Abstract
Background
Confocal laser endomicroscopy (CLE) enables “in vivo” microscopic tissue diagnosis based on tissue reflectance or tissue fluorescence upon application of fluorescence agents. The aim of the present study was to evaluate CLE as a new diagnostic approach for differentiation between malignant versus non-malignant pleural effusions.
Material/Methods
In 100 patients with pleural effusions, thoracentesis was performed. Cresyl violet and acriflavine were used as contrast agents for probe-based CLE of effusions. CLE video sequences were assessed by 4 independent investigators (2 experienced in this technique, 2 with only basic knowledge). In addition, all CLE samples were evaluated by an expert pathologist (p). Results were compared with conventional cytology of effusions and histology of cell blocks.
Results
CLE reliably permitted identification of malignant cells in pleural effusions. Sensitivity for detection of malignant effusions was 87% (p: 87%) and 81% (p: 72%) for acriflavine and cresyl violet, respectively. With regard to specificity, acriflavine and cresyl violet yielded a mean value of 99% (p: 100%) and 92% (p: 100%).
Conclusions
In this pilot study, CLE permitted simple and rapid detection of malignant pleural effusions. Larger prospective studies are warranted to corroborate our findings.
MeSH Keywords: Diagnostic Imaging; Microscopy, Confocal; Neoplastic Cells, Circulating; Pleural Effusion
Background
Confocal laser endomicroscopy (CLE) is a well-established method in gastroenterology. The principle of CLE is based on tissue reflectance or tissue fluorescence after application of fluorescence agents (mostly fluorescein sodium) [1] which enable the endoscopist to perform instant microscopic tissue diagnosis on site [2–4] with results comparable to those of traditional histology. For this purpose, it is widely used in the upper and lower gastrointestinal tract. In a pilot study, Tontini et al. differentiated pathologic changes of the mucosa between ulcerative colitis and Crohn disease applying CLE [5]. Additional applications of CLE include diagnosis and management of Barrett esophagus [6], pancreatic and bile duct strictures [7,8], gastric intestinal metaplasia/cancer [9,10], and colon polyps [11]. However, until now it has not replaced, but rather refined, pathological investigation.
The use of fluorescent CLE in the field of pulmonology is comparatively rare. A comprehensive literature review yielded only 28 publications reporting CLE in this field. In this context, pulmonary CLE was first described by Thiberville et al. in 2009 demonstrating feasibility and ease of use of this technique during fiberoptic or video-chip-based bronchoscopy under local anesthesia [12]. Beyond that, normal proximal and distal bronchial structures could be successfully illustrated [13–16]. Further encouraging attempts were made applying CLE to distinguish normal mucosa from cancer tissue both in vivo and ex vivo [17,18], to detect changes in the respiratory tract in case of acute lung allograft rejection [19], endobronchial hamartomas [20], and amiodarone-related pneumonia [21], as well as pulmonary alveolar microlithiasis [22] and radiation-induced pulmonary fibrosis [23]. Recent approaches even assessed the feasibility of CLE in solitary pulmonary nodules for different part of the lungs [24]. CLE was found to be helpful to precise positioning and to characterize the peripheral pulmonary nodule [25]. Moreover, Guisier et al. showed successful application of CLE for imaging of apoptosis in tumor xenografts of mice [26]. Despite these promising data, CLE is currently not routinely used in pulmonology.
Pleural effusions are a common symptom in a wide range of different diseases. They are caused by an imbalance between fluid production versus fluid reabsorption. More than 1.5 million pleural effusions occur in the United States every year as a consequence of a variety of inflammatory, infectious, and malignant conditions [27,28] (an estimated 150 000 of which proved to be malignant [29]). Most frequently, pleural effusions are seen in association with heart failure, malignancy, pneumonia, tuberculosis, and pulmonary embolism [30].
Presently, several tests are required for differential diagnosis: Initially, exudates are differentiated from transudates based on the Light’s criteria [30–32]. Especially in case of exudative effusions additional testing is needed: pH, glucose, lactate, triglyceride, cholesterol, leukocyte count including further characterization of subgroups such as neutrophils, lymphocytes, and mononuclear cells, microbiological analysis, as well as cytological and/or histological examination [31].
Regarding malignancy, the general aim is to minimize the required examinations for diagnosing patients with metastatic disease including those with malignant pleural effusions to avoid further complications and delay of therapy. A very elegant and relatively non-invasive method to reach this aim is to detect malignant cells in existing pleural effusions by cytology verifying stage IV disease [32–34].
Although cytological examination of suspected malignant pleural effusions is fast and efficient in cancer diagnosis (advanced stage), it can lead to false negative results in up to 40% of patients [30] resulting in a mean sensitivity averaging only 60% (with a range of 40% to 87%) [35–37]. Sending a second specimen taken on a different occasion may increase the sensitivity by 27%, while a third may only lead to a 5% increase in correct diagnosis, suggesting that sending more than 2 specimens should be avoided [37]. A combination of the cell block method with smears prepared from fluid samples was reported to improve diagnostic sensitivity by up to 15% [38–40].
First and foremost, the present study is a proof of concept study. Our main concern was whether CLE was applicable for diagnosing pleural fluids, and if so, is CLE able to distinguish malignant from non-malignant cells in pleural effusions? Second, we aimed to determine whether this would result in a potential clinical benefit.
Material and Methods
One hundred consecutive patients with pleural effusions (uni- or bilateral) were prospectively included between May 2011 and October 2012. Participants were enrolled if they met the following inclusion criteria: older than 18 years of age, ultrasound-guided possible thoracentesis, necessity of thoracentesis because of clinical reasons. Patients with 1 or more of following criteria were excluded from the study: severe uncontrolled coagulopathy without vital necessity of thoracentesis, residing in institutions (e.g., prison), and patients younger than18 years of age. Clinical data were noted including age, gender, patient history, secondary diagnoses, laboratory and histological/cytological data, as well as further interventions within the next 30 days such as pleural biopsies, re-thoracentesis, or computed tomography (CT) scans. The study was approved by the local ethical committee (https://www.ethikkommission.fau.de, Ethikkommission der Friedrich-Alexander-Universität Erlangen-Nürnberg, Krankenhausstraße 12, 91054 Erlangen) and was conducted according to the Declaration of Helsinki. Patients suffered from various underlying diseases such as different cancer types, active infections, hepatic cirrhosis, renal failure, or cardiac decompensation (Supplementary Tables 1, 2). For diagnostic and/or therapeutic reasons, all study participants underwent thoracentesis by single or repeat punctures or alternatively by inserting a pleural catheter. The procedure was explained to all patients in detail and written consent was obtained.
