Abstract
This review article aims to present the epidemiology and associated risk factors of allergic rhinitis (AR) in Asia. AR-related literature published on Asia was systematically reviewed and the associated risk factors were investigated.
The prevalence of AR in Asia varied considerably depending on the geographical location, study design and population involved. Several risk factors were observed to have strong association with disease presentation across multiple studies. Among these, family income, family size, daily personal computer usage time, personal and parental education attainment, and stress level have shown some level of biological gradient influence when multiple risk levels were analyzed. This suggests that AR manifestation and presentation possibly might be strongly affected by various personal and family factors. These findings are beneficial as they may provide insights into modifiable factors that may influence AR presentation. In addition, these results indicate that strategies to reduce personal and family-related risk factors have to be developed in order to alleviate the odds of AR expression.
Electronic supplementary material
The online version of this article (10.1186/s40413-018-0198-z) contains supplementary material, which is available to authorized users.
Keywords: Allergic rhinitis, Risk factor, Asia, Systematic review
Background
Allergic rhinitis epidemiology and symptoms
According to the Phase III International Study of Asthma and Allergies in Childhood (ISAAC), the prevalence of AR varied between 0.8 to 14.9% in 6-7 years old and 1.4 to 39.7% in 13-14 years old worldwide [1]. In Asia, this disease affects a large population, ranging from 27% in South Korea [2] to 32% in the United Arab Emirates [3].
It is a prevalent yet underappreciated atopic disorder which is commonly characterized by the presence of at least one of the following clinical symptoms: persistent nasal obstruction and mucous discharge, sneezing, and itching [4].
Although AR is commonly regarded as a mild and seasonal nuisance, it can trigger persistent mucosal inflammation which may synergize with other infective inflammation, resulting in severe outcomes including hospitalization [5]. As such, the odds of hospital admission for children with the allergic disease have been reported to increase by 19 times with the co-infection of rhinoviral diseases, allergic sensitization, and allergen exposure [6].
Risk factors affecting the presentation of allergic rhinitis
Apart from the demographic factors, smoking and drinking habits, pet adoption, education attainment, and family history were the risk factors of AR, commonly studied in Asian countries [7–11]. Conversely, Western countries focus more on the effects of pollens, drugs, pets, and family history on the presentation of AR [12–14]. The differences between the risk factors analyzed could be culturally induced or due to the climatic differences between Asian and Western countries. However, it was observed that pet adoption and family history are the common risk factors studied in both regions, suggesting their pervasiveness in inducing AR manifestation worldwide.
Disease diagnosis
While AR is influenced by genetic predisposition, the symptom presentation also depends on environmental exposures [15]. In addition, the disease can co-present with other diseases, such as asthma and other infectious diseases, which could further complicate the disease diagnosis. A robust association of rhinitis was found among individuals with allergic and non-allergic asthma [16]. Among patients with persistent and severe rhinitis, asthma was found prevailing [17].
Moreover, patients can experience adverse effects on social life, productivity at work and performance in school, especially for those who suffer from a more severe form of AR [16]. The use of suboptimum pharmacotherapy and antihistamines with sedative effects can further exacerbate the situation. This incurs a financial burden from both direct and indirect costs which adversely affects society [18]. Therefore, a prompt and accurate diagnosis, followed by appropriate disease management and awareness of the exacerbation risk factors, would be crucial to ease this burden.
Diagnosis of the disease is usually based on medical history of the patient in addition to skin prick test or blood test. However, misinterpretation can occasionally occur and this delays the golden treatment period which can result in other unexpected consequences, such as paying unnecessary medical expenditure and missing work [18].
The aim of the study
This review article aims to study the epidemiology of AR in Asia and identify significant modifiable risk factors associated with disease presentation. Several criteria have been employed to establish association between triggering factors and disease manifestation.
Methods
Search strategy and selection criteria
The epidemiology and potential factors associated with AR manifestation were obtained from the Web of Science using the search terms of ‘rhinitis’, ‘risk’ and Asian countries. The list of Asian countries and independent territories used in the search is listed in Additional file 1. ‘Rhinitis’ is used as it represents a general form of the disease which serves to capture as many risk factors, including both modifiable and non-modifiable, as possible. As Asian and Western countries are known to have different cultural and social backgrounds, our study only evaluated articles published on Asia and this articles serves to provide a detailed list of triggering risk factors associated with AR in Asia.
Using these search terms, 56 articles were first identified. The articles were carefully reviewed and those with unclear study design or disease definition or which were conducted in a narrow pool of individuals were excluded. Apart from these 14 articles, additional 6 cross-referencing articles were also included. These 20 articles, published between 1994 and 2017, were evaluated closely for their study design, disease prevalence, disease definition, and the AR risk factor analyzed.
Establishing the association link
The factors investigated in the 20 articles were further classified either as a potential risk factor or a co-morbidity. The association between potential modifiable risk factors and AR manifestation were evaluated using several important criteria established in literature. These criteria include the strength of association, consistency of the observed association, specificity, biologic gradient, biologic plausibility, coherence, analogy, and temporality. In addition, meta-analysis was conducted using the software/program-Stata/SE 11.2 with random effects model to evaluate the influence of modifiable risk factors with replicative results reported in at least three independent AR publications.
Results and discussion
AR epidemiology in Asia
Based on the methodology described, different articles published in Asia were reviewed. The reviewed articles have variable study design, disease definition and adopt different analysis parameters as shown in Table 1. The population size also varies from study to study, ranging from 200 in Kidoni et al. [19] to 30,000 in An et al. [2]
Table 1.
Summaries of allergic rhinitis-specific articles published in year 1994-2017 in Asia
| Country, location | No. of sample | Study design | Prevalence | Definition of the disease stage | Parameters analyzed | Reference, date |
|---|---|---|---|---|---|---|
| Singapore | 2868 adults aged 20-74 years | Cross-sectional population-based study | 4.5% | Allergic rhinitis: self-reported presence, in the previous year, of usual nasal blockage and discharge apart from colds or the flu, provoked by allergens, with or without conjunctivitis. | Significant parameters ➢ Age ➢ Fume exposure ➢ Housing estate ➢ Insect ➢ Occupational exposure ➢ Race ➢ Smoking Insignificant parameters ➢ Air pollution ➢ Carpet ➢ Gender ➢ Pet |
Ng & Tan, 1994 [36] |
| Korea | 10,054 residents | Cross-sectional interview based study with Physical examination | 1.14% | Perennial allergic rhinitis in this study was defined as the presence of typical nasal symptoms including watery rhinorrhea, sneezing, itching and nasal obstruction during a period greater than 12 months, positive history of known allergen or triggering factors, and the physical finding of pale nasal mucosa on endoscopic examination. | Significant parameters ➢ Educational attainment ➢ Residency Insignificant parameters ➢ Marital status ➢ Occupational exposure ➢ Smoking ➢ Social class |
Min et al., 1997 [20] |
| Thailand, Bangkok | 3124 residents | Cross-sectional questionnaire based study | 13.15% (95% CI = 13.13-13.17) with Chronic rhinitis (CR) | Rhinitis is defined as inflammation of the lining of the nose, characterized by one or more of the following symptoms, i.e. itching, sneezing, rhinorrhea and nasal obstruction (International Rhinitis Management Working Group, 1994). CR is diagnosed when one frequently has rhinitis symptoms without fever for a period of more than one year. | Significant parameters ➢ Associated allergic diseases ➢ Drinking ➢ Family history of atopy ➢ Household income ➢ Smoking Insignificant parameter ➢ Gender |
Bunnag et al., 2000 [37] |
| Israel | 10,057 schoolchildren, aged 13-14 years | Cross-sectional questionnaire based study | 41.6% with Ever AR, 9.4% with Current AR |
Ever AR: Children who reported having rhinitis and sneezing without flu ever Current AR: Answer ‘Yes’ to the question, “Do you have allergic rhinitis?” |
Significant parameters ➢ Asthma ➢ Family history of allergic diseases ➢ Gender ➢ Race ➢ Residency |
Graif et al., 2004 [38] |
| Singapore | 202 patients aged 2-14 years | Retrospective analysis with medical records from allergic rhinitis patients undergo SPT test in KK Children’s hospital (Jul 2001 to June 2002) | 33% (AR + asthma), 13% (AR + AD) & 7% (AR + asthma + AD) − 44% hospitalization rate |
Confirmation from a specialist in Pediatric Otolaryngology | Significant parameter ➢ Mold |
Kidoni et al., 2004 [19]. |
