Abstract
Background
Sirtuin 2 (SIRT2) is a member of the sirtuin family, nicotinamide adenine dinucleotide+-dependent deacylases, which participates in modulation of cell cycle control, neurodegeneration, and tumorigenesis. SIRT2 expression increases in acute myeloid leukemia blasts. Downregulation of SIRT2 using siRNA causes apoptosis of HeLa cells. Therefore, selective inhibitors of SIRT2 are candidate therapeutic agents for cancer. Adult T-cell leukemia/lymphoma (ATL) is a T-cell malignancy that has a poor prognosis and develops after long-term infection with human T-cell leukemia virus (HTLV)-1. Sirtuin 1 inhibition has been shown to induce apoptosis and autophagy in HTLV-1-infected cell lines, whereas the effects of SIRT2 inhibition alone have not been elucidated.
Methods
We assessed the efficacy of our small molecule selective SIRT2 inhibitors NCO-90/141 to induce leukemic cell death. Cell viability was examined using the cell proliferation reagent Cell Count Reagent SF. Apoptotic cells were detected by annexin V-FITC and terminal deoxynucleotidyl transferase dUTP nick end labeling assays by flow cytometry. Caspase activity was detected using an APOPCYTO Intracellular Caspase Activity Detection Kit. The presence of autophagic vacuoles was assessed using a Cyto-ID Autophagy Detection Kit.
Results
Our novel small molecule SIRT2-specific inhibitors NCO-90/141 inhibited cell growth of leukemic cell lines including HTLV-1-transformed T-cells. NCO-90/141 induced apoptosis via caspase activation and mitochondrial superoxide generation in leukemic cell lines. However, a caspase inhibitor did not prevent this caspase-associated cell death. Interestingly, NCO-90/141 increased the LC3-II level together with autophagosome accumulation, indicating autophagic cell death. Thus, NCO-90/141 simultaneously caused apoptosis and autophagy.
Conclusions
These results suggest that NCO-90/141 are highly effective against leukemic cells in caspase-dependent or -independent manners via autophagy, and they may have a novel therapeutic potential for treatment of leukemias including ATL.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4710-1) contains supplementary material, which is available to authorized users.
Keywords: Human T-cell leukemia virus-1, Adult T-cell leukemia/lymphoma, SIRT2, Apoptosis, Caspase-independent cell death
Background
Sirtuins (SIRT1–7) are nicotinamide adenine dinucleotide+-dependent deacylases or mono-[ADP-ribosyl] transferases that display diverse subcellular localizations and functions [1–3]. SIRT2 has an essential role in maintaining the integrity of mitosis and has been proposed to act as a tumor suppressor by preventing chromosomal instability during mitosis [4]. However, tumors that express high levels of SIRT2 are resistant to chemotherapy, specifically microtubule toxins [5]. SIRT2 mRNA levels are significantly elevated in acute myeloid leukemia (AML) blasts compared with those in bone marrow from healthy individuals [6]. High expression of SIRT2 is also an unfavorable prognostic biomarker for AML risk stratification [7]. A recent study has shown that pharmacological inhibition of both SIRT1 and SIRT2 reduces cell viability by apoptosis in adult T-cell leukemia/lymphoma (ATL) cells and delays tumor growth through p53 activation in melanoma [8, 9].
ATL is a T-cell malignancy derived from mature CD4+ T-cells and has a poor prognosis, which develops after long-term infection with human T-cell leukemia virus (HTLV)-1 [10–12]. Although the underlying mechanisms of ATL development have not been fully elucidated, genetic and epigenetic abnormalities have been implicated [13–16]. There are four subtypes of ATL, including acute, lymphoma, chronic, and smoldering [17]. Despite recent advances in chemotherapy, allogeneic hematopoietic stem cell transplantation, and antibody therapy, the prognoses of patients with acute lymphoma types are still unsatisfactory [18–21]. Therefore, there is a clear need for new molecular targets for the development of treatments for ATL.
We previously reported that NCO-01 and NCO-04 inhibit both SIRT1 and SIRT2 activities in enzyme assays and induce apoptotic cell death [8, 22]. SIRT1 and SIRT2 inhibition has been shown to induce apoptosis and autophagy, whereas the effects of SIRT2 inhibition alone have not been elucidated. In this study, we assessed the efficacy of our small molecule selective SIRT2 inhibitors NCO-90/141 to induce leukemic cell death. We found that NCO-90/141 induced apoptotic cell death by caspase activation in leukemic cell lines and induced caspase-independent cell death (CICD) by autophagosome accumulation and autophagy. This is the first evidence demonstrating the cell growth-inhibiting effect of SIRT2-specific inhibitors via caspase-dependent or -independent cell death such as autophagy in leukemic cells.
Methods
Cell lines
Cell lines S1T (HTLV-1-infected CD4+ T-cell line derived from an ATL patient; kindly provided by Dr. Naomichi Arima, Kagoshima University), [23] MT-2 (HTLV-1-infected T-cell line derived from normal human leukocytes transformed by leukemic T-cells from an ATL patient) purchased from Japanese Cancer Research Resources Bank (Osaka, Japan; catalogue number: JCRB1210), [24] Jurkat (T-lineage acute lymphoblastic leukemia cell line) purchased from RIKEN BioResource center (BRC) (Ibaraki, Japan; catalogue number: RBRC-RCB3053), and HL60 (acute myeloid leukemia cell line) purchased from RIKEN BRC (catalogue number: RBRC-RCB0041) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 0.1 mg/mL streptomycin, and 100 U/mL penicillin [8].