Beside routinely conducted tests (pH-value, lactate, glucose, albumin, microbiological examination, and conventional cytological or cell block analyses) we took an additional 30 mL of pleural effusion for CLE “ex-vivo” analysis. Cell block analyses and conventional cytological examinations served as the control for CLE. We used hematoxylin and eosin (H&E) staining, CK 20 and BerEP4 staining as well as the antibody for TTF-1. Immunohistochemical staining was conducted on 2 μm thick sections of formalin-fixed paraffin-embedded (FFPE) cyto-tumor blocks according to manufacturer’s protocol on Ventana Benchmark Ultra (Ventana Medical Systems, Inc., Tucson, AZ, USA). The antibody for TTF-1 (clone 8G7G3/1, dilution, 1: 500) was retrieved from Zytomed, Berlin, Germany.
To 50 samples, cresyl violet (1 mg cresyl violet dissolved in 2 mL sodium chloride 0.9% to each sample, Merck) was added. To the remaining 50 cases, acriflavine (2.5 mg acriflavine dissolved in 1 mL sodium chloride 0.9%, Sigma Aldrich) was added.
After centrifugation (15 minutes, 4°C, 1500 rpm) CLE was performed which required about 5–10 minutes for each sample including diagnostic rating.
Preferentially, the cell pellet was analyzed. The commercial Cellvizio® system (Mauna Kea Technologies, Paris) was used. A confocal miniprobe S type (1.4-mm diameter; SN: DM-2023, Mauna Kea Technologies) was applied which displayed a penetration depth of 0–50 μm, a lateral resolution of 3.5 μm, and a field of view of 600×500 μm. We selected a grey scaled imaging with the lower and upper level thresholds of the look-up table from 0 to 8,000 units (setting/parameters as used by Fuchs et al. [17]).
The video sequences were assessed with the included software (Cellvizio viewer, version 1.4.1; Mauna Kea Technologies). The completed video sequences were analyzed by 4 different physicians. Two physicians were the principle investigators who received detailed theoretical and practical training in this technique (experienced investigators A and B), the other 2 investigators (unexperienced investigators C and D) were just briefly introduced to CLE analysis by exercise sheets. Their rating was then compared to cytology results. Confirmation of malignant cells (presence of at least single tumor cells) resulted in the category “malignant – m”, all the others in the category “non-malignant – nm”. Assuming that cytology also affords shortcomings, we validated these data including clinical information. The latter were obtained retrospectively from the medical files. Alike, investigative results within 30 days following initial pleural puncture were considered: thoracoscopic-guided proof of pleural carcinomatosis, re-puncture with detection of malignant cells in cell block analyses, serious suspicion of pleural carcinomatosis in CT scan.
This approach was chosen to assess the analytical sensitivity and specificity of this method as well as the positive and negative predictive value.
Finally, all video sequences were reevaluated by an experienced pathologist blinded to the results of the evaluations by the non-pathologist investigators.
Results
During the study period, a total of 100 patients with pleural effusions were prospectively included. They were randomly assigned to 2 groups (acriflavine versus cresyl violet for effusion staining) consisting of 50 patients each at a 1: 1 ratio. Following staining with the well-established fluorescent agents acriflavine or cresyl violet, CLE was performed to allow real-time non-invasive cytological imaging.
With both dyes the main cell types could clearly be seen and differentiated: Cancer cells, mesothelial cells, and leukocytes. Similar to traditional cytology (please see Figures 1, 2; A2, B2, respectively) they showed typical characteristics with malignant cells had an unbalanced nucleus-plasma relation towards an enlarged nucleus. Carcinoma cells showed variation in cell size with hyperchromatic nuclei and irregular nuclear and cell membranes (Figures 1, 2; A1, B1 respectively). In only some cases, reactively transformed mesothelial cells were barely distinguishable from tumor cells. As a proof of concept study and as the 4 investigators of interest were internal specialists and not pathologists a subdivision of cancer cell types was not intended.
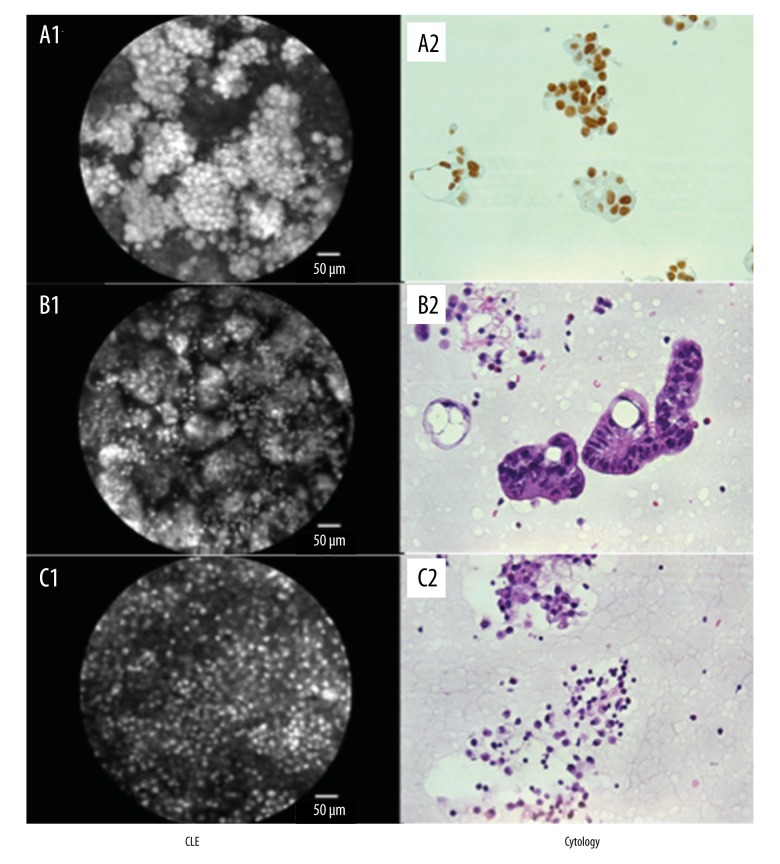
Figure 1.
