Abstract
Activity-based probes (ABPs) are reactive small molecules that covalently bind to active enzymes. When tagged with a fluorophore, ABPs serve as powerful tools to investigate enzymatic activity across a wide variety of applications. In this chapter, we provide detailed methods for using fluorescent ABPs to detect the activity of caspases during the onset of apoptosis in vitro. We describe how these probes can be used to biochemically profile caspase activity in vitro using fluorescent SDS-PAGE as well as their application to imaging protease activity in live animals and tissues.
Keywords: Caspases, Proteases, Apoptosis, Activity-based probes, Fluorescent SDS-PAGE, Imaging
1. Introduction
Apoptosis is a highly regulated form of cell death that is mediated by a family of cysteine proteases called caspases [1, 2]. The extrinsic cell death pathway is initiated at the cell surface through ligation of death receptors. This leads to the dimerization and activation of the initiator caspase-8. Alternatively, the intrinsic death pathway is stimulated in response to intracellular signals such as DNA damage or mitochondrial stress. This results in formation of the apoptosome and subsequent activation of caspase-9, another initiator caspase. Once active, caspase-8 and -9 can then cleave and activate the downstream executioner caspases (-3, -6, and -7), which are common to both pathways. These proteases can then hydrolyze peptide bonds after aspartic acid residues of numerous other proteins. These cleavages lead to inactivation of substrates and initiate signaling pathways that promote the controlled disassembly of the cell, and ultimately, death.
Because caspases are synthesized as inactive enzymes, measurement of their total expression using antibody-based methods does not always reflect their activity levels [3–6]. Further to this point, caspases are also subject to inhibition by IAPs (inhibitors of apoptosis) once activated. To study the function of proteases in normal cellular processes and disease states, tools are necessary to directly assess their activity.
Fluorogenic substrates are one type of tool that can be used to measure caspase activity [3]. These molecules contain a peptide sequence that is based on the preferred cleavage site of each caspase and a fluorophore. When an active caspase cleaves the substrate, a detectable shift in the fluorescent properties of the fluorophore occurs. Fluorescence emission therefore correlates with levels of active enzyme. Substrate-based probes are commercially available and are marketed as being specific for individual caspase family members. In reality, these probes are not truly specific given the overlap in specificity for multiple caspases and even other protease families. A particular substrate may be cleaved more efficiently by one caspase over the others; however, the others are likely able to cleave it as well. As a result, measuring the activity of an individual caspase is often difficult with this type of probe. Consequently, many reports in the literature that have used fluorogenic probes to ascribe functions to one caspase over another are difficult to interpret.
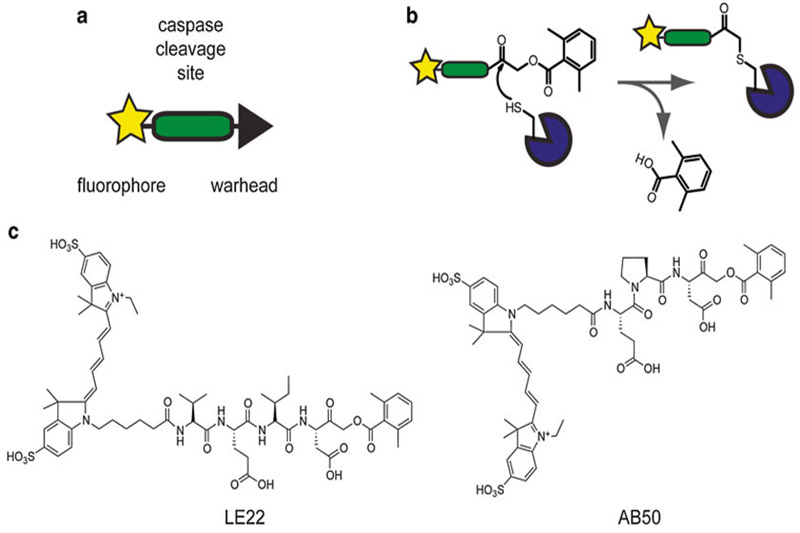
An alternative approach to measuring caspase activity utilizes activity-based probes (ABPs) [3, 5, 6]. These are small molecules that bind irreversibly to active caspases. Like the fluorogenic substrates, ABPs contain a peptide sequence based on the preferred cleavage site for caspases (including a P1 aspartate residue) and a fluorophore (see Fig. 1a, b). The key feature of an ABP, however, is a reactive functional group called a warhead that covalently modifies the enzyme in an activity-dependent manner. A common warhead for caspase probes (and other cysteine proteases) is the acyloxymethyl ketone, which binds selectively to the active-site cysteine residue.