Reagents
The novel SIRT2 inhibitors evaluated in this study, NCO-90 and NCO-141, have been described in a previous report [25]. Chemical structures of NCO-90 and NCO-141 are shown in Fig. 1. Molecular weights of NCO-90 and NCO-141 are 332.40 and 350.39, respectively. Caspase inhibitor Z-VAD-FMK and anti-Fas monoclonal antibody CH11 were purchased from Medical and Biological Laboratories (MBL) (Nagoya, Japan). STF-62247, an autophagy inducer, was purchased from Calbiochem (Darmstadt, Germany). Bafilomycin A1, a specific inhibitor of vacuolar proton ATPase, was purchased from Adipogen (Épalinges, Switzerland).
Fig. 1.
Chemical structures of NCO-90/141
We purchased primary antibodies against β-actin, histone H3, p53, acetyl-p53K382 (ac-p53), and SIRT2 from Cell Signaling Technology (Beverly, CA), a primary antibody against acetyl-histone H4K16 (ac-H4) from Abcam (Cambridge, UK), and a primary antibody against LC3 from MBL. Horseradish peroxidase-conjugated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA).
Protein extraction and western blot analysis
Cell lysates were obtained using RIPA Lysis Buffer RIPA Lysis Buffer (Santa Cruz Biotechnology, Dallas, TX). Nuclear extracts were obtained using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL), according to the manufacturer’s protocol. Western blotting was performed as described previously [26]. Images were obtained and analyzed using a ChemiDoc™ XRS (Bio-Rad, Hercules, CA).
Cell viability assay
Cell viability was examined using the cell proliferation reagent Cell Count Reagent SF (Nacalai Tesque) as described previously [8]. The half maximal inhibitory concentration for cell growth (GI50) was calculated using Grafit (Erithacus Software, Horley, UK).
Apoptosis analysis
Apoptotic cells were stained with annexin V-FITC (MBL) and 7-amino-actinomycin D (Beckman Coulter, Brea, CA), and analyzed by flow cytometry using a Cell Analyzer EC800 (Sony, Tokyo, Japan) as described previously [27, 28]. The percentages of specific apoptotic cells were calculated as follows: % specific apoptotic cells = (annexin V-positive cells − spontaneous annexin V-positive cells)/(100 − spontaneous annexin V-positive cells) × 100.
DNA fragmentation was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays using a MEBSTAIN Apoptosis Kit Direct (MBL) [8].
Mitochondrial superoxide generation assay
Measurements of mitochondrial superoxide generation were performed using MitoSOX Red (Molecular Probes, Invitrogen) as described previously [8].
Detection of caspase activity
Caspase activity was detected using an APOPCYTO Intracellular Caspase-3 or − 8 Activity Detection Kit (MBL) and CaspGLOW Fluorescein Active Caspase-9 Staining Kit (BioVision, Milpitas, CA) as described previously [8].
Analysis of autophagy by flow cytometry
The presence of autophagic vacuoles was assessed using a Cyto-ID Autophagy Detection Kit (Enzo Life Sciences, Farmingdale, NY), according to the manufacturer’s instructions [29, 30]. Autophagy analysis was performed by incubating cells with bafilomycin A1 for 30 min at 37 °C prior to treatment with CYTO-ID Green Detection Reagent, and then analyzing fluorescence by flow cytometry using the Cell Analyzer EC800 as described previously [8]. Autophagy was also evaluated using the FlowCellect™ Autophagy LC-3 Antibody-based Assay kit (Merck Millipore) to monitor lapidated LC-3-II as described previously [8]. Quantification of anti-LC3-FITC fluorescence was performed by pretreatment with a lysosomal inhibitor for 30 min prior to treatment of the 48-h NCO-90/141-treated sample to prevent lysosomal degradation of LC3.
Statistical analysis
Data are expressed as means ± SD. For data analyses, two-tailed Student’s t-tests and Wilcoxon matched-pairs tests were performed using Excel 2010 (Microsoft Japan, Tokyo, Japan) and Statcel2 software (OMS Publishing Inc., Tokyo, Japan). In all tests, values of P < 0.05 were considered as statistically significant.
Results
NCO-90/141 inhibit the cell growth of leukemic cell lines
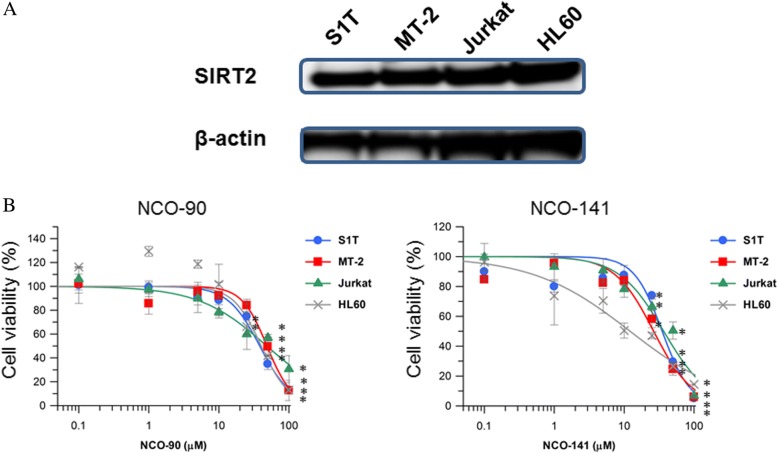
We examined whether NCO-90/141 affected the cell growth of S1T, MT-2, Jurkat, and HL60 cells that express SIRT2 protein (Fig. 2). Our small molecule selective SIRT2 inhibitors showed potent activities with IC50 values of 1.0 and 0.5 μM for NCO-90 and NCO-141, respectively, whereas NCO-90/141 did not inhibit SIRT1 at up to 300 μM. NCO-90/141 inhibited the growth of all four cell lines in a dose-dependent manner. NCO-90 showed potent activities with GI50 values of 38.3, 48.5, 48.2, and 40.2 μM in S1T, MT-2, Jurkat, and HL60 cells, respectively. NCO-141 showed potent activities with GI50 values of 34.9, 26.4, 36.7, and 12.1 μM in S1T, MT-2, Jurkat, and HL60 cells, respectively. In contrast, commercially available SIRT2 inhibitor AKG2 had no GI50 values in leukemic cell lines (Additional file 1: Figure S1). AKG2 inhibited SIRT1 and SIRT2 with IC50 values of 30 and 3.5 μM, respectively.
