significant differences were found between those patients according to the stages (P = 0.247, P = 0.791, respectively).
DISCUSSION
Inflammation has a critical role in tumor development, progression, clinical presentation, and prognosis. According to the type and prevalence of malignancy, systemic inflammatory and immune responses to the tumor cells and the peptides secreted by them vary. Tumor and the interaction of its host have important effects on tumor development. Today, in patients with malignancies, systemic inflammatory response indicators such as cytokines, CRP, albumin, serum amyloid A, and leukocytes have gained importance, and it is thought that they can be independent prognostic factors.[9]
Immune system has a binary function on the development and progression of cancer. It may eliminate the tumor cells or may cause the progression of the tumor by increasing the invasive capacities and metastatic skills of the active malignant cells. It is thought that excess number of circulating NEUs has an important role in the progression and angiogenesis of the tumor. Thus, the increased NEU count should be related to poor prognosis.[19]
High NLR occurring as a result of the added effect of increased NEU response to LYM suppression can support the development of cancer by inhibiting the antitumor immune response.[20,21] Neutrophilia can be a result of ectopic production of myeloid growth factors as a part of paraneoplastic syndrome or may form an inflammation response related to cancer together with the cytokines appearing as a result of tissue destruction.[22] Experimental studies have shown that activated NEUs can directly and indirectly activate tumor growth.[23] Molecular pathways used by inflammatory mediators can support the angiogenesis and metastasis of cancer cells and thus affect the tumor response of the treatments.[24]
Peripheral blood tests before treatment or during diagnosis may reflect the inflammatory situations in the tumor. Through complete blood count, which is a cheap and straightforward test, NLR, PLR, MPV, and other hematological parameters can be calculated and provide prognostic information for the patients in the treatment of diseases.[25]
In previous studies, correlation was determined between high NLR and tumor grade and stage, detected in 122 patients who underwent transurethral resection-bladder due to bladder tumor and were newly diagnosed with muscle noninvasive bladder tumor.[26] In most of the studies assessing the correlation between radical cystectomy results and NLR, high NLR results and worse ratios are found in disease-specific and general survival.[8,27]
Wei et al. published a meta-analysis from 17 published studies including 3159 cases to assess the prognostic value of NLR in the patients with urinary system cancer. In this meta-analysis, high NLR in the subgroup analyses performed according to cancer type is correlated with poor general survival in renal cell carcinoma, bladder cancer, and urothelial carcinomas.[28]
NLR threshold value is accepted as 2.5 in addition to determination of age, female gender, NLR, and PLT counts as an invasiveness indicator in urothelial carcinoma.[25] In a study conducted on RCC cases, it was shown that an NLR of 2.78 and above and a PLR of 185 and above were associated with decreased general survival.[29] In the present study, it was found that NLR was 3.07 ± 1.38 in the group of patients with testicular cancer and 2.01 ± 1.49 in the control group of patients undergoing varicocelectomy. We found that an NLR threshold value of 2.25 can be acceptable in testicular tumors.
In a study conducted on 36 patients with testicular tumor to examine NLR in patients with testicular tumor, a significant difference was found compared to the control group similar to the results obtained in this study.[29] In addition, in the present study, it was determined that NLR showed no statistically significant difference in various stages of the tumor. In the present study, a statistically significant difference was found between the groups in terms of PLR, like NLR (P < 0.05).
In the study of Song et al., conducted on patients with upper urinary system urothelial carcinoma undergoing nephroureterectomy, it was found that NLR and PLR were significantly higher in the patients having advanced-stage tumors, similar to the results obtained in the present study. In this study, it was found that lymphocyte/monocyte ratio (LMR) significantly decreased in advanced-stage tumors, and NLR was a superior predictive factor than LMR.[30] In the present study, even though LMR was lower in the patients with testicular tumor, the difference was not statistically significant.