| Laos, Vientiane | 536 (included students aged 6-7 years and 13-14 years) | Cross-sectional questionnaire based study from Dec 2006 to Feb 2007 with stool examination | 21.0% (6-7 years) & 22.3% (13-14 years) | Had a problem with sneezing, runny, or blocked nose when did not have cold or the flu in the past 12 months (ISAAC definition) | Significant parameters ➢ Household income ➢ Parasitic infection ➢ Past respiratory infection Insignificant parameters ➢ Age ➢ Air conditioning ➢ Birth order ➢ Family history of allergic diseases ➢ Food ➢ Gender ➢ Parity ➢ Past measles infection ➢ Pet ➢ Sharing bed ➢ Smoking ➢ Time on road |
Phathammavong et al., 2008 [9] |
| Singapore | 6794 children attending 120 randomly selected child care centres | Cross-sectional questionnaire based study | 25.6 (Rhinitis) | N.A. | Significant parameter ➢ Smoking |
Zuraimi et al., 2008 [39] |
| Taiwan, Taipei | 1368 elementary school children | Cross-sectional questionnaire based study with multi-stage clustered-stratified random method, physical examination | 50.1% | The presence of typical nasal symptoms including watery rhinorrhea, sneezing, and nasal obstruction of more than 12 months’ duration, positive history of known allergen or triggering factors, and pale nasal mucosa. | Significant parameters ➢ Air pollution ➢ Carpet ➢ Gender ➢ Parity Insignificant parameters ➢ Age of gestation ➢ Gestational complication ➢ Maternal education ➢ Mold ➢ Pet ➢ Smoking |
Hsu et al., 2009 [10] |
| United Arab Emirates, Al-Ain City | 7550 residents ≥13 years | Cross-sectional questionnaire based study | 32% | The definition of AR used in this study was having had AR symptoms of (nasal blockage, rhinorrhoea, sneezing and irritation), in the past 12 months. | Significant parameters ➢ Age ➢ Education attainment ➢ Family history of allergic diseases ➢ Gender ➢ Nationality |
Alsowaidi et al., 2010 [3] |
| Singapore | 2994 children living in homes without any indoor risk factors | Cross-sectional questionnaire based study | 24% (Rhinitis) | N.A. | Significant parameter ➢ Traffic Insignificant parameter ➢ Air conditioning |
Zuraimi et al., 2011 [21] |
| China, Guangzhou City | 9899 citizens | Cross-sectional questionnaire based study with stratified multistage cluster sampling method | 6.24% | According to the diagnostic criteria of AR in the ARIA 2001 Guideline, the ENT specialists verified the screening questionnaires and made the diagnosis based on the typical AR symptoms within the last 12 months. Intermittent AR was determined when the symptoms occur, 4 days/week or, 4 consecutive weeks/year; while persistent AR was determined when symptoms last 4 days/week or 4 consecutive weeks/year. |
Significant parameters ➢ Computer usage ➢ Family history of allergic diseases ➢ Home renovation ➢ Pet ➢ Residency ➢ Smoking Insignificant parameters ➢ Age ➢ Breastfeeding ➢ Car ownership ➢ Hair coloring ➢ Household income |
Li et al., 2014 [7] |
| Korea | 31,217 subjects aged 6-97 years | Cross-sectional study, data from Korea National Health and Nutrition Examination Survey | 27% | N.A. | Significant parameters ➢ Marital status ➢ Occupational exposure ➢ Sleep time ➢ Stress level Insignificant parameters ➢ BMI ➢ Drinking ➢ Education attainment ➢ Family size ➢ Household income ➢ Residency ➢ Smoking |
An et al., 2015 [2] |
| China | 20,803 elementary school students | Cross-sectional questionnaire based study | 9.8% | AR: yes for “Has your child had allergic rhinitis in the past 12 months?” | Significant parameters ➢ Age ➢ Age of gestation ➢ Breastfeeding ➢ Family size ➢ Gender ➢ Household income ➢ Housing estate ➢ Maternal education ➢ Mode of delivery ➢ Maternal pre- or postnatal depression ➢ Paternal education Insignificant parameters ➢ Drinking ➢ Smoking |
Li et al., 2015 [22] |
| Malaysia | 695 Malaysia office works aged 18-60 years | Cross-sectional questionnaire based study, SPT test, building inspection | 53% with current rhinitis | Doctor diagnosis | Significant parameters ➢ Age ➢ House dust mite Insignificant parameters ➢ Gender ➢ Pet ➢ Smoking |
Lim et al., 2015 [11] |
| China, Wuhan | 3327 | Cross-sectional questionnaire based study, physical examination | 17.67% | Doctor diagnosis | Significant parameter ➢ Gender Insignificant parameter ➢ BMI |
Lei, Yang & Zhen, 2016 [40] |
| Malaysia, Johor Bahru | 462 students from 8 random schools | Cross-sectional questionnaire based study, building inspections | 18.8% for students from junior high schools | N.A. | Significant parameter ➢ Fungi |
Norbäck et al., 2016 (1) [41] |
| Malaysia, Johor Bahru | 462 students from 8 random schools | Cross-sectional questionnaire based study, building inspections | 18.8% for students from junior high schools | N.A. | Significant parameters ➢ Atopy ➢ Family history of allergic disease ➢ Fungi ➢ House dust mite ➢ Race Insignificant parameters ➢ Gender ➢ Smoking |
Norbäck et al., 2016 (2) [35] |
| China, Shanghai | 13,335 children, aged 4-6 years | Cross-sectional questionnaire based study | 12.6% | Answer yes for “Has your child ever had a problem with sneezing, or a runny, or blocked nose when he/she did not have a cold or the flu in the past years” | Significant parameters ➢ Breastfeeding ➢ Gruel introduction |
Huang et al., 2017 [34] |
| Taiwan | 1497 newborns | Birth cohort follow-up, questionnaire survey, physician-verified and serological testing | Non-atopic parents & one atopic parent & atopic parents : 30.8% vs 39.9% vs 54.7% |
Doctor diagnosis | Significant parameters ➢ Age of gestation ➢ Gender ➢ Residency |
Lee et al., 2017 [42] |
| Kuwait | 1154 students, aged 18-26 years attending Kuwait University | Cross-sectional questionnaire based study | 20.4% (95% Cl- 18.1-22.9) | Current rhinitis: “ever doctor-diagnosed rhinitis” plus “having problems with sneezing, runny, or blocked nose in the absence of cold or flu in the last 12 months” | Significant parameters ➢ Age ➢ Family history of allergic diseases ➢ Pet Insignificant parameters ➢ Birth order ➢ Gender ➢ Mode of delivery ➢ Smoking |
Ziyab, 2017 [8] |
Though similar parameters were used to study the epidemiology of AR, a larger population group will help to further establish the prevalence of the disease as it better represents the targeted population. In addition, apart from the country of study, the disease prevalence differs depending on the disease definition and the study population. In the study conducted by Min et al. [20], AR prevalence is 1.14% among Korean residents; while in a retrospective study published by Alsowaidi et al., 2010 [3], 32% of United Arab Emirates residents are AR patients.
Risk factors and co-morbidities of AR
Apart from the general demographic factors, many modifiable risk factors for allergic diseases, such as smoking and drinking habits were investigated as summarised in Table 1. Furthermore, cultural- or socioeconomic-related factors specific to an individual country have been explored in some studies to identify their association with AR presentation. For instance, heavy traffic and individual stress level are two factors investigated in a Singapore [21] and Korea [2] study, respectively. These factors were identified worrying elements in the respective countries, thus finding their association with AR presentation is crucial.
We further classified these factors into a potential risk factors or co-morbidities category based on the following definitions. A typical risk factor is a demographical, physical, sociological or environmental component which potentially increases the risk of presenting a disease or is protective against the expression of an illness. On the other hand, if AR manifestation is linked to another disease occurrence, it will be known as a co-morbid of AR. As listed in Table 2, most of the factors analysed are in the risk factor category. However, diseases such as asthma are co-morbidities which can possibly induce AR expression as shown in Table 3. In this article, only the modifiable risk factors were evaluated for their relationship with AR manifestation.
Table 2.
The list of risk factors analyzed in the literature reviewed
| No. | Risk Factors |
|---|---|
| 1 | Age |
| 2 | Age of gestation |
| 3 | Air conditioning |
| 4 | Air pollution |
| 5 | Alcohol consumption (self/parent) |
| 6 | Birth order |
| 7 | BMI |
| 8 | Breastfeeding |
| 9 | Car ownership |
| 10 | Carpet |
| 11 | Computer usage |
| 12 | Drinking (self/parent) |
| 13 | Education attainment |
| 14 | Family history of allergic diseases |
| 15 | Family history of atopy |
| 16 | Family size |
| 17 | Food |
| 18 | Fume exposure |
| 19 | Fungi |
| 20 | Gender |
| 21 | Gestational complication |
| 22 | Gruel introduction period |
| 23 | Hair coloring |
| 24 | Home renovation |
| 25 | House dust mite |
| 26 | Household income |
| 27 | Housing estate |
| 28 | Insect |
| 29 | Marital status |
| 30 | Maternal education |
| 31 | Maternal pre- or postnatal depression |
| 32 | Mode of delivery |
| 33 | Mold |
| 34 | Nationality |
| 35 | Occupational exposure |
| 36 | Parasitic infection |
| 37 | Parity |
| 38 | Past measles infection |
| 39 | Past respiratory infection |
| 40 | Paternal education |
| 41 | Pet |
| 42 | Race |
| 43 | Residency |
| 44 | Sharing bed |
| 45 | Sleep time |
| 46 | Smoking (self/parent) |
| 47 | Social class |
| 48 | Stress level |
| 49 | Time on road |
| 50 | Traffic |
Table 3.
The list of co-morbidities analyzed in the literature reviewed
| No. | Co-morbidities |
|---|---|
| 1 | Atopy |
| 2 | Associated allergy |
| 3 | Current asthma |
Demographical factors affecting the AR presentation
Multiple papers have suggested the importance of age, gender, race, and nationality in affecting AR presentation (Table 4). The association of race and nationality on the disease expression could signify the difference in social and cultural backgrounds, as well as genetics, which can potentially influence the presentation of AR. However, as these factors are non-modifiable, they are only useful in evaluating the risk of presenting AR, but not for prevention.
Table 4.