Fig. 2.
NCO-90/141 reduce cell viability in leukemic cell lines. a SIRT2 protein in leukemic cell lines was analyzed by western blotting. b Cell lines were incubated at 2 × 105 cells/mL in the presence of various concentrations of NCO-90 and NCO-141 for 72 h. The viabilities of the cultured cells were measured by Cell Count Reagent SF. Cells cultured in the absence of each SIRT2 inhibitor were assigned a relative viability of 1. Data represent mean percentages ± SD of three independent experiments. *P < 0.05 vs. 0 μM
NCO-90/141 induce apoptosis by caspase activation and mitochondrial superoxide generation in leukemic cell lines
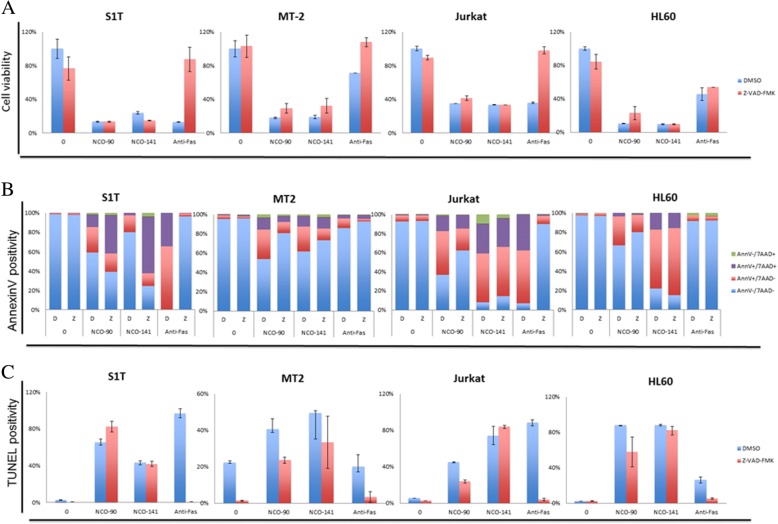
To examine whether NCO-90/141 induce apoptosis, we analyzed NCO-90/141-induced cell death by annexin V staining (Fig. 3). We observed that NCO-90/141 induced annexin V-positive cells in leukemic cell lines. The percentages of specific annexin V-positive cells induced by NCO-90 (100 μM) were 52.5%, 34.7%, 42.3%, and 66.5% in S1T, MT-2, Jurkat, and HL60 cell lines, respectively. The percentages of specific annexin V-positive cells induced by NCO-141 (100 μM) were 85.3%, 39.0%, 85.9%, and 86.7% in S1T, MT-2, Jurkat, and HL60 cell lines, respectively. NCO-90/141 induced the early phase of apoptosis (annexin V+/7-amino-actinomycin D−; Fig. 5b). We also observed that NCO-90/141 induced DNA fragmentation and caspase activation in the leukemic cell lines (Figs. 5c and 6).
Fig. 3.
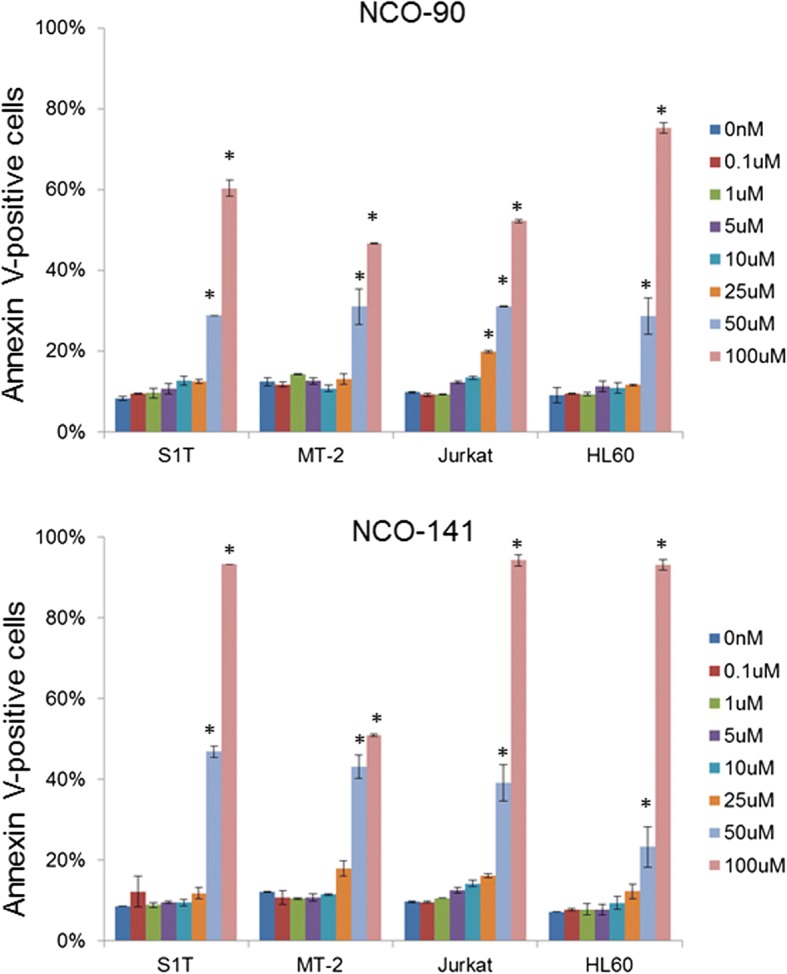
NCO-90/141 induce annexin V-positive cells in leukemic cell lines. Cell lines were incubated at 2 × 105 cells/mL in the presence of various concentrations of NCO-90 and NCO-141 for 72 h. Annexin V-positive cells were detected by flow cytometry. Data represent mean percentages ± SD of apoptotic cells in three independent experiments. *P < 0.05 vs. 0 μM
Fig. 5.