Comparison between CLE + acriflavine staining and cytology. (A) Lung adenocarcinoma: (A1) probe-based confocal laser endomicroscopy with positive proof of malignant cells, suspicion of glandular growth; (A2) cytological analysis, detection of TTF-1 positive tumor cells corresponding to a lung adenocarcinoma. (B) Rectal cancer: (B1) CLE aided detection of malignant cells, glandular growth; (B2) cytological confirmation of tumor cells, hematoxylin and eosin staining. (C) Esophageal cancer as underlying disease: (C1) CLE, no proof of tumor cells, detection of smaller cells with an intense dyeing of cell nuclei, classification as inflammatory cells; (C2) detection of neutrophils in cytology, hematoxylin and eosin staining.
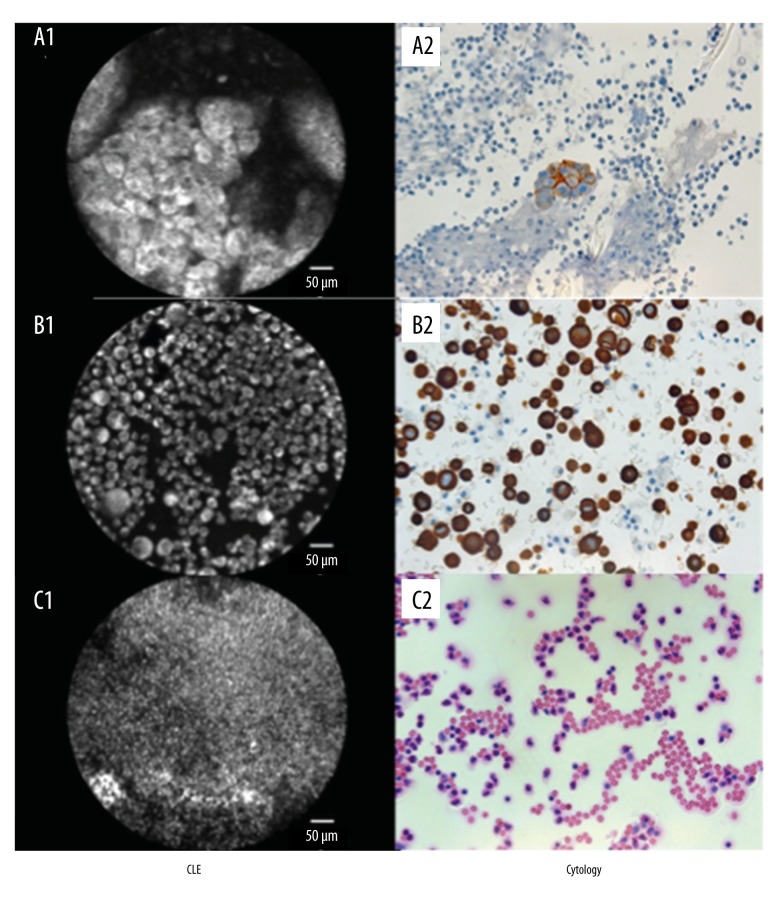
Figure 2.
Comparison between CLE + cresyl violet staining and cytology. (A) Lung adenocarcinoma: (A1) pCLE with detection of malignant cells, glandular growth; (A2) BerEP4-positive cells in cytology matching finally a lung adenocarcinoma. (B) Gastric cancer: (B1) large cells with shifted nucleus-cytoplasma-relation, suspected tumor cells. (B2) CK20 staining, cytological detection of tumor cells, gastrointestinal origin. (C) Empyema. (C1) CLE based detection of inflammatory cells with small cytoplasma seam and strong stained nuclei; (C2) Cytological proof of neutrophils corresponding to empyema diagnosis, hematoxylin and eosin staining.
The CLE mesothelial cells were not as brightly shining as tumor cells. They also showed a balanced nucleus-plasma relation and regular contour of nucleus and cell membrane.
Leukocytes could be identified by their small cell size. Also, they were more brightly shining than all the other cell components (Figures 1, 2; C1 respectively).
In the acriflavine group, 24 patients did not have a tumor diagnosis as underlying disease. In 23 cases a malignancy was known. Despite joint efforts, in 3 cases the diagnosis remained unclear (Supplementary Table 1). Eight pleural effusions were cytologically identified as malignant. Among these 7 were correctly classified by all 4 investigators leading to a sensitivity of 87%. One specimen was misjudged by all 4, whereas another sample was falsely categorized as “malignant” by 1 investigator only (with basic knowledge). This resulted in a mean specificity of 99% (range, 97% to 100%).
Taking also the available clinical data into account, the sensitivity was 70% and the specificity had the same range (97% to 100%) with a mean value of 99%. Upon consideration of the clinical information, 2 more malignant diagnoses were made compared with cytology/cell block analysis alone decreasing the sensitivity. Comparing cytological results taking the additional clinical information into account yielded similar sensitivity (80%) and specificity (100%).
The pathologist classified 49 out of 50 probes correctly. Only in case number 23 the pathologist failed to detect malignancy as he classified the tissue as necrotic. Therefore, the sensitivity for malignant effusions was 87% while the specificity was 100%.
In the cresyl violet group 39 out of 50 patients suffered from a known malignancy while the remaining 11 patients were tumor-free (Supplementary Table 2). Eleven pleural effusions were cytologically classified as malignant. In 1 case, sequential cytological analyses were necessary to reveal the presence of malignant cells in the pleural effusion.
Based on CLE analysis 9 specimens were correctly categorized as malignant (by all 4 investigators), in 2 samples no malignant cells could be found. Without considering the additional clinical information, 2 (by all investigators) to 4 (2 more samples misjudged by 1 and the other time by both investigators with only a short briefing in this technique) samples had to be assessed as “false positive”. Thus, sensitivity scored 81% and specificity 92% (range, 89% to 94%).
When adding additional information obtained clinically, 2 samples that were initially misjudged as false positive could be in fact verified as positive. Intensive inquiries revealed in 1 case a profound suspicion of progressive pleural carcinomatosis on CT scan matching the location of sample acquisition. In the other case, a second pleural puncture shortly after the first was performed revealing malignant cells on cell block analysis. Thus, the sensitivity only reached 73% and the mean specificity scored 97% (range, 94% to 100%) because the addition of clinical data lead to detection of 15 malignant effusions.