Fig. 1.
Activity-based probes for caspases. (a) Schematic of the key features of an activity-based probe. (b) Mechanism of a cysteine protease binding to an ABP with an acyloxymethyl ketone warhead. (c) Structures of two fluorescent activity-based probes for caspases, AB50 and LE22. Figure adapted from Ref. 3
The binding of an ABP to the caspase is strong enough to survive the denaturing conditions of SDS-PAGE, and fluorescence emitted by the fluorophore can be detected by scanning the gel with a flatbed laser scanner. This unique feature of ABPs enables identification of the specific caspases that are active within a particular sample at a given time. ABPs can also be administered in vivo, allowing for in situ determination of caspase activity by imaging the accumulation of fluorescence in live mice or excised tissues. Probe-labeled caspases can then be analyzed biochemically by fluorescent SDS-PAGE, allowing for identification of the exact enzymes that lead to fluorescence accumulation in vivo.
Two examples of ABPs for caspases are AB50 and LE22 [7, 8]. Both these probes contain an acyloxymethyl ketone warhead and are tagged with a Cy5 fluorophore. They differ slightly in their peptide specificity region and their target caspases. AB50 contains a Glu-Pro-Asp sequence and binds to active caspase-3 and -7 (see Fig. 1c). The peptide sequence in LE22 is Val-Glu-Ile-Asp and it labels active caspase-6 in addition to -3 and -7 (see Fig. 1c). Both these probes are also able to label the initiator caspases-8 and -9; however, given that the levels of these enzymes are dramatically lower than the executioner caspases, they are not often detected in whole cell extracts. LE22 and AB50 suffer from slight cross-reactivity with another cysteine protease called legumain; however, this is not usually a problem since all probe-labeled targets can be tracked biochemically.
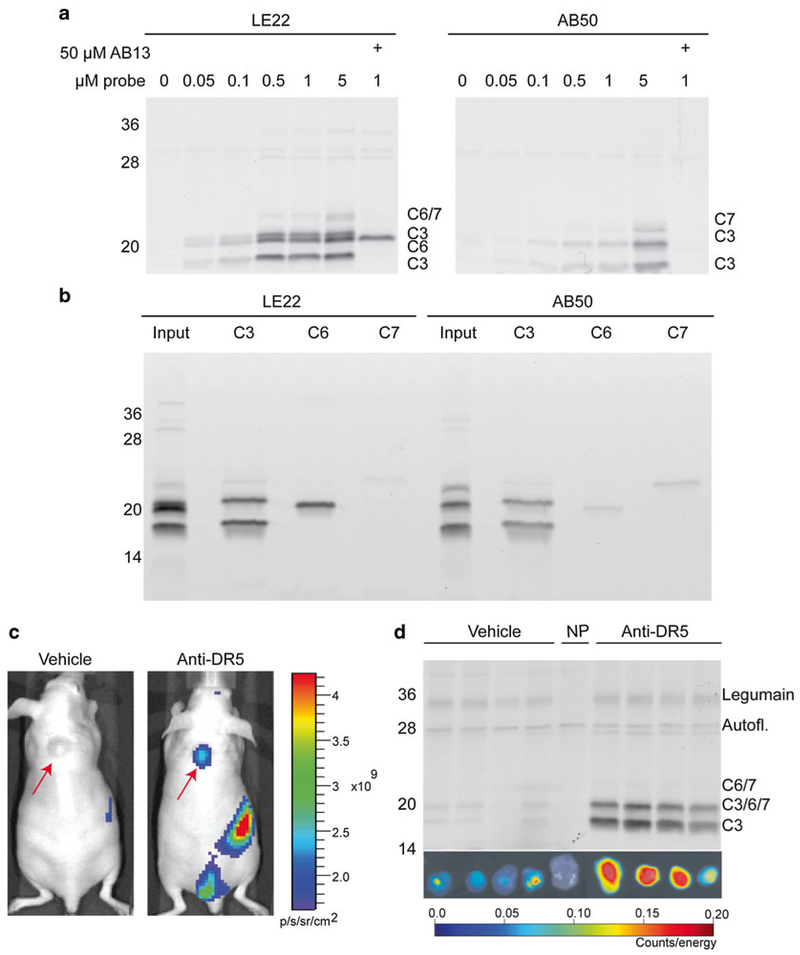
We previously utilized AB50 and LE22 to detect caspase activity in COLO205 colon cancer cells after inducing apoptosis with an anti-death receptor 5 antibody (anti-DR5) [7, 8]. Both probes labeled caspases in a dose-dependent manner (see Fig. 2a). As outlined above, their specificities were slightly different, with AB50 labeling active caspase-3/-7 and LE22 labeling -3/-6/-7. Band identity was confirmed