Strength of association of demographic factors with AR manifestation
| Study | Study population, N | OR/PRa | Values (95% CI) | p-value | References |
|---|---|---|---|---|---|
| Age | |||||
| Alsowaidiet al., 2010 [3] | 7550 | OR | 0.66 (0.54 - 0.81) | < 0.0005 | > 19 years in ref. to 13-19 years: OR adjusted for nationality, gender, family history of AR, and education |
| Li et al., 2015 [22] | 20,803 | OR | 1.05 (1.02-1.07) | < 0.05 | Continuous variable, 1 year increase (elementary school student) |
| Lim et al., 2015 [11] | 695 | OR | 0.72 (0.58 - 0.88) | < 0.01 | Continuous variable, 10 year increase (18 - 60 years): OR adjusted for gender, smoking, house dust mite allergy, cat allergy, home dampness, and home renovation |
| Ng & Tan, 1994 [36] | 2868 | OR | 0.19 (0.10 – 0.35) | < 0.0001 | 60-74 years in ref. to 20-39 years: OR adjusted for race, flat size, housing estate, smoking, insect exposure, occupational exposure, and fume |
| Ziyab, 2017 [8] | 1154 | PR | 1.04 (1.01 - 1.07) | < 0.01 | Continuous variable (18-26 years): PR adjusted for gender, cat exposure, maternal AR, and paternal AR |
| Gender | |||||
| Alsowaidi et al., 2010 [3] | 7550 | OR | 0.75 (0.63 - 0.88) | < 0.005 | Male in ref. to female: OR adjusted for nationality, age, family history of AR, and education |
| Graif et al., 2004 [38] | 10,057 | OR | 0.85 (0.74 – 0.97) | – | Male in ref. to female: OR adjusted for current asthma, family history of asthma, race, residency, and smoking |
| Hsu et al., 2009 [10] | 1368 | OR | 0.58 (0.47 – 0.72) | < 0.001 | Male in ref. to female: OR adjusted for birth weight, parity, gestational age, maternal education, gestational complications, smoking, pets, carpets, molds, and air pollutions |
| Lee et al., 2017 [42] | 1497 | OR | 1.57 | < 0.01 | Male in ref. to female |
| Lei, Yang & Zhen, 2016 [40] | 3327 | OR | 0.68 (0.46 - 1.00) | < 0.05 | Male in ref. to female |
| Li et al., 2015 [22] | 20,803 | OR | 1.55 (1.41 - 1.70) | < 0.001 | Male in ref. to female |
| Race | |||||
| Graif et al., 2004 [38] | 10,057 | OR | 1.75 (1.45 - 2.13) | – | Jews in ref. to Arabs: OR adjusted for current asthma, family history of asthma, gender, residency, and smoking |
| Ng & Tan, 1994 [36] | 2, 868 | OR | 2.02 (1.29 - 3.14) | < 0.005 | Indian in ref. to Malay: OR adjusted for age, flat size, housing estate, smoking, insect exposure, occupational exposure, and fume |
| Norbäck et al., 2016 (2) [35] | 462 | OR | 0.33 (0.13 - 0.88) | < 0.05 | Indian in ref. to Malay: OR adjusted for gender, smoking, atopy, and family history of allergic diseases |
| Nationality | |||||
| Alsowaidi et al., 2010 [3] | 7550 | OR | 0.48 (0.34 - 0.68) | < 0.005 | Others in ref. to Arabs: OR adjusted for age, gender, family history of AR, and education |
| Residency | |||||
| Graif et al., 2004 [38] | 10,057 | OR | 0.84 (0.90 - 1.40) | – | Urban in ref. to rural: OR adjusted for current asthma, family history of asthma, gender, gender, and smoking |
| Lee et al., 2017 [42] | 1497 | OR | 0.71 | < 0.05 | Townhouse in ref. to others |
| Li et al., 2014 [7] | 9899 | OR | 1.91 (1.37 - 2.68) | < 0.001 | Urban in ref. to rural |
| Min et al., 1997 [20] | 10,054 | OR | 5.26 (2.27 - 12.50) | < 0.05 | Urban in ref. to rural: OR adjusted for age |
| Housing estate | |||||
| Li et al., 2015 [22] | 20,803 | OR | 2.19 (1.97 - 2.43) | < 0.001 | Cities SH, GZ, WH, CD in ref. to XA, HA, HO, UR |
| Ng & Tan, 1994 [36] | 2868 | OR | 1.92 (1.07 - 3.46) | < 0.05 | Toa Payoh in ref. to Yishun : OR adjusted for age, flat size, race, smoking, insect exposure, occupational exposure, and fume |
| Household income | |||||
| Bunnag et al., 2000 [37] | 3124 | OR | 1.97 (1.23 - 3.16) | < 0.05 | High income in ref. to medium income: adjusted OR |
| Li et al., 2015 [22] | 20,803 | OR | 1.42 (1.21 - 1.68) | < 0.001 | 800-1500 RMB/month in ref. to 800 RMB/month |
| 1.93 (1.64 - 2.27) | < 0.001 | 1500-2500 RMB/month in ref. to 800 RMB/month | |||
| 2.88 (2.47 - 3.37) | < 0.001 | > 2500 RMB/month in ref. to 800RMB/month | |||
| Phathammavong et al., 2008 [9] | 536 | OR | 2.23 (1.04 - 4.81) | < 0.05 | High income in ref. to low income: OR adjusted for gender, age, parity, parents education, pets ownership, sharing bed, air conditioning, measles infection, respiratory infection, time on road, meat, fish, vegetables, cow milk, fast food and eggs consumptions, and intestinal parasitic infestation |
| Parity | |||||
| Hsu et al., 2009 [10] | 1368 | OR | 1.42 (1.02 - 1.97) | < 0.025 |
N = 1 in ref. to N ≥ 3 : OR adjusted for birth weight, gender, gestational age, maternal education, gestational complications, smoking, pets, carpets, molds, and air pollutions |
| 1.43 (1.01 - 2.01) | < 0.025 | N = 2 in ref. to N ≥ 3: OR adjusted for birth weight, gender, gestational age, maternal education, gestational complications, smoking, pets, carpets, molds, and air pollutions | |||
| Family size | |||||
| Li et al., 2015 [22] | 20,803 | OR | 1.26 (1.05 - 1.51) | < 0.005 | N < 3 in ref. to N ≥ 4 |
| 1.18 (1.0 - 1.30) | < 0.005 | N = 3 in ref. to N ≥ 4 | |||
| Marital status | |||||
| An et al., 2015 [2] | 31,217 | OR | 0.85 (0.74 - 0.97) | < 0.05 | Married in ref. to unmarried: OR adjusted for age, gender, family size, residency, educational, Household income, and occupation |
aOR odds ratio, PR prevalence ratio
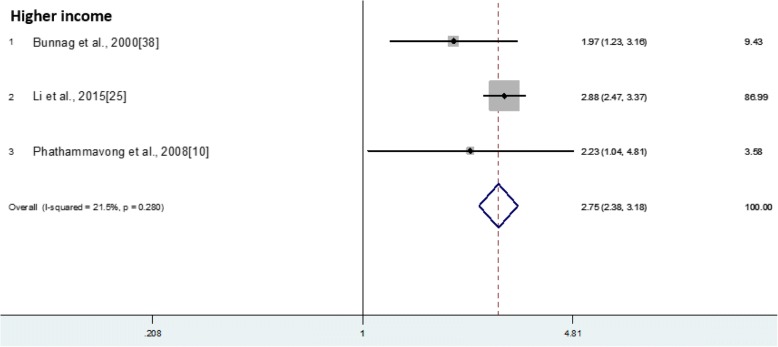
In Li et al. [22], the odds of AR have shown to increase with the rise of household income when different household income groups are compared. For the household with an income of > 2500 RMB/month, the odds of AR is 2.88 times of those with an income of 800 RMB/month. A similar trend is observed in another two independent studies. A pooled odds ratio of 2.75 has been obtained which suggests the significant role of household income in affecting AR expression (Fig. 1).
Fig. 1.
Individual and combined odds ratio and 95% confidence intervals for higher income group in association with Allergic Rhinitis presentation
Moreover, being married, a large number of members in the household, and parity were indicated to be beneficial for protecting one against AR. However, their influences towards protection of AR are likely to be interrelated as married individuals are usually with children and are therefore likely to report an increased parity number and household members.
Personal risk factors affecting AR presentation
Apart from the demographical factors that are usually non-changeable to an individual, one’s behaviours, attitude, and encounters might have direct and indirect influences to the disease presentation. These factors are highly varied from one person to another and are often affected by their background and the social group they interact with.
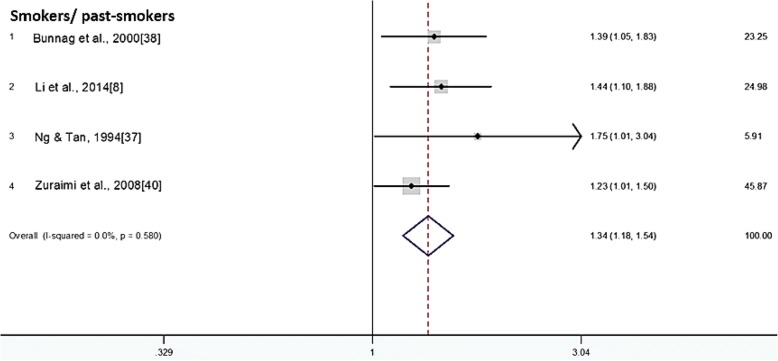
As stated in Table 5, like the case with many other infectious diseases [23, 24], alcohol consumption and smoking habits have shown to increase the odds of presenting AR. This is especially true for the smoking habit; which shows higher odds of expressing AR among present smokers, past smokers, and even passive smokers as compared to non-smokers and those who are not exposed to passive smoking. The result is consistent across four independent articles and a pooled odds ratio of 1.34 was obtained indicating smoking habit does associate with the increased AR manifestation (Fig. 2).
Table 5.