A comparison between cytology and cytology supplemented with clinical data revealed a similar sensitivity of 73% and a specificity of 100%. The pathologist rated 8 (out of 11 cytologically proven cases) as malignant pleural effusions correctly (sensitivity 72%, specificity 100%). Three were not detected as malignant (case number 20, 28, and 31).
Discussion
In pulmonology, CLE is a comparatively “young technique”. Despite some promising data on this topic, CLE has not been implemented in routine clinical practice. In 2009, Thiberville et al. illustrated normal proximal and distal bronchial tissue structures [12]. Later on, Fuchs et al. and Sorokina et al. differentiated normal mucosa from tumor-infiltrated mucosa in vivo and ex vivo [17,18]. Also, other disease entities, such as acute lung allograft rejection [19], amiodaron-induced pneumonia [21] or alveolar mircolithiasis [22], showed structural changes detected by CLE. While this method is widely used in gastroenterology, its use is still experimental in the lung without a clearly defined field of application [17]. Nevertheless, these studies raised the expectation that CLE may be a potential novel diagnostic tool in pulmonology, especially in cytology and not only tissue-based diagnosis (in vivo histology).
With our current study, we show that CLE indeed represents a useful, reliable, and feasible tool for diagnostic evaluation of pleural effusions of different origins. Almost all malignant pleural effusions were correctly classified both by experienced investigators and those with only basic knowledge in this technique. Considering the clinical data as well, no sample was misjudged as false positive by the experienced physicians. In line with expectations, the error rate of the investigators with only a short briefing in this technique was higher than in the experienced group. Albeit the latter difference did not reach statistical significance.
In a few cases no consensus regarding the state of malignancy could be reached comparing the results from cytological findings with CLE. In 2 cases, CLE detected malignant cells while cytology remained negative. Taking into account additional clinical data, the CLE results could be confirmed. These data demonstrate that cytology – though being the current gold standard – also comes with a relatively high rate of error. Inversely, 3 times cytology correctly classified pleural effusions as malignant while CLE did not.
Both methods had a similar false negative rate according to clinical data: cytology in 4 and 2 cases, respectively; CLE in 4 and 3 samples leading to an almost equal specificity (100%/100% vs. mean 97%/99%) and sensitivity (73%/80% vs. mean 73%/70%). As a second control, all probes were double-checked by an experienced pathologist. Of all 100 samples only 4 were misjudged indicating that this technique (CLE) should be implemented in routine clinical practice.
Certainly, there were some limitations to the design and the interpretation of this study:
Of course, every new technique needs to prove an advantage over the current gold standard to change practice. However, we view our study as a proof of concept study to provide an answer whether CLE may be able to reliably detect tumorous effusions since it has not been applied in this setting before. It is not intended nor powered by sample size to demonstrate non-inferiority, let alone superiority, to cytology. Studies with larger samples sizes will have to come up with a pertinent answer to this question.
Another limitation of this study was that the samples analyzed by CLE or cytology could not be identical due to the different technical approaches. Due to the different staining methods, we had to use different parts of pleural effusions. For this reason, it is theoretically possible that malignant cells were present and detectable in one sample and not in the other one leading to the possibility of false results.
Another difficulty was the interpretation of the additional clinical data. Postulating malignant cells were missed either by conventional cytology and/or CLE because of CT scan, thoracoscopic findings, or re-puncture results within 30 days after the initial examination is not unproblematic.
Also, our results on CLE cytology primarily need to be compared with the current golden standard which is still cytology/cell block analyses.
A further limitation of the study was the small number of cytologically positive (malignant) pleural effusions despite many probes were from cancer patients. However, this is a known phenomenon and diagnostic caveat described by Sahn et al. [41]. For all these reasons, further studies should be performed to confirm our findings showing CLE has a role in this application field. Finally, costs may be another limitation precluding a wide implementation of this technique. Similar to other new and captivating technologies, CLE is without doubt a quite expensive tool. One probe for up to 20 samples usually costs up to 4000 EUR. As an exception, we were able to obtain a miniprobe from the producer which was not provided for “in vivo” use. With this special probe (costs: 4000 EUR) up to 1 000 000 samples could be analyzed which is cost-cutting (0.4 cent per examination).
To our knowledge this was the first report using CLE for cytological analysis of pleural effusions. Based upon this fact drawing direct comparisons to earlier CLE studies might be difficult. In other specialties, particularly in gastroenterology, CLE is widely used. The method gives support in more precise localization of biopsies, that may lead to improved results and reduced numbers of biopsies needed for definite diagnosis [42]. Similar findings could be obtained in lung cancer demonstrating excellent phenotyping of normal and tumor-infiltrated mucosa; the sensitivity in detecting malignant lesions was found to be 96% and the specificity 87% [17].
Our findings suggest that CLE might be of clinical relevance for the analysis of pleural effusions. Moreover, the results open new avenues for research on CLE-based analysis in order to refine conventional cytology/histology.
In the future, it would be very interesting to compare CLE findings with other innovative approaches of targeted molecular diagnosis such as PCR for tumor-specific markers. Several groups have shown that such techniques may help to refine tumor diagnosis in the setting of pleural effusions [43]. Also, it may be a promising future strategy to test CLE using antigen-specific probes as has been done in gastroenterology already [44]. In addition, the application of CLE in other pulmonary examination methods would be desirable (e.g., EBUS-TBNA) as an encouraging case report has already shown [45].
Conclusions
CLE is versatile, easy to handle and a rapidly applicable method on cytological specimen. At a high percentage, a clear differentiation between malignant and non-malignant pleural effusions could be achieved. In the future, CLE may have a value as a fast and reliable “bedside test”. However, currently, we believe that CLE may lead to complementary information in addition to traditional cytological examination and may help to improve cytological-aided diagnosis of malignant effusions. Finally, a combination of the techniques of CLE and cytology may significantly decrease the number of false negative results. As a consequence, in more cases (and more quickly) the accurate diagnosis of malignancies involving the pleura could be made without major interventions (particularly in lung cancer patients). Further studies should be initiated with the intention of subdivision of cancer types CLE-based. As expected the diagnostic efficiency is examiner-dependent though without statistical significance in our study.
Supplementary Tables
Supplementary Table 1.