NCO-90/141 induce caspase-independent cell death. S1T, MT-2, Jurkat, and HL60 cells were treated with NCO-90 (50 μM) or NCO-141 (S1T and MT-2 cells, 50 μM; Jurkat and HL-60 cells, 100 μM) and Z-VAD-FMK (40 μM) for 72 h. a Viability of cultured cells was measured by Cell Count Reagent SF. b and c Annexin V-, 7-amino-actinomycin D (7AAD)-, and TUNEL-positive cells were detected by flow cytometry. Data represent mean percentages ± SD of three independent experiments
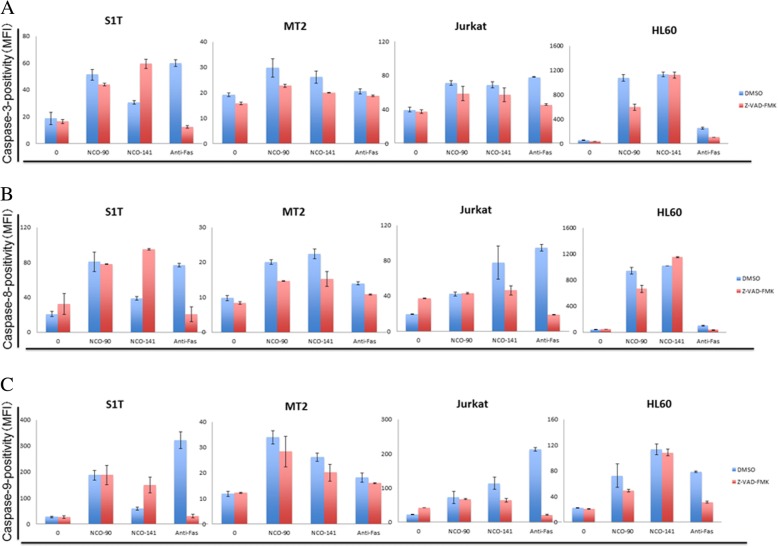
Fig. 6.
NCO-90/141 induce both caspase-dependent and -independent cell death with caspase activities. S1T, MT-2, Jurkat, and HL60 cells were treated with NCO-90 (50 μM) or NCO-141 (S1T and MT-2 cells, 50 μM; Jurkat and HL-60 cells, 100 μM) and Z-VAD-FMK (40 μM) for 72 h. a–c Caspase-positive cells were detected by flow cytometry. Data represent mean percentages ± SD of three independent experiments
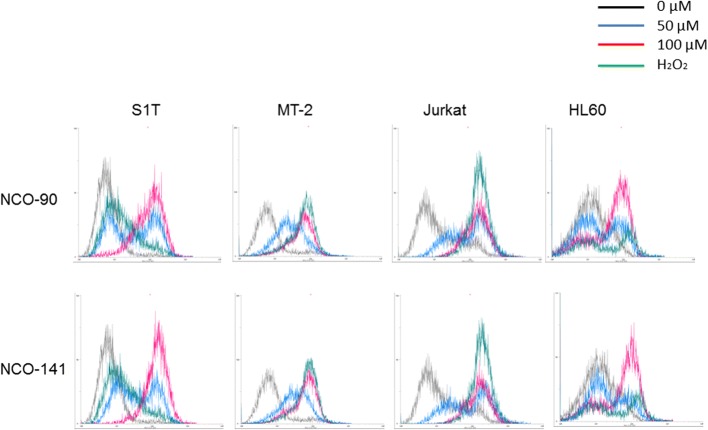
During apoptosis, mitochondria play key roles including the release of caspase activators such as cytochrome c following loss of mitochondrial transmembrane potential [31]. In addition to this essential role of mitochondria during the execution phase of apoptosis, it appears that reactive oxygen species produced by mitochondria are involved in cell death [32]. MitoSOX Red detects superoxides in mitochondria of live cells. The positive charge on the phosphonium group in MitoSOX Red selectively targets this cell-permeable hydroethidine derivative to mitochondria where it accumulates as a function of mitochondrial membrane potential and exhibits fluorescence upon oxidation and subsequent binding to mitochondrial DNA. By measuring the shift in fluorescence emission by flow cytometry, mitochondrial superoxide generation was detected in NCO-90/141-treated cells (Fig. 4).