Strength of association of personal risk factor with AR manifestation
| Study | Study population, N | ORa | Values (95% CI) | p-value | References |
|---|---|---|---|---|---|
| Alcohol | |||||
| Bunnag et al., 2000 [37] | 3124 | OR | 1.46 (1.15 - 1.86) | < 0.05 | Drinker in ref. to non-drinker: adjusted OR |
| Smoking | |||||
| Bunnag et al., 2000 [37] | 3124 | OR | 1.39 (1.05 - 1.83) | < 0.05 | Smoker in ref. to non-smoker: adjusted OR |
| Li et al., 2014 [7] | 9899 | OR | 1.44 (1.10 - 1.88) | < 0.01 | Smoker in ref. to non-smoker |
| Ng & Tan, 1994 [36] | 2868 | OR | 1.75 (1.01 – 3.04) | < 0.05 | Past smoker in ref. to non- smoker: OR adjusted for age, flat size, housing estate, race, insect exposure, occupational exposure, and fume |
| Zuraimi et al., 2008 [39] | 6794 | OR | 1.23 (1.01 - 1.50) | – | Passive smoker in ref. to non-passive smoker: OR adjusted for age, gender, race, socioeconomic status, housing type, family atopy, breastfeeding, food allergy, respiratory infections, home dampness, air conditioning, home wall paper, carpet, home traffic density, childcare centre ventilation and dampness |
| Computer usage | |||||
| Li et al., 2014 [7] | 9899 | OR | 1.45 (1.10 - 1.91) | < 0.01 | Occasionally in ref. to never |
| 1.46 (1.10 - 1.93) | < 0.01 | < 2 h daily in ref. to never | |||
| 1.58 (1.14 - 2.19) | < 0.01 | 2-4 h daily in ref. to never | |||
| Education | |||||
| Alsowaidi et al., 2010 [3] | 7550 | OR | 1.42 (1.05 - 1.93) | < 0.05 | University in ref. to illiterate and primary school: OR adjusted for nationality, gender, family history of AR, and age |
| Min et al., 1997 [20] | 10,054 | OR | 1.83 (0.82 - 4.02) | < 0.05 | Elementary in ref. to illiterate: OR adjusted for age |
| 2.11 (0.93 - 4.79) | < 0.05 | Junior in ref. to illiterate: OR adjusted for age | |||
| 2.81 (1.34 - 5.86) | < 0.05 | Senior in ref. to illiterate: OR adjusted for age | |||
| 2.54 (1.08 - 5.96) | < 0.05 | College in ref. to illiterate: OR adjusted for age | |||
| Stress | |||||
| An et al., 2015 [2] | 31,217 | OR | 1.14 (1.01 - 1.28) | < 0.001 | A little in ref. to little: OR adjusted for age, gender, height, weight, body mass index, smoking status, sleep time and drinking |
| 1.46 (1.28 - 1.66) | < 0.001 | Moderate in ref. to little : OR adjusted for age, gender, height, weight, body mass index, smoking status, sleep time and drinking |
|||
| 1.47 (1.21 - 1.79) | < 0.001 | Severe in ref. to little | |||
| : OR adjusted for age, gender, height, weight, body mass index, smoking status, sleep time and drinking | |||||
| Sleep time | |||||
| An et al., 2015 [2] | 31,217 | OR | 0.92 (0.84 - 1.00) | < 0.05 | > 7 h in ref. to ≤7 h : OR adjusted for age, gender, height, weight, body mass index, smoking status, stress and drinking |
| Parasitic infection | |||||
| Phathammavong et al., 2008 [9] | 536 | OR | 3.41 (1.03 - 11.29) | < 0.05 | With parasitic infection in ref. to without : OR adjusted for gender, age, parity, parents education, pets ownership, sharing bed, air conditioning, measles infection, respiratory infection, time on road, meat, fish, vegetables, cow milk, fast food and eggs consumptions, and family income |
| Past respiratory infection | |||||
| Phathammavong et al., 2008 [9] | 536 | OR | 4.06 (1.83 - 9.01) | < 0.05 | With past respiratory infection in ref. to without : OR adjusted for gender, age, parity, parents education, pets ownership, sharing bed, air conditioning, measles infection, family income, time on road, meat, fish, vegetables, cow milk, fast food and eggs consumptions, and intestinal parasitic infestation |
aOR odds ratio
Fig. 2.
Individual and combined odds ratio and 95% confidence intervals for smokers/past-smokers in association with Allergic Rhinitis presentation
Coincidentally, people with more computer usage, higher education, higher stress level and lesser sleeping time were presented with higher AR susceptibility. Though several pathways were speculated for such association, the effects of confounders and bias could not be ruled out and further study is required to establish the direct association link between these factors.
Stress might be one of the critical risk factors for AR presentation. Studies have shown the association between the level of stress in individuals with more frequent drinking and smoking habits, having higher daily computer usage, and higher education levels but with less sleeping time [25–27]. Being in a stressful situation can trigger the expression of cortisol which can induce allergic responses and enhance AR expression (Table 8) [28]. In addition, literature has suggested the possibility of dust trapped on the computer [7] and higher indoor allergen exposure [2] to explain the higher odds of AR manifestation among office workers who usually have higher education qualifications. Dose-response effects were also observed in computer usage, education attainment and stress level as odds of AR increase with higher level of risk exposure, with the exception for AR odds of college students to illiterate individuals in Min et al. [20].
Table 8.
Collated potential risk factors for AR presentation
| No | Potential risk factor | No. of studies | No. of studies with significant results | Possible explanations | Sources |
|---|---|---|---|---|---|
| 1 | Age | 7 | 5 | The allergic condition is highest in young adults, declining with age [43]. However, the reason remains unclear. | Alsowaidi et al., 2010 [3] Li et al., 2015 [22] Lim et al., 2015 [11] Ng & Tan, 1994 [36] Ziyab, 2017 [8] Li et al., 2014a [7] Phathammavong et al., 2008a [9] |
| 2 | Age of gestation | 3 | 2 | Preterm baby, who is characterized by lower birth weight and earlier exposure to the mother microflora, have prematurity protection against AR [42]. In contrast, successful pregnancy shifted the T lymphocytes production to Th2 which increases the risk of atopy and AR [22]. | Lee et al., 2017 [42] Li et al., 2015 [22] Hsu et al., 2009a [10] |
| 3 | Air conditioning | 2 | 0 | Home dampness has been shown to be related to allergic rhinitis exacerbations [44], probably in relation to the development of mold or mildew. As air-conditional areas usually have higher dampness, it may lead to increase in AR expression [45]. | Phathammavong et al., 2008a [9] Zuraimi et al., 2011a [21] |
| 4 | Air pollution | 2 | 1 | The pollutants might provoke and exacerbate the allergic conditions of the current patients. Besides, it might also make a person more susceptible to certain allergens [45]. | Hsu et al., 2009 [10] Ng & Tan, 1994a [36] |
| 5 | Alcohol consumption (self/parent) | 3 | 1 | Alcohol consumption is related to increased stress level which is one of the provoking factors potentially enhancing AR presentation [2]. | Bunnag C et al., 2000 [37] An et al., 2015a [2] Li et al., 2015a [22] |
| 6 | Birth order | 2 | 0 | An allergic mother might be more prone to provide low-exposure environment for the next children [29]. | Phathammavong et al., 2008a [9] Ziyab, 2017a [8] |
| 7 | BMI | 2 | 0 | Higher BMI and greater weight-to-height ratio is associated with higher atopic and higher allergic diseases incidence regardless of gender and age [43]. | An et al., 2015a [2] Lei, Yang & Zhen, 2016a [40] |
| 8 | Breastfeeding | 3 | 2 | Breastfeeding for more than 6 months has shown to enhance the presentation of AR [30, 31], but the reason remains unknown. Contrary plausibility has also shown that food proteins consumed by the mother [32] or breastfeeding might help to reduce the inflammatory responses by destroying microbes [33] and is thus protective against AR presentation. | Huang et al., 2017 [34] Li et al., 2015 [22] Li et al., 2014a [7] |
| 9 | Car ownership | 1 | 0 | Car owners spend more time outdoor and are thus exposed to higher levels of outdoor pollutants [46]. | Li et al., 2014a [7] |
| 10 | Carpet | 2 | 1 | Having carpets at home increases the risk of accumulating mite allergens, thus resulting in more AR cases [47]. | Hsu et al., 2009 [10] Ng & Tan, 1994a [36] |
| 11 | Computer usage | 1 | 1 | Studies suggested that when the computer is not properly cleaned, prolong usage of the computer will likely result in higher allergen exposure and thus an increase in AR cases [7]. | Li et al., 2014 [7] |
| 12 | Drinking (self/parent) | 3 | 1 | Alcohol consumption is related to increased stress level which is one of the provoking factors potentially enhancing AR presentation [2]. | Bunnag C et al., 2000 [37] An et al., 2015a [2] Li et al., 2015a [22] |
| 13 | Education attainment | 3 | 2 | People with higher education usually work in an indoor environment, thus exposing them to indoor allergens [2]. | Alsowaidi et al., 2010 [3] Min et al., 1997 [20] An et al., 2015a [2] |
| 14 | Family history of allergic diseases | 6 | 5 | Allergic diseases can be hereditary, with incomplete genetic penetrance [48]. | Alsowaidi et al., 2010 [3] Li et al., 2014 [7] Graif et al., 2004 [38] Norbäck et al., 2016 (2) [35] Ziyab, 2017 [8] Phathammavong et al., 2008a [9] |
| 15 | Family history of atopy | 1 | 1 | Atopy is usually used as a marker for other allergic diseases, and genetic factors usually play a role in allergic disease presentation. As such, higher family history of atopy usually suggests higher chance of contracting allergic diseases [43]. | Bunnag et al., 2000 [37] |
| 16 | Family size | 2 | 1 | Crowding increases the contact of an individual with allergens and is thus protective against manifestation of allergic reaction [47]. | Li et al., 2015 [22] An et al., 2015a [2] |
| 17 | Food | 1 | 0 | Some foods are protective against AR, most likely through shifting the macromolecules production, such as fatty acid balance, which later results in the reduction of inflammatory mediators required for disease presentation [30]. | Phathammavong et al., 2008a [9] |