Patients characteristics: staining with acriflavin.
| Patient No. | Age years | Sex | Cell block/cytology | Underlying disease | Detection* 1/2/3/4/ |
|---|---|---|---|---|---|
| 1 | 74 | M | Non-malignant | Pneumonia | n/n/n/n |
| 2 | 81 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 3 | 76 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 4 | 76 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 5 | 85 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 6 | 87 | M | Malignant | Adenocarcinoma, TTF1- | m/m/m/m |
| 7 | 88 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 8 | 84 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 9 | 78 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 10 | 36 | F | Non-malignant | Suspected empyema | n/n/n/n |
| 11 | 71 | M | Malignant | Esophageal cancer | m/m/m/m |
| 12 | 72 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 13 | 78 | M | Non-malignant | Haematothorax | n/n/n/n |
| 14 | 73 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 15 | 77 | M | Non-malignant | Esophageal cancer | n/n/n/n |
| 16 | 55 | M | Non-malignant | Squamous cell lung cancer | n/n/n/n |
| 17 | 73 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 18 | 67 | M | Malignant | Rectal cancer | m/m/m/m |
| 19 | 68 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 20 | 38 | F | Non-malignant | LAM | n/n/n/n |
| 21 | 76 | F | Non-malignant | Lung embolism | n/n/n/n |
| 22 | 86 | M | Non-malignant | Not clear | n/n/n/n |
| 23 | 63 | F | Malignant | Pneumonia, cervical ca. | m/m/m/m |
| 24 | 66 | M | Non-malignant | Colon carcinoma | n/n/m/n |
| 25 | 41 | M | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 26 | 41 | F | Non-malignant | Haematothorax | n/n/n/n |
| 27 | 86 | M | Non-malignant | Suspected tbc | n/n/n/n |
| 28 | 44 | F | Malignant | Breast cancer | n/n/n/n |
| 29 | 70 | M | Non-malignant | Chronic pleuritis | n/n/n/n |
| 30 | 23 | F | Non-malignant | Hepatic cirrhosis | n/n/n/n |
| 31 | 23 | F | Non-malignant | Hepatic cirrhosis | n/n/n/n |
| 32 | 64 | M | Non-malignant | Empyema | n/n/n/n |
| 33 | 86 | M | Non-malignant | Not clear | n/n/n/n |
| 34 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 35 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 36 | 75 | M | Non-malignant | Crohn’s disease | n/n/n/n |
| 37 | 41 | M | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 38 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 39 | 24 | F | Nonmalignant | Cervical carcinoma | n/n/n/n |
| 40 | 91 | M | Non-malignant | Suspected lung cancer | n/n/n/n |
| 41 | 22 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 42 | 56 | F | Malignant | Gastric cancer | m/m/m/m |
| 43 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 44 | 68 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 45 | 76 | F | Non-malignant | Ovarian carcinoma | n/n/n/n |
| 46 | 80 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 47 | 86 | M | Non-malignant | Not clear | n/n/n/n |
| 48 | 54 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 49 | 75 | M | Non-malignant | Hepatic cirrhosis | n/n/n/n |
| 50 | 75 | M | Non-malignant | Hepatic cirrhosis | n/n/n/n |
Detection of the four different investigators (first the two principle investigators 1+2, second the two others with basic knowledge 3+4): m – malignant; n – non-malignant.
Supplementary Table 2.
Patients characteristics: staining with cresyl violet.
| Patient No. | Age years | Sex | Cell block/cytology | Underlying disease | Detection* 1/2/3/4/ |
|---|---|---|---|---|---|
| 1 | 52 | F | Malignant | Gastric cancer | m/m/m/m |
| 2 | 66 | M | Non-malignant | Anaplastic thyroid cancer | n/n/n/n |
| 3 | 70 | M | Non-malignant | Hypopharyngeal cancer | n/n/n/n |
| 4 | 52 | F | Malignant | Gastric cancer | m/m/m/m |
| 5 | 66 | M | Non-malignant | Anaplastic thyroid cancer | n/n/n/n |
| 6 | 75 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 7 | 84 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 8 | 49 | F | Non-malignant | Cholangiocellular cancer | n/n/n/n |
| 9 | 80 | M | Malignant | Squamous cell lung cancer | m/m/m/m |
| 10 | 81 | F | Non-malignant | Suspected pancreas cancer | n/n/m/n |
| 11 | 80 | F | Non-malignant | Pneumonia | n/n/n/n |
| 12 | 86 | M | Non-malignant | Fibrothorax | n/n/n/n |
| 13 | 68 | M | Non-malignant | Squamous lung cancer | n/n/n/n |
| 14 | 60 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 15 | 65 | M | Non-m./M | Lung adenocarcinoma | m/m/m/m |
| 16 | 71 | M | Non-malignant | Empyema | n/n/n/n |
| 17 | 67 | M | Non-malignant | Oropharyngeal carcinoma | m/m/m/m |
| 18 | 55 | F | Non-malignant | Small cell lung cancer | n/n/n/n |
| 19 | 71 | F | Non-malignant | Ovarian cancer | n/n/n/n |
| 20 | 51 | F | Non-malignant | Empyema | n/n/n/n |
| 21 | 86 | F | Non-malignant | Small cell lung cancer | n/n/n/n |
| 22 | 54 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 23 | 72 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 24 | 60 | M | Non-malignant | Hypopharyngeal cancer | n/n/n/n |
| 25 | 75 | M | Non-malignant | Squamous cell lung cancer | n/n/m/m |
| 26 | 54 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 27 | 72 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 28 | 78 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 29 | 74 | M | Non-malignant | Pneumonia | n/n/n/n |
| 30 | 86 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 31 | 65 | M | Malignant | Cholangiocellular cancer | n/n/n/n |
| 32 | 74 | M | Non-malignant | Pneumonia | n/n/n/n |
| 33 | 68 | M | Non-malignant | Suspected hypernephroma | n/n/n/n |
| 34 | 41 | M | Non-malignant | Lymphoma | n/n/n/n |
| 35 | 74 | M | Non-malignant | Empyema | n/n/n/n |
| 36 | 60 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 37 | 48 | M | Non-malignant | Oral squamous cell cancer | n/n/n/n |
| 38 | 77 | M | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 39 | 47 | F | Non-malignant | Squamous cell cup | n/n/n/n |
| 40 | 77 | M | Malignant | Squamous cell lung cancer | n/n/n/n |
| 41 | 84 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 42 | 60 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 43 | 90 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 44 | 85 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 45 | 71 | F | Non-malignant | Ovarian cancer | n/n/n/n |
| 46 | 55 | F | Non-malignant | Small cell lung cancer | n/n/n/n |
| 47 | 37 | M | Non-malignant | Acute pancreatitis | n/n/n/n |
| 48 | 68 | F | Non-malignant | Esophageal cancer | n/n/n/n |
| 49 | 82 | M | Non-malignant | Aspiration pneumonia | n/n/n/n |
| 50 | 67 | M | Non-malignant | Esophageal cancer | n/n/n/n |
Detection of the four different investigators (first the two principle investigators 1+2, second the two others with basic knowledge 3+4): m – malignant; n – non-malignant.