Fig. 4.
NCO-90/141 induce mitochondrial superoxide generation in leukemic cell lines. S1T, MT-2, Jurkat, and HL60 cells were treated with NCO-90 or NCO-141 for 6 h and then analyzed using MitoSOX Red by flow cytometry
NCO-90/141 induce both caspase-dependent and -independent cell death
These data suggested that NCO-90/141 might induce apoptosis via a caspase-dependent pathway. To test this, we assessed the effects of a pan-caspase inhibitor, Z-VAD-FMK, on NCO-90/141-induced cell death (Figs. 5 and 6). NCO-90/141 induced significant growth inhibition with annexin V-positive cells and DNA fragmentation in leukemic cell lines. NCO-90/141 also activated caspase activity (caspase-3, − 8, and − 9). Z-VAD-FMK was able to suppress anti-Fas antibody-induced cell death as a positive control. However, Z-VAD-FMK, which inhibits caspase-1, − 3, − 4, − 7, and − 8, did not suppress the cell death, annexin V-positive cells, or DNA fragmentation (Fig. 6a–c). Z-VAD-FMK also did not inhibit caspase-3, − 8, or − 9 activities (Fig. 6a–c).
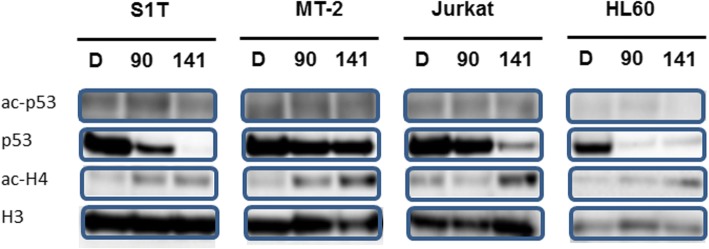
An early increase in reactive oxygen species has been found to precede mitochondrial membrane permeabilization, and in some case to be independent of caspases in various models including apoptosis induced by p53 [32]. Acetylation of p53 induced by cellular stress stimulates the DNA-binding capacity of p53 and enhances its biological functions. NCO-90/141 increased levels of acetylated H4, which is a SIRT2 substrate, but did not increase acetylated p53 (Fig. 7). In contrast, degradation of p53 in the nucleus was increased by NCO-90/141.
Fig. 7.
NCO-90/141 induce p53 degradation. S1T, MT-2 Jurkat, and HL60 cells were treated with NCO-90 or NCO-141 for 72 h. Protein levels in the nucleus were detected by western blotting with the indicated antibodies
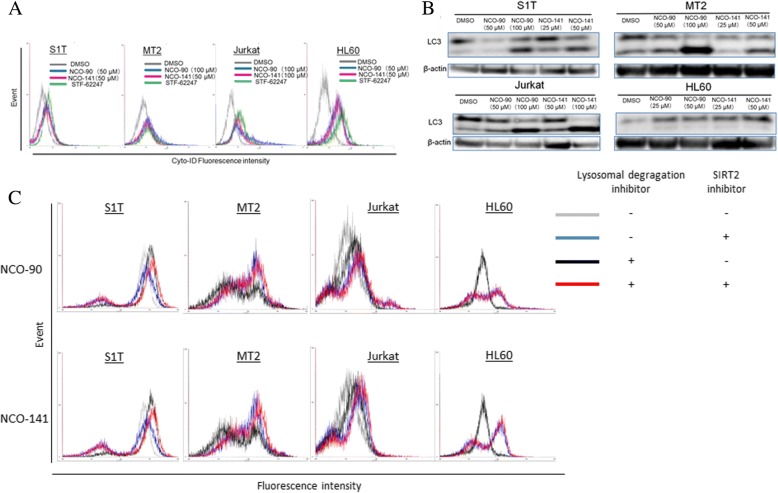
NCO-90/141 induce autophagy in leukemic cell lines
Some mitochondrial proteins, such as apoptosis-inducing factor, which are released as a result of mitochondrial outer membrane permeabilization, promote CICD [33]. The incidence of CICD concomitant with increased autophagic activity may be indicative of autophagic type II cell death [34]. Autophagy degrades cellular components, so that cells eventually activate their apoptosis machinery [35]. Autophagy was detected using a Cyto-ID Autophagy Detection Kit [29]. Autophagy levels were increased in the presence of NCO-90/141 (Fig. 8a). A shift of the soluble form LC3-I to the autophagic vesicle-associated form LC3-II is a specific marker for autophagosome promotion. NCO-90/141 significantly increased the levels of LC3-II (lapidated LC3; Fig. 8b). Thus, NCO-90/141 increased autophagosome accumulation and autophagy in leukemic cell lines. Next, we confirmed whether NCO-90/141 increased activation of autophagic flux or inhibited autophagosome degradation (Fig. 8c). The shift of the LC-3 level was observed after NCO-90/141 treatment together with the lysosome inhibitor (red) in comparison with no treatments (black). NCO-90/141 induced LC3 translocation. However, there was a shift of the LC-3 level by treatment with NCO-90/141 in the absence of the lysosome inhibitor (blue) in comparison with no NCO-90/141 treatment (gray). Thus, NCO-90/141 also inhibited autophagosome degradation.
Fig. 8.