CONCLUSIONS
In the present study, it was found that particularly NLR, PLR, and MPV were significantly higher in testicular tumor compared to the control group as similar to other cancers. While there are numerous studies on other cancers related to hematological parameters, there is a limited amount of data for testicular tumor in the literature. These practical and simple hematological parameters may be associated with the diagnosis of testicular cancer and its stage. Especially, NLR, PLR, and MPV may be helpful in diagnosis of germ cell testicular malignancies in addition to other accepted serum tumor markers. However, these parameters were insufficient in predicting the stage of the tumor as indicated by the results of this study, which was a limitation of our study due to the low number of the patients in each stage subgroup.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We appreciate to Selim Çam for their great helps in analysis of the statistics.
REFERENCES
- 1.Gregory AD, Houghton AM. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011;71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–64. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 4.Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:5591–7. doi: 10.3748/wjg.v21.i18.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–64. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 6.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 7.Zahorec R. Ratio of neutrophil to lymphocyte counts – Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 8.Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–91. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 9.Moore MM, Chua W, Charles KA, Clarke SJ. Inflammation and cancer: Causes and consequences. Clin Pharmacol Ther. 2010;87:504–8. doi: 10.1038/clpt.2009.254. [DOI] [PubMed] [Google Scholar]
- 10.Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Murri AM, Wilson C, Lannigan A, Doughty JC, Angerson WJ, McArdle CS, et al. Evaluation of the relationship between the systemic inflammatory response and cancer-specific survival in patients with primary operable breast cancer. Br J Cancer. 2007;96:891–5. doi: 10.1038/sj.bjc.6603682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson NB, Glen P, McMillan DC, McKay CJ, Foulis AK, Carter R, et al. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br J Cancer. 2005;92:21–3. doi: 10.1038/sj.bjc.6602305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George JN. Platelets. Lancet. 2000;355:1531–9. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: A meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemal Y, Demiraǧ G, Ekiz K, Yücel I. Mean platelet volume could be a useful biomarker for monitoring epithelial ovarian cancer. J Obstet Gynaecol. 2014;34:515–8. doi: 10.3109/01443615.2014.912620. [DOI] [PubMed] [Google Scholar]
- 16.Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, et al. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Intern Med. 2011;22:e95–8. doi: 10.1016/j.ejim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Kilincalp S, Çoban Ş, Akinci H, Hamamcı M, Karaahmet F, Coşkun Y, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015;24:328–33. doi: 10.1097/CEJ.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 19.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 20.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134:230–4. [PubMed] [Google Scholar]
- 21.Schaider H, Oka M, Bogenrieder T, Nesbit M, Satyamoorthy K, Berking C, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer. 2003;103:335–43. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- 22.Vassilatou E, Fisfis M, Morphopoulos G, Savva S, Voucouti E, Stefanoudaki K, et al. Papillary thyroid carcinoma producing granulocyte-macrophage colony-stimulating factor is associated with neutrophilia and eosinophilia. Hormones (Athens) 2006;5:303–9. doi: 10.14310/horm.2002.11196. [DOI] [PubMed] [Google Scholar]
- 23.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gueron G, De Siervi A, Vazquez E. Advanced prostate cancer: Reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer Prostatic Dis. 2012;15:213–21. doi: 10.1038/pcan.2011.64. [DOI] [PubMed] [Google Scholar]
- 25.Can C, Baseskioglu B, Yılmaz M, Colak E, Ozen A, Yenilmez A, et al. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int. 2012;89:468–72. doi: 10.1159/000343278. [DOI] [PubMed] [Google Scholar]
- 26.Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2015;33:67e1–7. doi: 10.1016/j.urolonc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Krane LS, Richards KA, Kader AK, Davis R, Balaji KC, Hemal AK, et al. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. 2013;27:1046–50. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: A meta-analysis. PLoS One. 2014;9:e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuksel OH, Verit A, Sahin A, Urkmez A, Uruc F. White blood cell counts and neutrophil to lymphocyte ratio in the diagnosis of testicular cancer: A simple secondary serum tumor marker. Int Braz J Urol. 2016;42:53–9. doi: 10.1590/S1677-5538.IBJU.2014.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song X, Zhang GM, Ma XC, Luo L, Li B, Chai DY, et al. Comparison of preoperative neutrophil-lymphocyte, lymphocyte-monocyte, and platelet-lymphocyte ratios in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Onco Targets Ther. 2016;9:1399–407. doi: 10.2147/OTT.S97520. [DOI] [PMC free article] [PubMed] [Google Scholar]