Acknowledgment
We want to thank the bronchoscopic assistance personnel (Mrs. Slavica Alickovic and Mr. Tomislav Vilusic) for their active support in this study.
Footnotes
Source of support: Departmental sources
References
- 1.Peter S, Council L, Bang JY, et al. Poor agreement between endoscopists and gastrointestinal pathologists for the interpretation of probe-based confocal laser endomicroscopy findings. World J Gastroenterol. 2014;20(47):17993–8000. doi: 10.3748/wjg.v20.i47.17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rey JW, Kiesslich R, Hoffman A. New aspects of modern endoscopy. World J Gastrointest Endosc. 2014;6(8):334–44. doi: 10.4253/wjge.v6.i8.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127(3):706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 4.De Palma GD, Wallace MB, Giovannini M. Confocal laser endomicroscopy. Gastroenterol Res Pract. 2012;2012:216209. doi: 10.1155/2012/216209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tontini GE, Mudter J, Vieth M, et al. Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: A pilot study. Endoscopy. 2015;47(5):437–43. doi: 10.1055/s-0034-1391226. [DOI] [PubMed] [Google Scholar]
- 6.Leggett CL, Gorospe EC. Application of confocal laser endomicroscopy in the diagnosis and management of Barrett’s esophagus. Ann Gastroenterol. 2014;27(3):193–99. [PMC free article] [PubMed] [Google Scholar]
- 7.Kahaleh M, Turner BG, Bezak K, et al. Probe-based confocal laser endomicroscopy in the pancreatic duct provides direct visualization of ductal structures and aids in clinical management. Dig Liver Dis. 2015;47(3):202–4. doi: 10.1016/j.dld.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Caillol F, Bories E, Autret A, et al. Evaluation of pCLE in the bile duct: final results of EMID study: pCLE: Impact in the management of bile duct strictures. Surg Endosc. 2015;29(9):2661–68. doi: 10.1007/s00464-014-3986-8. [DOI] [PubMed] [Google Scholar]
- 9.Lim LG, Yeoh KG, Srivastava S, et al. Comparison of probe-based confocal endomicroscopy with virtual chromoendoscopy and white-light endoscopy for diagnosis of gastric intestinal metaplasia. Surg Endosc. 2013;27(12):4649–55. doi: 10.1007/s00464-013-3098-x. [DOI] [PubMed] [Google Scholar]
- 10.Gong S, Ge ZZ, Xue HB. In vivo diagnosis of gastric signet-ring cell carcinoma by confocal laser endomicroscopy. J Dig Dis. 2014;15(1):46–49. doi: 10.1111/1751-2980.12093. [DOI] [PubMed] [Google Scholar]
- 11.Nakai Y, Isayama H, Shinoura S, et al. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases. Dig Endosc. 2014;26(Suppl 1):86–94. doi: 10.1111/den.12152. [DOI] [PubMed] [Google Scholar]
- 12.Thiberville L, Salaun M, Lachkar S, et al. Confocal fluorescence endomicroscopy of the human airways. Proc Am Thorac Soc. 2009;6(5):444–49. doi: 10.1513/pats.200902-009AW. [DOI] [PubMed] [Google Scholar]
- 13.Thiberville L, Salaun M. Bronchoscopic advances: On the way to the cells. Respiration. 2010;79(6):441–49. doi: 10.1159/000313495. [DOI] [PubMed] [Google Scholar]
- 14.Salaun M, Bourg-Heckly G, Thiberville L. [Confocal endomicroscopy of the lung: from the bronchus to the alveolus]. Rev Mal Respir. 2010;27(6):579–88. doi: 10.1016/j.rmr.2009.12.009. [in French] [DOI] [PubMed] [Google Scholar]
- 15.Fuchs FS, Zirlik S, Hildner K, et al. Fluorescein-aided confocal laser endomicroscopy of the lung. Respiration. 2011;81(1):32–38. doi: 10.1159/000320365. [DOI] [PubMed] [Google Scholar]
- 16.Wijmans L, d’Hooghe JN, Bonta PI, Annema JT. Optical coherence tomography and confocal laser endomicroscopy in pulmonary diseases. Curr Opin Pulm Med. 2017;23(3):275–83. doi: 10.1097/MCP.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs FS, Zirlik S, Hildner K, et al. Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur Respir J. 2013;41(6):1401–8. doi: 10.1183/09031936.00062512. [DOI] [PubMed] [Google Scholar]
- 18.Sorokina A, Danilevskaya O, Averyanov A, et al. Comparative study of ex vivo probe-based confocal laser endomicroscopy and light microscopy in lung cancer diagnostics. Respirology. 2014;19(6):907–13. doi: 10.1111/resp.12326. [DOI] [PubMed] [Google Scholar]
- 19.Yserbyt J, Dooms C, Decramer M, Verleden GM. Acute lung allograft rejection: Diagnostic role of probe-based confocal laser endomicroscopy of the respiratory tract. J Heart Lung Transplant. 2014;33(5):492–98. doi: 10.1016/j.healun.2014.01.857. [DOI] [PubMed] [Google Scholar]
- 20.Shafiek H, Gomez C, Pierola J, et al. Probe-based confocal laser endomicroscopy imaging of endobronchial hamartomas. Respiration. 2014;88(6):484–86. doi: 10.1159/000368085. [DOI] [PubMed] [Google Scholar]
- 21.Salaun M, Roussel F, Bourg-Heckly G, et al. In vivo probe-based confocal laser endomicroscopy in amiodarone-related pneumonia. Eur Respir J. 2013;42(6):1646–58. doi: 10.1183/09031936.00191911. [DOI] [PubMed] [Google Scholar]