NCO-90/141 induce autophagy. S1T, MT-2, and Jurkat cells were treated with NCO-90/141 and STF-62247 (50 μM), an autophagy inducer, for 48 h. a Autophagy was measured by quantifying the mean fluorescence intensity using a Cyto-ID Autophagy Detection Kit. b Protein levels were detected by western blotting with the indicated antibodies. c Cellular autophagic flux monitored by lapidated LC3-II was evaluated using the FlowCellect™ Autophagy LC3 Antibody-based Assay Kit. S1T, MT-2 Jurkat, and HL60 cells were treated with 50 μM NCO-90 (upper) or NCO-141 (lower) for 24 h
Discussion
SIRT2, a nicotinamide adenine dinucleotide+-dependent deacetylase, has been proposed to be a tumor suppressor associated with aging, the cell cycle, and carcinogenesis [36]. SIRT2 knockout mice develop cancers in multiple organs via aurora-A and -B that direct centrosome amplification, aneuploidy, and mitotic cell death [4]. However, SIRT2 mRNA levels are significantly elevated in AML blasts [6]. SIRT2-overexpressing cells also exhibit prolongation of the cell cycle [37]. SIRT2 activity in glioma cells is required for survival [38]. Furthermore, SIRT2 downregulation using siRNA causes apoptosis of HeLa cells [39]. Therefore, SIRT2 inhibitors are emerging as antitumor drugs [40]. Here, SIRT2 protein expression in leukemic cell lines indicated that SIRT2 is a target for treatment of leukemia (Fig. 2). We have developed specific SIRT2 inhibitors NCO-90 and NCO-141 [22]. NCO-90/141 inhibited cell growth of leukemic cell lines including HTLV-1-transformed T cells by apoptosis and CICD (Figs. 2, 3, 4, 5 and 6). Selective SIRT2 inhibitors induce cell death in non-small cell lung cancer and breast cancer cell lines [41, 42]. Thus, SIRT2 inhibitors are promising lead candidates for use in cancer treatments. However, studies of SIRT2 functions in cancers have obtained contradictory results, indicating that further studies will be required to estimate the therapeutic potential of targeting SIRT2 in cancer [1, 36, 43].
In this study, a caspase inhibitor did not prevent NCO-90/141-induced cell death, indicating that NCO-90/141 induced CICD (Figs. 5 and 6). Under the conditions of CICD, glyceraldehyde-3-phosphate dehydrogenase in glycolysis participates in transcriptional upregulation of ATG12 and enhances autophagy [34]. Furthermore, caspase inhibition often leads to autophagic cell death when caspase inhibition does not inhibit cell death [33]. Autophagy is the regulated and destructive mechanism by which long-lived proteins, organelles, and protein aggregates are captured within autophagosomes [44–46]. Here, we found that NCO-90/141 inhibited the growth of leukemic cell lines and increased LC3-II levels by mitochondrial superoxide generation and caspase activation (Figs. 4 and 8). Autophagy caused by NCO-90/141 may induce degradation of p53. SIRT2 interferes with autophagy-mediated degradation of protein aggregates in neuronal cells under proteasome inhibition [47]. SIRT2 knockdown also increases basal autophagy [48]. Mitochondrial outer membrane permeabilization triggers the removal of permeabilized mitochondria by the autophagic machinery [49]. Thus, SIRT2 inhibition induces autophagy by mitochondrial superoxide generation. Furthermore, NCO-90/141-induced cell death was not inhibited in combination with bafilomycin A, an autophagy flux inhibitor, for 72 h (data not shown). Therefore, these results suggest that the molecules involved in caspase-independent DNA fragmentation may augment caspase activity, and secondary caspase activation and autophagy induced by NCO-90/141 may be the result, but not the cause, of cell death.
Conclusions
In the present study, the novel SIRT2-specific inhibitors NCO-90/141 induced caspase-dependent cell death such as apoptosis or CICD such as autophagic cell death in leukemic cell lines. Although apoptosis and autophagy share many common mechanisms, current knowledge of the molecular interactions between autophagic and apoptotic pathways is incomplete and fragmented. Therefore, it may be necessary to further elucidate the relationship between apoptosis and autophagy following NCO-90/141 treatment in primary leukemic cells.
Additional file
Figure S1. AGK2 does not reduce cell viability of leukemic cell lines. Cell lines were incubated at 2 × 105 cells/mL in the presence of various concentrations of AGK2 for 48 h. The viabilities of the cultured cells were measured by Cell Count Reagent SF. Cells cultured in the absence of AGK2 were assigned a relative viability of 1. Data represent mean percentages ± SD of three independent experiments. (TIF 532 kb)
Acknowledgments
We thank Dr. Yosuke Ota, Dr. Yukihiro Itho, Ms. Nanako Ohtsubo, Ms. Kana Imaishi, Ms. Risako Oka, Ms. Naho Kato, Ms. Kaoru Takeuchi, and Ms. Nanae Yamashaki for technical assistance. We also thank Mitchell Arico from Edanz Group (http://www.edanzediting.com) for editing a draft of this manuscript.
Funding
This work was supported by KAKENHI (Grant-in-Aid for Scientific Research C, Grant No. 16 K09863 to T.K.) and the Central Research Institute of Fukuoka University (Grant No. 177107 to T.K.). The funding agencies had no role in the design of the study, collection, analysis, and interpretation of data, or writing the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article in the form of graphs. The raw data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AML
Acute myeloid leukemia
- ATL
Adult T-cell leukemia/lymphoma
- CICD
Caspase-independent cell death
- HTLV
Human T-cell leukemia virus
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Authors’ contributions
TK designed and performed the experiments, analyzed the data, and wrote the manuscript. PM and TS supervised the project and produced NCO-90 and NCO-141. TO, AA, YU, and SH performed experiments and provided advice. All authors read and approved the final version of the manuscript.