| 18 | Fume exposure | 1 | 1 | Fume released into the air by various means is also one of the potential triggering factors in AR presentation [45]. | Ng & Tan, 1994 [36] |
| 19 | Fungi | 2 | 2 | Airborne fungi spores induce type I hypersensitivity and hence AR presentation [49]. | Norbäck et al., 2016 (1) [41] Norbäck et al., 2016 (2) [35] |
| 20 | Gender | 12 | 7 | The allergic diseases appear more frequently in males at infant age, but with equal burden as females at mid-teens, and then become more frequent in females with the reason remain largely unknown [43]. | Alsowaidi et al., 2010 [3] Graif et al., 2004 [38] Hsu et al., 2009 [10] Lee et al., 2017 [42] Lei, Yang & Zhen, 2016 [40] Li et al., 2015 [22] Bunnag et al., 2000a [37] Lim et al., 2015a [11] Ng & Tan, 1994a [36] Norbäck et al., 2016 (2)a [35] Phathammavong et al., 2008a [9] Ziyab, 2017a [8] |
| 21 | Gestational complication | 1 | 0 | Uterus complication during gestation periods affects the immune system development of the fetus and increases the risk of atopy-related diseases [29]. | Hsu et al., 2009a [10] |
| 22 | Gruel introduction period | 1 | 1 | Study shows that gruel introduction between 4 to 6 months, in complementary with breastfeeding, induces IL-10 and TGFβ production which is protective against AR [34]. | Huang et al., 2017 [34] |
| 23 | Hair coloring | 1 | 0 | Oxidative hair dye can induce hypersensitivity reactions, thus increasing the risk of expressing AR [50]. | Li et al., 2014a [7] |
| 24 | Home renovation | 1 | 1 | The materials used during the home renovation, such as formaldehyde might have an impact in causing cell sensitization and later AR presentation [7, 31]. | Li et al., 2014 [7] |
| 25 | House dust mite | 2 | 2 | Long term exposure to threshold concentrations of dust mite fecal proteins causes the presentation of allergens by antigen presenting cells (APC) to CD4+ T lymphocytes, leading to the production of downstream mediators and manifestation of AR symptoms [49]. | Lim et al., 2015 [11] Norbäck et al., 2016 (2) [35] |
| 26 | Household income | 5 | 3 | Higher income is associated with better living conditions and hygiene behavior, thus reducing the exposure to a variety of allergens, which possibly increases their odds of AR [42]. | Bunnag et al., 2000 [37] Li et al., 2015 [22] Phathammavong et al., 2008 [9] An et al., 2015a [2] Li et al., 2014a [7] |
| 27 | Housing estate | 2 | 2 | Living in a housing estate with poor environmental conditions has resulted in more allergic cases [47]. | Li et al., 2015 [22] Ng & Tan, 1994 [36] |
| 28 | Insect | 1 | 1 | Prolonged exposure to insects, which is one of the common allergens may trigger hypersensitivity reactions with production of mediators and hence, the expression of AR symptoms [49]. | Ng & Tan, 1994 [36] |
| 29 | Marital status | 2 | 1 | Being married is hypothesized to be associated with positive physical and mental outcomes and is therefore protective against AR [2]. | An et al., 2015 [2] Min et al., 1997a [20] |
| 30 | Maternal education | 2 | 1 | Educated parents will have higher awareness of their children health status, and thus adopt protective measures to combat against AR starting from a young age [45]. | Li et al., 2015 [22] Hsu et al., 2009a [10] |
| 31 | Maternal pre- or postnatal depression | 1 | 1 | Pre- or postnatal depression results in excessive cortisol expression, which will affect the immune system development of the fetus [22]. | Li et al., 2015 [22] |
| 32 | Mode of delivery | 2 | 1 | Exposure of the fetus to the mother microflora during birth is an advantage to protect them against allergic sensitization [29, 51]. In contrast, cesarean birth is associated with higher AR risk [51]. | Li et al., 2015 [22] Ziyab, 2017a [8] |
| 33 | Mold | 2 | 1 | Mold spores induce type I hypersensitivity and hence, AR presentation [49]. | Kidoni et al., 2004 [19] Hsu et al., 2009a [10] |
| 34 | Nationality | 1 | 1 | AR prevalence is especially high in Asia probably due to the higher humidity, more extensive smoking and vaccination habits [43]. | Alsowaidi et al., 2010 [3] |
| 35 | Occupational exposure | 3 | 2 | Some occupations have higher risk of exposure to allergens, thus increasing their risk of expressing AR [50]. | An et al., 2015 [2] Ng & Tan, 1994 [36] Min et al., 1997a [20] |
| 36 | Parasitic infection | 1 | 1 | Parasitic infection might have some effects to a person’s gut microbiota, which could later offer some protection against allergic sensitization as stated in hygiene hypothesis [52]. However, some literature also show that parasitic infection influences the allergy development due to its competition with human immune response [9]. | Phathammavong et al., 2008 [9] |
| 37 | Parity | 2 | 1 | Being allergic might cause reduced reproductivity in females, resulting in a lower parity which is associated with AR presentation [29]. | Hsu et al., 2009 [10] Phathammavong et al., 2008a [9] |
| 38 | Past measles infection | 1 | 0 | The association of measles with AR is not clear, but it was hypothesized that measles infection might protect against AR development or could promote allergic sensitization [52]. | Phathammavong et al., 2008a [9] |
| 39 | Past respiratory infection | 1 | 1 | Evidence shows that past respiratory infection, such as tuberculosis caused by Mycobacterium tuberculosis could be protective against AR, possibly through reduction of allergy sensitization [52]. In contrast, some studies have shown that past respiratory infection is directly associated with AR development [9]. | Phathammavong et al., 2008 [9] |
| 40 | Paternal education | 1 | 1 | Educated parents are more likely to keep a hygienic living environment, thus possibly increasing the incidence of allergic conditions in their children [45]. | Li et al., 2015 [22] |
| 41 | Pet | 6 | 3 | For individuals sensitive to pet furs, long term exposure to the pet induces hypersensitivity reaction and could later result in AR presentation [49]. | Li et al., 2014 [7] Ziyab, 2017 [8] Phathammavong et al., 2008a [9] Hsu et al., 2009a [10] Lim et al., 2015a [11] Ng & Tan, 1994a [36] |
| 42 | Race | 3 | 3 | Cultural differences between the races probably have some effects on AR presentation; however, there is currently no specific research addressing the impact of races on AR disease presentation. | Graif et al., 2004 [38] Ng & Tan, 1994 [36] Norbäck et al., 2016 (2) [35] |
| 43 | Residency | 5 | 4 | For people who lived in urban areas, they are more prevalent in developing allergic reaction [47], probably due to a poorer housing or environmental conditions. Modern building techniques increase indoor humidity and temperature, facilitates mold development and hence, contributes to AR presentation [2]. | Graif et al., 2004 [38] Lee et al., 2017 [42] Li et al., 2014 [7] Min et al., 1997 [20] An et al., 2015a [2] |
| 44 | Sharing bed | 1 | 0 | Sharing bed is hypothesized as one of the potential risk factors for AR [9], probably due to increased risk of getting infections from other people. | Phathammavong et al., 2008a [9] |
| 45 | Sleep time | 1 | 1 | People with lesser sleep are usually with higher levels of stress, which is a potential trigger factor for AR expression [2]. | An et al., 2015 [2] |
| 46 | Smoking (self/parent) | 12 | 4 | Tobacco smoke is one of the trigger factors which precipitates the hypersensitivity reactions, thus exacerbating the AR conditions [47]. On the other hand, parents with AR children will also try to reduce their children exposure to external allergic stimuli through changing their smoking habits, thus explaining the negative association of AR and smoking habit [45]. | Bunnag et al., 2000 [37] Li et al., 2014 [7] Ng & Tan, 1994 [36] Zuraimi et al., 2008 [39] An et al., 2015a [2] Hsu et al., 2009a [10] Li et al., 2015a [22] Lim et al., 2015a [11] Min et al., 1997a [20] Norbäck et al., 2016 (2)a [35] Phathammavong et al., 2008a [9] Ziyab, 2017a [8] |
| 47 | Social class | 1 | 0 | As stated in hygiene hypothesis, people in lower social class are likely to have a greater exposure to infections. This may have direct and indirect impacts to their gut microbiota, which might offer protection against allergic sensitization [45, 52]. | Min et al., 1997a [20] |
| 48 | Stress level | 1 | 1 | Stress can trigger the production of cortisol, and later induce allergic responses [28]. | An et al., 2015 [2] |
| 49 | Time on road | 1 | 0 | Longer time spent on road is associated with higher AR risk, probably due to prolonged exposure to air contaminant [9]. | Phathammavong et al., 2008a [9] |
| 50 | Traffic | 1 | 1 | The release of motor vehicles such as NOx and CO provokes and exacerbates the conditions of the current AR patients, and might have consequences on changes in susceptibility towards allergens, thus affecting AR presentation [45]. Depending on the outdoor environmental pollution, long term exposure to heavy traffic might lead to allergic sensitization and resulted in AR expression [21]. | Zuraimi et al., 2011 [21] |
aIndicates the publication with insignificant results
In contrast, people with parasitic or past respiratory infections were reported to have higher odds of AR presentation. The results are contradictory with biological plausibility discussed in other literature. Phathammavong et al. [9], proposed that AR and other respiratory infections compete for immune responses, resulting in a higher odds of presenting AR among the respiratory infection patients. This hypothesis is supported by the reported odds of AR for individuals with either parasitic infection or past respiratory infection are exceptionally high (3.41 and 4.06 respectively). However, this factor has only been studied in Phathmmavong et al. among the articles reviewed and further analysis is essential to confirm the effects of these infections on AR presentation, which could be one of the most important factors in predicting AR risk.