- 22.Yserbyt J, Alame T, Dooms C, Ninane V. Pulmonary alveolar microlithiasis and probe-based confocal laser endomicroscopy. J Bronchology Interv Pulmonol. 2013;20(2):159–63. doi: 10.1097/LBR.0b013e31828abc03. [DOI] [PubMed] [Google Scholar]
- 23.Perez JR, Ybarra N, Chagnon F, et al. Image-guided fluorescence endomicroscopy: From macro- to micro-imaging of radiation-induced pulmonary fibrosis. Sci Rep. 2017;7(1):17829. doi: 10.1038/s41598-017-18070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan T, Thiberville L, Hermant C, et al. Assessing the feasibility of confocal laser endomicroscopy in solitary pulmonary nodules for different part of the lungs, using either 0.6 or 1.4 mm probes. PLoS One. 2017;12(12):e0189846. doi: 10.1371/journal.pone.0189846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Z, Zhong C, Li S, et al. Needle-based confocal laser endomicroscopy in the diagnosis of peripheral pulmonary nodule: A preliminary report. J Thorac Dis. 2017;9(8):2608–12. doi: 10.21037/jtd.2017.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guisier F, Bohn P, Patout M, et al. In- and ex-vivo molecular imaging of apoptosis to assess sensitivity of non-small cell lung cancer to EGFR inhibitors using probe-based confocal laser endomicroscopy. PLoS One. 2017;12(7):e0180576. doi: 10.1371/journal.pone.0180576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas AR, Sterman DH. Advances in pleural disease management including updated procedural coding. Chest. 2014;146(2):508–13. doi: 10.1378/chest.13-2250. [DOI] [PubMed] [Google Scholar]
- 28.Sahn SA. Getting the most from pleural fluid analysis. Respirology. 2012;17(2):270–77. doi: 10.1111/j.1440-1843.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 29.Xia H, Wang XJ, Zhou Q, et al. Efficacy and safety of talc pleurodesis for malignant pleural effusion: A meta-analysis. PLoS One. 2014;9(1):e87060. doi: 10.1371/journal.pone.0087060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopcinovic LM, Culej J. Pleural, peritoneal and pericardial effusions – a biochemical approach. Biochem Med (Zagreb) 2014;24(1):123–37. doi: 10.11613/BM.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Na MJ. Diagnostic tools of pleural effusion. Tuberc Respir Dis (Seoul) 2014;76(5):199–210. doi: 10.4046/trd.2014.76.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam HS. Malignant pleural effusion: Medical approaches for diagnosis and management. Tuberc Respir Dis (Seoul) 2014;76(5):211–17. doi: 10.4046/trd.2014.76.5.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froudarakis ME. Pleural effusion in lung cancer: More questions than answers. Respiration. 2012;83(5):367–76. doi: 10.1159/000338169. [DOI] [PubMed] [Google Scholar]
- 34.Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Malignant pleural effusion and algorithm management. J Thorac Dis. 2013;5(Suppl 4):S413–19. doi: 10.3978/j.issn.2072-1439.2013.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest. 1975;67(5):536–39. doi: 10.1378/chest.67.5.536. [DOI] [PubMed] [Google Scholar]
- 36.Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii4–17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 37.Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7(6):665–68. [PubMed] [Google Scholar]
- 38.Shivakumarswamy U, Arakeri SU, Karigowdar MH, Yelikar B. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol. 2012;29(1):11–15. doi: 10.4103/0970-9371.93210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhanvadia VM, Santwani PM, Vachhani JH. Analysis of diagnostic value of cytological smear method versus cell block method in body fluid cytology: Study of 150 cases. Ethiop J Health Sci. 2014;24(2):125–31. doi: 10.4314/ejhs.v24i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekker A, Bupp PA. Cytology of serous effusions. An investigation into the usefulness of cell blocks versus smears. Am J Clin Pathol. 1978;70(6):855–60. doi: 10.1093/ajcp/70.6.855. [DOI] [PubMed] [Google Scholar]
- 41.Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J. 1997;10(8):1907–13. doi: 10.1183/09031936.97.10081907. [DOI] [PubMed] [Google Scholar]
- 42.Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132(3):874–82. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 43.Zhong J, Li X, Bai H, et al. Malignant pleural effusion cell blocks are substitutes for tissue in EML4-ALK rearrangement detection in patients with advanced non-small-cell lung cancer. Cytopathology. 2016;27(6):433–43. doi: 10.1111/cyt.12322. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Zuo XL, Li CQ, et al. In vivo molecular imaging of gastric cancer by targeting MG7 antigen with confocal laser endomicroscopy. Endoscopy. 2013;45(2):79–85. doi: 10.1055/s-0032-1325762. [DOI] [PubMed] [Google Scholar]
- 45.Wijmans L, de Bruin DM, Meijer Sl, Annema JT. Real-time optical biopsy of lung cancer. Am J Respir Crit Care Med. 2016;194(8):e10–e11. doi: 10.1164/rccm.201603-0657IM. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Patients characteristics: staining with acriflavin.