This article does not contain any experiments with human participants or animal performed by any of the authors.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tomohiro Kozako, Phone: +81-92-871-6631, Email: kozako@fukuoka-u.ac.jp.
Paolo Mellini, Email: paolo.mellini@uniroma1.it.
Takeo Ohsugi, Email: ohsugi@rakuno.ac.jp.
Akiyoshi Aikawa, Email: aikawa@fukuoka-u.ac.jp.
Yu-ichiro Uchida, Email: k7288319@kadai.jp.
Shin-ichiro Honda, Email: sihonda@fukuoka-u.ac.jp.
Takayoshi Suzuki, Email: suzukit@koto.kpu-m.ac.jp.
References
- 1.Gomes P, Outeiro TF, Cavadas C. Emerging role of Sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci. 2015;36(11):756–768. doi: 10.1016/j.tips.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97(11):5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99(7):735–745. doi: 10.1016/S0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20(4):487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita N, Takami Y, Kimura M, Tachiiri S, Ishiai M, Nakayama T, Takata M. Role of NAD-dependent deacetylases SIRT1 and SIRT2 in radiation and cisplatin-induced cell death in vertebrate cells. Genes Cells. 2005;10(4):321–332. doi: 10.1111/j.1365-2443.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Li Y, Chen L, Wang C, Wang Q, Zhang H, Lin Y, Li Q, Pang T. SIRT2 mediates multidrug resistance in acute myelogenous leukemia cells via ERK1/2 signaling pathway. Int J Oncol. 2016;48(2):613–623. doi: 10.3892/ijo.2015.3275. [DOI] [PubMed] [Google Scholar]
- 7.Deng A, Ning Q, Zhou L, Liang Y. SIRT2 is an unfavorable prognostic biomarker in patients with acute myeloid leukemia. Sci Rep. 2016;6:27694. doi: 10.1038/srep27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozako T, Suzuki T, Yoshimitsu M, Uchida Y, Kuroki A, Aikawa A, Honda S, Arima N, Soeda S. Novel small-molecule SIRT1 inhibitors induce cell death in adult T-cell leukaemia cells. Sci Rep. 2015;5:11345. doi: 10.1038/srep11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks CL, Gu W. p53 activation: a case against sir. Cancer Cell. 2008;13(5):377–378. doi: 10.1016/j.ccr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchiyama T: Human T cell leukemia virus type I (HTLV-I) and human diseases. In: Annu Rev Immunol. vol. 15, 1997/01/01 edn; 1997: 15–37. [DOI] [PubMed]
- 11.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7(4):266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- 13.Kozako T, Arima N, Toji S, Masamoto I, Akimoto M, Hamada H, Che XF, Fujiwara H, Matsushita K, Tokunaga M, et al. Reduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patients. J Immunol. 2006;177(8):5718–5726. doi: 10.4049/jimmunol.177.8.5718. [DOI] [PubMed] [Google Scholar]
- 14.Kozako T, Yoshimitsu M, Fujiwara H, Masamoto I, Horai S, White Y, Akimoto M, Suzuki S, Matsushita K, Uozumi K, et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia. 2009;23(2):375–382. doi: 10.1038/leu.2008.272. [DOI] [PubMed] [Google Scholar]
- 15.Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24(39):6047–6057. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 17.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the lymphoma study group (1984-87) Br J Haematol. 1991;79(3):428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 18.Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, Saburi Y, Miyahara M, Sueoka E, Uike N, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 19.Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, Ikeda S, Masuda M, Nagoshi H, Ueda R, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan clinical oncology group study JCOG9801. J Clin Oncol. 2007;25(34):5458–5464. doi: 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Tomonaga M, Fukuda H, Hanada S, Utsunomiya A, Tara M, Sano M, Ikeda S, Takatsuki K, Kozuru M, et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan clinical oncology group study 9303. Br J Haematol. 2001;113(2):375–382. doi: 10.1046/j.1365-2141.2001.02737.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimitsu M, White Y, Arima N. Prevention of human T-cell lymphotropic virus type 1 infection and adult T-cell leukemia/lymphoma. Recent Results Cancer Res. 2014;193:211–225. doi: 10.1007/978-3-642-38965-8_12. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Imai K, Imai E, Iida S, Ueda R, Tsumoto H, Nakagawa H, Miyata N. Design, synthesis, enzyme inhibition, and tumor cell growth inhibition of 2-anilinobenzamide derivatives as SIRT1 inhibitors. Bioorg Med Chem. 2009;17(16):5900–5905. doi: 10.1016/j.bmc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Arima N, Arimura K, Tokito Y, Sakaki Y, Matsushita K, Orihara K, Akimoto M, Ozaki A, Kukita T, Hagiwara T, et al. HTLV-I tax protein inhibits apoptosis induction but not G1 arrest by pyrrolidinedithiocarbamate, an anti-oxidant, in adult T cell leukemia cells. Exp Hematol. 2004;32(2):195–201. doi: 10.1016/j.exphem.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Khan MN, Sawada H, Imai E, Itoh Y, Yamatsuta K, Tokuda N, Takeuchi J, Seko T, Nakagawa H, et al. Design, synthesis, and biological activity of a novel series of human sirtuin-2-selective inhibitors. J Med Chem. 2012;55(12):5760–5773. doi: 10.1021/jm3002108. [DOI] [PubMed] [Google Scholar]