Family risk factors affecting AR presentation
In Table 6, mother depression and cesarean delivery are positively correlated with the odds of AR presentation. As stated in Li et al. [22], pre- and postnatal depression stimulates the production of cortisol, and this secretion affects the immune development of a fetus and increases the odds of presenting AR. Apart from this, cesarean delivery might further exacerbate this situation as unlike vaginal delivery, the infants are not exposed to the mother’s birth canal microflora, which has shown to be protective against AR expression [29] as illustrated in Table 8.
Table 6.
Strength of association of family factor with AR manifestation
| Study | Study population, N | OR/PRa | Values (95% CI) | p-value | References |
|---|---|---|---|---|---|
| Age of gestation | |||||
| Lee et al., 2017 [42] | 1497 | OR | 0.51 | < 0.05 | Preterm in ref. to term |
| Li et al., 2015 [22] | 20,803 | OR | 1.07 (0.88 - 1.30) | < 0.001 | Preterm in ref. to term |
| 1.42 (1.20 - 1.69) | < 0.001 | Post-term in ref. to term | |||
| Mother depression | |||||
| Li et al., 2015 [22] | 20,803 | OR | 1.16 (1.05 - 1.29) | < 0.05 | Mother with pre- or postnatal depression in ref. to without |
| Mode of delivery | |||||
| Li et al., 2015 [22] | 20,803 | OR | 1.36 (1.23 - 1.49) | < 0.001 | Cesarean in ref. to vaginal delivery |
| Breastfeeding | |||||
| Huang et al., 2017 [34] | 13,335 | OR | 0.97 (0.94 - 0.99) | < 0.05 | With exclusive for > 6 months breastfeeding in ref. to never breastfeeding : OR adjusted for family atopy, gender, age, district of the current residence, home ownership, early pet-keeping, parental smoking, and home dampness |
| Li et al., 2015 [22] | 20,803 | OR | 0.67 (0.61 – 0.73) | < 0.001 | With exclusive breastfeeding in the first 4 months in ref. to without |
| Maternal education | |||||
| Li et al., 2015 [22] | 20,803 | OR | 1.55 (1.36 - 1.77) | < 0.001 | High school in ref. to middle school or below |
| 2.11 (1.86 - 2.39) | < 0.001 | College or above in ref. to middle school or below | |||
| Paternal education | |||||
| Li et al., 2015 [22] | 20,803 | OR | 1.52 (1.32 - 1.74) | < 0.001 | High school in ref. to middle school or below |
| 2.02 (1.77 - 2.30) | < 0.001 | College or above in ref. to middle school or below | |||
| Gruel introduction | |||||
| Huang et al., 2017 [34] | 13,335 | OR | 0.95 (0.90 - 1.00) | < 0.05 | For > 6 months-old in ref. to < 3 months-old : OR adjusted for family atopy, gender, age, district of the current residence, home ownership, early pet-keeping, parental smoking, and home dampness |
| Family history of atopy | |||||
| Bunnag et al., 2000 [37] | 3124 | OR | 1.96 (1.56 - 2.46) | < 0.05 | With family history of atopy in ref. to without: adjusted OR |
| Family history of allergic diseases | |||||
| Alsowaidi et al., 2010 [3] | 7550 | OR | 6.08 (4.93 - 7.50) | < 0.0005 | With family history of AR in ref. to without : OR adjusted for nationality, gender, age, and education |
| Li et al., 2014 [7] | 9899 | OR | 3.51 (2.65 - 4.64) | < 0.001 | With family history of AR in ref. to without |
| Graif et al., 2004 [38] | 10,057 | OR | 1.30 (1.02 - 1.66) | – | With family history of asthma in ref. to without: OR adjusted for current asthma, gender, gender, gender, and smoking |
| Norbäck et al., 2016 (2) [35] | 462 | OR | 3.49 (1.97 - 6.20) | < 0.001 | With family history of allergic reactions in ref. to without: OR adjusted for gender, smoking, atopy, and race |
| Ziyab, 2017 [8] | 1154 | PR | 1.82 (1.39 - 2.39) | < 0.001 | With maternal allergy in ref. to without: PR adjusted for gender, cat exposure, and age |
| 1.87 (1.25 - 2.77) | < 0.005 | With paternal allergy in ref. to without: PR adjusted for gender, cat exposure, and age | |||
aOR odds ratio, PR prevalence ratio
Conversely, inconsistent results are observed for the association of breastfeeding with AR presentation across multiple studies [30, 31]. This refutes the commonly accepted hypothesis which states breastfeeding as protective through the antibodies present in the milk and the additional nutrients from the mother’s diet transferred to the milk [32, 33]. In contrast, parental education and awareness encourages a hygienic environment which is unfavorable for AR protection as this reduces the chance of exposing their children to a larger variety of allergens in early life. Similarly, for gruel consumption, the subtle protection might be due to the effect of gruel to stimulate inflammatory cytokines which suppress the allergic reaction [34].
On the other hand, genetic factor is long established to play an influential role in AR presentation [15] and a family history of atopy and allergic diseases might predispose children to AR. Multiple studies have shown that family history is a key risk factor associated with the increased risk of AR expression. This is particularly true for children with a family history of AR as high odds ratios of 6.08 and 3.51 have been reported in studies conducted by Alsowaidi et al. [3] and Li et al. [7], respectively. However, genetic factor is non-modifiable and hence, it needs to be complemented with other preventive measures in order to reduce the risk of presenting the disease.
Environmental risk factors affecting AR presentation
As suggested in multiple studies investigated, environmental factors are highly important in triggering AR. For instance, Table 7 has shown that the presence of allergens such as fungi, molds, insects and house dust mites could increase the odds of presenting AR. Among the allergens studied, the presence of fungi and molds were reported to have very high odds of association to AR with 3.44 for fungi in Norbäck et al. [35] and 9.40 for molds in Kidoni et al. [19] Moreover, insect exposure and house dust mite have been identified as two of the most important risk factors for AR as indicated in Table 7. These common indoor allergens, such as mold and fungal spores, insect wastes and house dust mite fecal proteins can induce Type I hypersensitivity reaction by promoting the expression of a range of allergic-causing mediators, thus increasing the odds of expressing AR (Table 8). In addition, the utilization of carpets, which trap dust, and home renovation, which introduces a variety of allergic-causing renovation materials, further exacerbate the situation.
Table 7.
Strength of association of environmental risk factors with AR manifestation
| Study | Study population, N | OR/PRa | Values (95% CI) | p-value | References |
|---|---|---|---|---|---|
| Fungi | |||||
| Norbäck et al., 2016 (1) [41] | 462 | OR | 0.76 (0.58 - 0.99) | < 0.05 | With fungi in ref. to without: OR adjusted for gender, ethnicity, smoking, atopy and heredity |
| Norbäck et al., 2016 (2) [35] | 462 | OR | 3.44 (1.81 - 6.59) | < 0.001 | With fungal endotoxin C14 3-OH FA in ref. to without: OR adjusted for classroom level |
| Mold | |||||
| Kidoni et al., 2004 [19] | 202 | OR | 9.40 (3.80 - 22.90) | – | With mold sensitization vs without |
| Insect | |||||
| Ng & Tan, 1994 [36] | 2868 | OR | 2.08 (1.29 – 3.35) | < 0.005 | Once every day in ref. to once every few months : OR adjusted for age, flat size, housing estate, race, race, occupational exposure, and fume |
| House dust mite | |||||
| Lim et al., 2015 [11] | 695 | OR | 1.66 (1.08 - 2.56) | < 0.05 | With house dust mite allergy in ref. to without : OR adjusted for gender, current smoking status, age, cat allergy, home dampness, and indoor home painting in last 12 months |
| Norbäck et al., 2016 (2) [35] | 462 | OR | 2.91 (1.35 - 6.24) | < 0.01 | Continuous variable, 1000 mg increase in fine dust : OR adjusted for classroom level |
| Carpet | |||||
| Hsu et al., 2009 [35] | 1368 | OR | 1.60 (1.09 - 2.35) | < 0.025 | With carpets in ref. to without : OR adjusted for birth weight, gender, gestational age, maternal education, gestational complications, smoking, pets, parity, molds, and air pollutions |
| Home renovation | |||||
| Li et al., 2014 [7] | 9899 | OR | 1.39 (1.06 - 1.81) | < 0.05 | With home renovation in ref. to without |
| Air pollution | |||||
| Hsu et al., 2009 [10] | 1368 | OR | 1.44 (1.10 - 1.88) | < 0.01 | With air pollution in ref. to without : OR adjusted for birth weight, gender, gestational age, maternal education, gestational complications, smoking, pets, carpets, molds, and parity |
| Fume exposure | |||||
| Ng & Tan, 1994 [36] | 2868 | OR | 2.29 (1.32 - 3.99) | < 0.005 | Often in ref. to rarely: OR adjusted for age, flat size, housing estate, race, race, occupational exposure, and race |
| Traffic | |||||
| Zuraimi et al., 2011 [21] | 2994 | PR | 1.58 (1.04 - 2.39) | < 0.05 | Heavy traffic in ref. to low traffic for all children : PR adjusted for gender, age, race, socioeconomic status, housing type, parental atopy, breastfeeding, food allergy, and resident height |
| 1.73 (1.00 - 2.99) | < 0.05 | Heavy traffic in ref. to low traffic for all lifetime residents : PR adjusted for gender, age, race, socioeconomic status, housing type, parental atopy, breastfeeding, food allergy, and resident height |
|||
| Occupational exposure | |||||
| An et al., 2015 [2] | 31,217 | OR | 1.28 (1.11 - 1.47) | < 0.01 | Unemployed in ref. to engineer : OR adjusted for age, gender, family size, residency, educational, household income, and marriage |
| 1.29 (1.09 - 1.52) | < 0.01 | Manager, expert, specialist & clerks in ref. to engineer : OR adjusted for age, gender, family size, residency, educational, household income, and marriage |
|||
| 1.18 (1.01 - 1.39) | < 0.01 | Service worker & seller in ref. to engineer : OR adjusted for age, gender, family size residency, educational, household income, and marriage |
|||
| 1.32 (1.11 - 1.58) | < 0.01 | Technician, mechanics & production worker in ref. to engineer : OR adjusted for age, gender, family size, residency, educational, household income, and marriage |
|||
| Ng & Tan 1994 [36] | 2868 | OR | 1.95 (1.36 - 2.80) | < 0.0005 | Wth occupational exposure vs without: OR adjusted for age, flat size, housing estate, race, race, fume, and race |
aOR odds ratio, PR prevalence ratio
Similarly, outdoor exposures to heavy traffic, air pollution, and fume exposure were also reported to be positively correlated with AR manifestation. These factors are especially crucial for those whose occupations expose them to the allergens [36]. Constant outdoor encounters with pollutants released from motor vehicles and heavy fumes during work promote AR presentation by changing a person’s susceptibility towards allergens [2, 10, 21, 36].