| Patient No. | Age years | Sex | Cell block/cytology | Underlying disease | Detection* 1/2/3/4/ |
|---|---|---|---|---|---|
| 1 | 74 | M | Non-malignant | Pneumonia | n/n/n/n |
| 2 | 81 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 3 | 76 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 4 | 76 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 5 | 85 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 6 | 87 | M | Malignant | Adenocarcinoma, TTF1- | m/m/m/m |
| 7 | 88 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 8 | 84 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 9 | 78 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 10 | 36 | F | Non-malignant | Suspected empyema | n/n/n/n |
| 11 | 71 | M | Malignant | Esophageal cancer | m/m/m/m |
| 12 | 72 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 13 | 78 | M | Non-malignant | Haematothorax | n/n/n/n |
| 14 | 73 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 15 | 77 | M | Non-malignant | Esophageal cancer | n/n/n/n |
| 16 | 55 | M | Non-malignant | Squamous cell lung cancer | n/n/n/n |
| 17 | 73 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 18 | 67 | M | Malignant | Rectal cancer | m/m/m/m |
| 19 | 68 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 20 | 38 | F | Non-malignant | LAM | n/n/n/n |
| 21 | 76 | F | Non-malignant | Lung embolism | n/n/n/n |
| 22 | 86 | M | Non-malignant | Not clear | n/n/n/n |
| 23 | 63 | F | Malignant | Pneumonia, cervical ca. | m/m/m/m |
| 24 | 66 | M | Non-malignant | Colon carcinoma | n/n/m/n |
| 25 | 41 | M | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 26 | 41 | F | Non-malignant | Haematothorax | n/n/n/n |
| 27 | 86 | M | Non-malignant | Suspected tbc | n/n/n/n |
| 28 | 44 | F | Malignant | Breast cancer | n/n/n/n |
| 29 | 70 | M | Non-malignant | Chronic pleuritis | n/n/n/n |
| 30 | 23 | F | Non-malignant | Hepatic cirrhosis | n/n/n/n |
| 31 | 23 | F | Non-malignant | Hepatic cirrhosis | n/n/n/n |
| 32 | 64 | M | Non-malignant | Empyema | n/n/n/n |
| 33 | 86 | M | Non-malignant | Not clear | n/n/n/n |
| 34 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 35 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 36 | 75 | M | Non-malignant | Crohn’s disease | n/n/n/n |
| 37 | 41 | M | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 38 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 39 | 24 | F | Nonmalignant | Cervical carcinoma | n/n/n/n |
| 40 | 91 | M | Non-malignant | Suspected lung cancer | n/n/n/n |
| 41 | 22 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 42 | 56 | F | Malignant | Gastric cancer | m/m/m/m |
| 43 | 24 | F | Non-malignant | Cervical carcinoma | n/n/n/n |
| 44 | 68 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 45 | 76 | F | Non-malignant | Ovarian carcinoma | n/n/n/n |
| 46 | 80 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 47 | 86 | M | Non-malignant | Not clear | n/n/n/n |
| 48 | 54 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 49 | 75 | M | Non-malignant | Hepatic cirrhosis | n/n/n/n |
| 50 | 75 | M | Non-malignant | Hepatic cirrhosis | n/n/n/n |
Detection of the four different investigators (first the two principle investigators 1+2, second the two others with basic knowledge 3+4): m – malignant; n – non-malignant.
Supplementary Table 2.
Patients characteristics: staining with cresyl violet.
| Patient No. | Age years | Sex | Cell block/cytology | Underlying disease | Detection* 1/2/3/4/ |
|---|---|---|---|---|---|
| 1 | 52 | F | Malignant | Gastric cancer | m/m/m/m |
| 2 | 66 | M | Non-malignant | Anaplastic thyroid cancer | n/n/n/n |
| 3 | 70 | M | Non-malignant | Hypopharyngeal cancer | n/n/n/n |
| 4 | 52 | F | Malignant | Gastric cancer | m/m/m/m |
| 5 | 66 | M | Non-malignant | Anaplastic thyroid cancer | n/n/n/n |
| 6 | 75 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 7 | 84 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 8 | 49 | F | Non-malignant | Cholangiocellular cancer | n/n/n/n |
| 9 | 80 | M | Malignant | Squamous cell lung cancer | m/m/m/m |
| 10 | 81 | F | Non-malignant | Suspected pancreas cancer | n/n/m/n |
| 11 | 80 | F | Non-malignant | Pneumonia | n/n/n/n |
| 12 | 86 | M | Non-malignant | Fibrothorax | n/n/n/n |
| 13 | 68 | M | Non-malignant | Squamous lung cancer | n/n/n/n |
| 14 | 60 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 15 | 65 | M | Non-m./M | Lung adenocarcinoma | m/m/m/m |
| 16 | 71 | M | Non-malignant | Empyema | n/n/n/n |
| 17 | 67 | M | Non-malignant | Oropharyngeal carcinoma | m/m/m/m |
| 18 | 55 | F | Non-malignant | Small cell lung cancer | n/n/n/n |
| 19 | 71 | F | Non-malignant | Ovarian cancer | n/n/n/n |
| 20 | 51 | F | Non-malignant | Empyema | n/n/n/n |
| 21 | 86 | F | Non-malignant | Small cell lung cancer | n/n/n/n |
| 22 | 54 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 23 | 72 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 24 | 60 | M | Non-malignant | Hypopharyngeal cancer | n/n/n/n |
| 25 | 75 | M | Non-malignant | Squamous cell lung cancer | n/n/m/m |
| 26 | 54 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 27 | 72 | M | Non-malignant | Small cell lung cancer | n/n/n/n |
| 28 | 78 | M | Malignant | Lung adenocarcinoma | m/m/m/m |
| 29 | 74 | M | Non-malignant | Pneumonia | n/n/n/n |
| 30 | 86 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 31 | 65 | M | Malignant | Cholangiocellular cancer | n/n/n/n |
| 32 | 74 | M | Non-malignant | Pneumonia | n/n/n/n |
| 33 | 68 | M | Non-malignant | Suspected hypernephroma | n/n/n/n |
| 34 | 41 | M | Non-malignant | Lymphoma | n/n/n/n |
| 35 | 74 | M | Non-malignant | Empyema | n/n/n/n |
| 36 | 60 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 37 | 48 | M | Non-malignant | Oral squamous cell cancer | n/n/n/n |
| 38 | 77 | M | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 39 | 47 | F | Non-malignant | Squamous cell cup | n/n/n/n |
| 40 | 77 | M | Malignant | Squamous cell lung cancer | n/n/n/n |
| 41 | 84 | F | Non-malignant | Lung adenocarcinoma | n/n/n/n |
| 42 | 60 | F | Malignant | Lung adenocarcinoma | m/m/m/m |
| 43 | 90 | M | Non-malignant | Cardiac decompensation | n/n/n/n |
| 44 | 85 | F | Non-malignant | Cardiac decompensation | n/n/n/n |
| 45 | 71 | F | Non-malignant | Ovarian cancer | n/n/n/n |
| 46 | 55 | F | Non-malignant | Small cell lung cancer | n/n/n/n |
| 47 | 37 | M | Non-malignant | Acute pancreatitis | n/n/n/n |
| 48 | 68 | F | Non-malignant | Esophageal cancer | n/n/n/n |
| 49 | 82 | M | Non-malignant | Aspiration pneumonia | n/n/n/n |
| 50 | 67 | M | Non-malignant | Esophageal cancer | n/n/n/n |
Detection of the four different investigators (first the two principle investigators 1+2, second the two others with basic knowledge 3+4): m – malignant; n – non-malignant.