- 26.Kozako T, Aikawa A, Shoji T, Fujimoto T, Yoshimitsu M, Shirasawa S, Tanaka H, Honda S, Shimeno H, Arima N, et al. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int J Cancer. 2012;131(9):2044–2055. doi: 10.1002/ijc.27481. [DOI] [PubMed] [Google Scholar]
- 27.Kozako T, Fukada K, Hirata S, White Y, Harao M, Nishimura Y, Kino Y, Soeda S, Shimeno H, Lemonnier F, et al. Efficient induction of human T-cell leukemia virus-1-specific CTL by chimeric particle without adjuvant as a prophylactic for adult T-cell leukemia. Mol Immunol. 2009;47(2–3):606–613. doi: 10.1016/j.molimm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Kozako T, Hirata S, Shimizu Y, Satoh Y, Yoshimitsu M, White Y, Lemonnier F, Shimeno H, Soeda S, Arima N. Oligomannose-coated liposomes efficiently induce human T-cell leukemia virus-1-specific cytotoxic T lymphocytes without adjuvant. FEBS J. 2011;278(8):1358–1366. doi: 10.1111/j.1742-4658.2011.08055.x. [DOI] [PubMed] [Google Scholar]
- 29.Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, Kuksin D, Lin B, Qiu J. A novel image-based cytometry method for autophagy detection in living cells. Autophagy. 2012;8(9):1371–1382. doi: 10.4161/auto.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stankov MV, El Khatib M, Kumar Thakur B, Heitmann K, Panayotova-Dimitrova D, Schoening J, Bourquin JP, Schweitzer N, Leverkus M, Welte K, et al. Histone deacetylase inhibitors induce apoptosis in myeloid leukemia by suppressing autophagy. Leukemia. 2014;28(3):577–588. doi: 10.1038/leu.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 32.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84(2–3):131–141. doi: 10.1016/S0300-9084(02)01369-X. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11(7):725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 34.Tait SW, Green DR. Caspase-independent cell death: leaving the set without the final cut. Oncogene. 2008;27(50):6452–6461. doi: 10.1038/onc.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21(7):387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SH, Zhu Y, Ozden O, Kim HS, Jiang H, Deng CX, Gius D, Vassilopoulos A. SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis. Transl Cancer Res. 2012;1(1):15–21. [PMC free article] [PubMed] [Google Scholar]
- 37.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23(9):3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X, Nie H, Hong Y, Sheng C, Xia W, Ying W. SIRT2 activity is required for the survival of C6 glioma cells. Biochem Biophys Res Commun. 2012;417(1):468–472. doi: 10.1016/j.bbrc.2011.11.141. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Matsumori H, Nakayama Y, Osaki M, Kojima H, Kurimasa A, Ito H, Mori S, Katoh M, Oshimura M, et al. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells. 2011;16(1):34–45. doi: 10.1111/j.1365-2443.2010.01460.x. [DOI] [PubMed] [Google Scholar]
- 40.Kozako T, Suzuki T, Yoshimitsu M, Arima N, Honda SI, Soeda S. Anticancer agents targeted to Sirtuins. Molecules. 2014;19(12):20295–20313. doi: 10.3390/molecules191220295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann G, Breitenbucher F, Schuler M, Ehrenhofer-Murray AE. A novel sirtuin 2 (SIRT2) inhibitor with p53-dependent pro-apoptotic activity in non-small cell lung cancer. J Biol Chem. 2014;289(8):5208–5216. doi: 10.1074/jbc.M113.487736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah AA, Ito A, Nakata A, Yoshida M. Identification of a selective SIRT2 inhibitor and its anti-breast Cancer activity. Biol Pharm Bull. 2016;39(10):1739–1742. doi: 10.1248/bpb.b16-00520. [DOI] [PubMed] [Google Scholar]
- 43.McGlynn LM, Zino S, MacDonald AI, Curle J, Reilly JE, Mohammed ZM, McMillan DC, Mallon E, Payne AP, Edwards J, et al. SIRT2: tumour suppressor or tumour promoter in operable breast cancer? Eur J Cancer. 2014;50(2):290–301. doi: 10.1016/j.ejca.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 46.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gal J, Bang Y, Choi HJ. SIRT2 interferes with autophagy-mediated degradation of protein aggregates in neuronal cells under proteasome inhibition. Neurochem Int. 2012;61(7):992–1000. doi: 10.1016/j.neuint.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Inoue T, Nakayama Y, Li Y, Matsumori H, Takahashi H, Kojima H, Wanibuchi H, Katoh M, Oshimura M. SIRT2 knockdown increases basal autophagy and prevents postslippage death by abnormally prolonging the mitotic arrest that is induced by microtubule inhibitors. FEBS J. 2014;281(11):2623–2637. doi: 10.1111/febs.12810. [DOI] [PubMed] [Google Scholar]
- 49.Tait SW, Green DR. Mitochondria and cell death: outer membran permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. AGK2 does not reduce cell viability of leukemic cell lines. Cell lines were incubated at 2 × 105 cells/mL in the presence of various concentrations of AGK2 for 48 h. The viabilities of the cultured cells were measured by Cell Count Reagent SF. Cells cultured in the absence of AGK2 were assigned a relative viability of 1. Data represent mean percentages ± SD of three independent experiments. (TIF 532 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article in the form of graphs. The raw data used and/or analyzed during the current study are available from the corresponding author on reasonable request.