Evaluation of risk factors associated with AR manifestation using several criteria
Various risk factors have shown strong association with AR presentation. Results are consistent for several risk factors across studies with different experimental setups and countries.
In addition to the ORs, criteria such as biological gradient, biological plausibility and temporality are important in evaluating the association between risk factors and AR. The biological gradient of the factor can be established especially when it is studied in a continuous manner or in multiple exposure levels. This was demonstrated in various demographical factors such as in family income, family size, personal factors like computer usage, education attainment, stress levels and even in parental education attainment. Moreover, the association between the risk factors and AR manifestation are further strengthened when factors with similar roles in AR presentation, such as the common allergens like house dust mites, fungi, and molds, display comparable results.
Furthermore, the listed factors can only be considered as a potential risk if its exposure is reasonably affected or altered the risk of AR development. Its biologic plausibility must also be coherent to the study results found. However, with reference to Table 7, breastfeeding, parasitic infection and past respiratory infections show contradictory results as to what is hypothesized and further analysis and interpretation is thus needed.
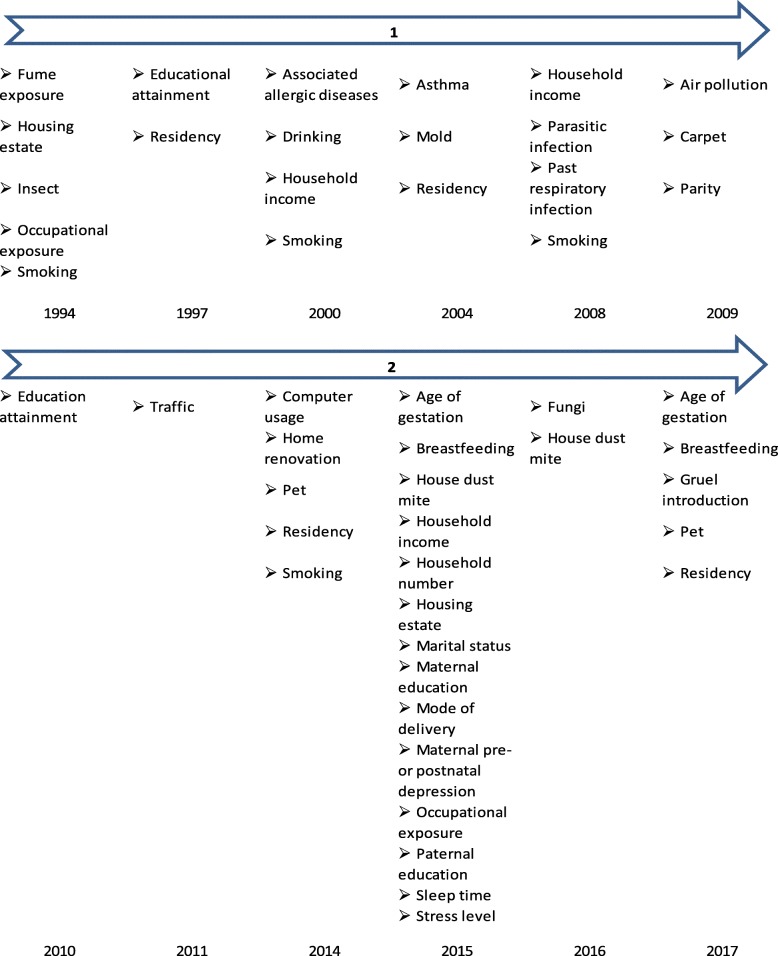
Last but not least, with reference to Fig. 3, the two risk factors, family income and smoking, analyzed using meta-analysis are consistently being identified as significant AR risk factor before and after 2010. In addition, education attainment and occupational exposure are two other significant modifiable risks that appeared in AR publications before and after 2010 in Asia. In contrast, it was observed that after the year 2010, more family-related risks were analyzed and shown to be significant AR risk factors, such as the age of gestation and breastfeeding. This suggests a shift in focus to consider more family-related risk factors among the Asian population.
Fig. 3.
Years in which significant AR risk factors were identified
Limitations and conclusion
The studies chosen for this review are limited to articles published in Asia. Thus, the result might not be relevant and applicable to other nations outside Asia. In addition, the analysis might still be biased though several criteria have been used in establishing the significance of the potential AR risk factor in triggering or protecting against AR presentation. The analysed data could be affected by personal viewpoints in addition to errors occurred when translating data from primary literature to the review summaries, such as misrepresentation and misinterpretation of the original data. Thus, it is highly recommended for readers to refer to the original articles before extracting any information from this article. Furthermore, as most of the studies used in this review are observational studies, confounding effects cannot be ruled out and the association of a particular risk factor with the disease presentation might not be as straightforward as what is illustrated here.
From the articles reviewed, family income, family size, computer usage, personal and parental education attainment and stress level are identified as risk factors with the greatest potential to influence AR presentation, and when compared to other factors, they fulfill most of the criteria listed. In contrast, more considerations are required in interpreting the effects of breastfeeding, parasitic infections and past respiratory infections to AR presentation. These factors show incoherent biological plausibility and more in-depth investigation and analysis is thus required.
The results obtained from this review article can be used to improve the diagnosis of AR in clinical settings by identifying patients with risk factors strongly associated with AR manifestation. In addition, as personal and family-related modifiable factors are found to be strong AR triggering factors, strategies to alleviate personal stress levels and increase the awareness of allergy risk in a hygienic environment have to be developed.
Additional file
List of countries and dependent territories used in the literature review search. (PDF 322 kb)
Acknowledgements
The authors would like to thank all authors involved in the studies reviewed above as well as the individuals that volunteered in these studies. In addition, we also would like to express my special thanks of gratitude to Ng Yu Ting, Sri Anusha Matta, and Sio Yang Yie for language editing of this manuscript.
Abbreviations
- AR
Allergic rhinitis
- OR
Odds ratio
- PR
Prevalence ratio
Authors’ contributions
FTC supported and guided the literature review process. SNC carried out the literature review and translated the information into the manuscript. Both authors read and approved the final version of the article.
Not applicable.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sher Ney Chong, Email: e0002357@u.nus.edu.
Fook Tim Chew, Email: dbscft@nus.edu.sg.
References
- 1.Strachan D, Sibbald B, Weiland S, Aït-Khaled N, Anabwani G, Anderson HR, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the international study of asthma and allergies in childhood (ISAAC) Pediatr Allergy Immunol. 1997;8:161–176. doi: 10.1111/j.1399-3038.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 2.An S-Y. Analysis of various risk factors predisposing subjects to allergic rhinitis. Asian Pacific J Allergy Immunol. 2015;:143–52. doi:10.12932/AP0554.33.2.2015. [DOI] [PubMed]
- 3.Alsowaidi S, Abdulle A, Shehab A, Zuberbier T, Bernsen R. Allergic rhinitis: prevalence and possible risk factors in a gulf Arab population. Allergy Eur J Allergy Clin Immunol. 2010;65:208–212. doi: 10.1111/j.1398-9995.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy. 2008;63:1052–1055. [Google Scholar]
- 5.Murray CS. Allergens, viruses, and asthma exacerbations. Proc Am Thorac Soc. 2004;1:99–104. doi: 10.1513/pats.2306027. [DOI] [PubMed] [Google Scholar]
- 6.Cookson W. The alliance of genes and environment in asthma and allergy. Nature 1999;402 November:B5–11. [DOI] [PubMed]
- 7.Li CW, De Chen H, Zhong JT, Bin LZ, Peng H, Lu HG, et al. Epidemiological characterization and risk factors of allergic rhinitis in the general population in Guangzhou City in China. PLoS One. 2014;9:1–16. doi: 10.1371/journal.pone.0114950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziyab AH. Prevalence and risk factors of asthma, rhinitis, and eczema and their multimorbidity among young adults in Kuwait: a cross-sectional study. Biomed Res Int. 2017;2017:2184193. doi: 10.1155/2017/2184193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phathammavong O, Ali M, Phengsavanh A, Xaysomphou D, Odajima H, Nishima S, et al. Prevalence and potential risk factors of rhinitis and atopic eczema among schoolchildren in Vientiane capital, Lao PDR: ISAAC questionnaire. Biosci Trends 2008;2:193–199. [PubMed]
- 10.Hsu S-P, Lin K-N, Tan C-T, Lee F-P, Huang H-M. Prenatal risk factors and occurrence of allergic rhinitis among elementary school children in an urban city. Int J Pediatr Otorhinolaryngol. 2009;73:807–810. doi: 10.1016/j.ijporl.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Lim FL, Hashim Z, LTL T, Said SM, Hashim JH, Norbäck D. Asthma, airway symptoms and rhinitis in office workers in Malaysia: associations with house dust mite (HDM) allergy, cat allergy and levels of house dust mite allergens in office dust. PLoS One. 2015;10:1–21. doi: 10.1371/journal.pone.0124905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamay Z, Akcay A, Ones U, Guler N, Kilic G, Zencir M. Prevalence and risk factors for allergic rhinitis in primary school children. Int J Pediatr Otorhinolaryngol. 2007;71:463–471. doi: 10.1016/j.ijporl.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Sultész M, Katona G, Hirschberg A, Gálffy G. Prevalence and risk factors for allergic rhinitis in primary schoolchildren in Budapest. Int J Pediatr Otorhinolaryngol. 2010;74:503–509. doi: 10.1016/j.ijporl.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Kuyucu S, Saraclar Y, Tuncer A, Geyik PO, Adalioglu G, Akpinarli A, et al. Epidemiologic characteristics of rhinitis in Turkish children: the international study of asthma and allergies in childhood (ISAAC) phase 2. Pediatr Allergy Immunol. 2006;17:269–277. doi: 10.1111/j.1399-3038.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 15.Magnan A, Meunier JP, Saugnac C, Gasteau J, Neukirch F. Frequency and impact of allergic rhinitis in asthma patients in everyday general medical practice: a French observational cross-sectional study. Allergy Eur J Allergy Clin Immunol. 2008;63:292–298. doi: 10.1111/j.1398-9995.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- 16.Cirillo I, Marseglia G, Klersy C, Ciprandi G. Allergic patients have more numerous and prolonged respiratory infections than nonallergic subjects. Allergy Eur J Allergy Clin Immunol. 2007;62:1087–1090. doi: 10.1111/j.1398-9995.2007.01401.x. [DOI] [PubMed] [Google Scholar]
- 17.Cardell LO, Olsson P, Andersson M, Welin KO, Svensson J, Tennvall GR, et al. TOTALL: high cost of allergic rhinitis - a national Swedish population-based questionnaire study. npj Prim Care Respir Med 2016;26. [DOI] [PMC free article] [PubMed]
- 18.Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assess Econ Impact. J Allergy Clin Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- 19.Kidoni MI, See Y, Goh A, Chay OM, Balakrishnan A. Aeroallergen sensitization in pediatric allergic rhinitis in Singapore: is air-conditioning a factor in the tropics? Pediatr Allergy Immunol. 2004;15:340–343. doi: 10.1111/j.1399-3038.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 20.Min YG, Jung HW, Kim HS, Park SK, Yoo KY. Prevalence and risk factors for perennial allergic rhinitis in Korea: results of a nationwide survey. Clin Otolaryngol Allied Sci. 1997;22:139–44. [DOI] [PubMed]
- 21.Zuraimi MS, Tham KW, Chew FT, Ooi PL, Koh D. Home air-conditioning, traffic exposure, and asthma and allergic symptoms among preschool children. Pediatr Allergy Immunol. 2011;22(1 PART 2):112–118. doi: 10.1111/j.1399-3038.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Jiang Y, Li S, Shen X, Liu J, Jiang F. Pre-and postnatal risk factors in relation to allergic rhinitis in school-aged children in China. PLoS One. 2015;10:1–11. doi: 10.1371/journal.pone.0114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talamini G, Bassi C, Falconi M, Sartori N, Salvia R, Rigo L, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44:1303–1311. doi: 10.1023/A:1026670911955. [DOI] [PubMed] [Google Scholar]
- 24.Poikolainen K, Karvonen J, Pukkala E. Excess mortality related to alcohol and smoking among hospital-treated patients with psoriasis. Arch Dermatol. 1999;135:1490–1493. doi: 10.1001/archderm.135.12.1490. [DOI] [PubMed] [Google Scholar]
- 25.Conway TL, Vickers RR, Ward HW, Rahe RH. Occupational stress and variation in cigarette, coffee, and alcohol consumption. J Health Soc Behav. 1981;22:155. doi: 10.2307/2136291. [DOI] [PubMed] [Google Scholar]
- 26.Robotham D, Julian C. Stress and the higher education student: a critical review of the literature. J Furth High Educ. 2006;30:107–117. doi: 10.1080/03098770600617513. [DOI] [Google Scholar]
- 27.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder : a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 28.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nafstad P, Magnus P, Jaakkola JJ. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000;106:867–873. doi: 10.1067/mai.2000.110558. [DOI] [PubMed] [Google Scholar]
- 30.Nafstad P, Nystad W, Magnus P, Jaakkola JJK. Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J Asthma. 2003;40:343–8. [DOI] [PubMed]
- 31.Wang X, Liu W, Hu Y, Zou Z, Shen L, Huang C. Home environment, lifestyles behaviors, and rhinitis in childhood. Int J Hyg Environ Health. 2016;219:220–231. doi: 10.1016/j.ijheh.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Spiekermann GM, Walker WA. Oral tolerance and its role in clinical disease. J Pediatr Gastroenterol Nutr. 2001;32:237–255. doi: 10.1097/00005176-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Hanson LÅ. Session 1: feeding and infant development breast-feeding and immune function. Proc Nutr Soc. 2007;66:384–396. doi: 10.1017/S0029665107005654. [DOI] [PubMed] [Google Scholar]
- 34.Huang C, Liu W, Cai J, Weschler LB, Wang X, Hu Y, et al. Breastfeeding and timing of first dietary introduction in relation to childhood asthma, allergies, and airway diseases: a cross-sectional study. J Asthma. 2017;54:488–497. doi: 10.1080/02770903.2016.1231203. [DOI] [PubMed] [Google Scholar]
- 35.Norbäck D, Hashim JH, Markowicz P, Cai GH, Hashim Z, Ali F, et al. Endotoxin, ergosterol, muramic acid and fungal DNA in dust from schools in Johor Bahru, Malaysia - associations with rhinitis and sick building syndrome (SBS) in junior high school students. Sci Total Environ. 2016;545–546:95–103. doi: 10.1016/j.scitotenv.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 36.Ng TP, Tan WC. Epidemiology of allergic rhinitis and its associated risk-factors in Singapore. Int J Epidemiol. 1994;23:553–558. doi: 10.1093/ije/23.3.553. [DOI] [PubMed] [Google Scholar]
- 37.Bunnag C, Jareoncharsri P, Voraprayoon S, Kongpatanakul S. Epidemiology of rhinitis in Thais : characteristics and risk factors. Asian Pacific J Allergy Immunol. 2000;18:1. [PubMed] [Google Scholar]
- 38.Graif Y, Garty B-Z, Livne I, Green MS, Shohat T. Prevalence and risk factors for allergic rhinitis and atopic eczema among schoolchildren in Israel: results from a national study. Ann Allergy Asthma Immunol. 2004;92:245–249. doi: 10.1016/S1081-1206(10)61555-4. [DOI] [PubMed] [Google Scholar]
- 39.Zuraimi MS, Tham KW, Chew FT, Ooi PL, David K. Home exposures to environmental tobacco smoke and allergic symptoms among young children in Singapore. Int Arch Allergy Immunol. 2008;146:57–65. doi: 10.1159/000112503. [DOI] [PubMed] [Google Scholar]
- 40.Lei Y, Yang H, Zhen L. Obesity is a risk factor for allergic rhinitis in children of Wuhan (China) Asia Pac Allergy. 2016;6:101–104. doi: 10.5415/apallergy.2016.6.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norbäck D, Hashim JH, Cai GH, Hashim Z, Ali F, Bloom E, et al. Rhinitis, ocular, throat and dermal symptoms, headache and tiredness among students in schools from Johor Bahru, Malaysia: associations with fungal DNA and mycotoxins in classroom dust. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0147996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M-T, Wu C-C, Ou C-Y, Chang J-C, Liu C-A, Wang C-L, et al. A prospective birth cohort study of different risk factors for development of allergic diseases in offspring of non-atopic parents. Oncotarget. 2017;8:10858–10870. doi: 10.18632/oncotarget.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao TC, Ou LS, Yeh KW, Lee WI, Chen LC, Huang JL. Associations of age, gender, and BMI with prevalence of allergic diseases in children: PATCH study. J Asthma. 2011;48:503–510. doi: 10.3109/02770903.2011.576743. [DOI] [PubMed] [Google Scholar]
- 44.Kilpeläinen M, Terho EO, Helenius H, Koskenvuo M. Home dampness, current allergic diseases, and respiratory infections among young adults. Thorax. 2001;56:462–467. doi: 10.1136/thorax.56.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YL, Shaw CK, Su HJ, Lai JS, Ko YC, Huang SL, et al. Climate, traffic-related air pollutants and allergic rhinitis prevalence in middle-school children in Taiwan. Eur Respir J. 2003;21:964–970. doi: 10.1183/09031936.03.00094602. [DOI] [PubMed] [Google Scholar]
- 46.Duggan EM, Sturley J, Fitzgerald AP, Perry IJ, Hourihane JOB. The 2002-2007 trends of prevalence of asthma, allergic rhinitis and eczema in Irish schoolchildren. Pediatr Allergy Immunol. 2012;23:464–471. doi: 10.1111/j.1399-3038.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- 47.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills T a. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 48.Dold S, Wjst M, von Mutius E, Reitmeir P, Stiepel E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch Dis Child. 1992;67:1018–1022. doi: 10.1136/adc.67.8.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(1 SUPPL):S2–S8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 50.Helaskoski E, Suojalehto H, Virtanen H, Airaksinen L, Kuuliala O, Aalto-Korte K, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46–52. doi: 10.1016/j.anai.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–279. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl 1):S2–10. doi: 10.1136/thorax.55.suppl_1.S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of countries and dependent territories used in the literature review search. (PDF 322 kb)