by pretreatment with the caspase-3/-7-specific inhibitor AB13 (see Fig. 2a) and by immunoprecipitation with caspase-specific antibodies (see Fig. 2b). We also utilized these probes to image caspase activation in tumor-bearing mice treated with anti-DR5 chemotherapy. Upon induction of apoptosis, LE22 fluorescence accumulated in the tumor, as demonstrated by whole animal imaging (see Fig. 2c) and ex vivo imaging (see Fig. 2d). The increase in fluorescence between vehicle- and drug-treated mice corresponded with an increase in caspase labeling as shown by fluorescent SDS-PAGE analysis (see Fig. 2d).
Fig. 2.
Detection of caspase activity with ABPs in vitro and in vivo. (a) Dose curve of activity-dependent caspase labeling in apoptotic cells. COLO205 colon cancer cells were treated with anti-DR5 antibody for 4 h to induce apoptosis. For the last 30 min of treatment, the ABPs LE22 or AB50 were added at the indicated dose to detect caspase activity, followed by fluorescent SDS-PAGE. Where indicated, AB13, a caspase-3/-7-selective inhibitor was added prior to ABP addition. (b) Confirmation of the identity of the caspases labeled in (a) by immunoprecipitation with cleaved caspase antibodies. (c) In vivo detection of caspase activity. Nude mice bearing subcutaneous COLO205 tumors (red arrows) were treated with anti-DR5 antibody or vehicle for 11 h, followed by injection of the ABP LE22. After 1 h, mice were imaged for Cy5 fluorescence using an IVIS100 whole animal imaging machine. (d) Tumors were extracted from the mice in (c), imaged ex vivo, and then lysed for fluorescent SDS-PAGE analysis. Panels (a–d) reproduced with permission from Ref. 9
The following protocols describe the application of covalent ABPs to detect caspase activity in vitro and in vivo [9]. We indicate appropriate conditions for using LE22 and AB50 to detect caspase activity; however, these proteases can be adapted for any covalent ABP that targets any protease. Optimization of timing, dosage, and buffer conditions may be required.
2. Materials
2.1. Components for Labeling Caspases with Activity-Based Probes
Cells (chosen based on model).
6-well plates.
Cell scrapers.
Complete medium for cells (varies by cell type).
Caspase activation agent (typically an apoptosis stimulant; chosen based on model).
Cold Phosphate buffered saline (PBS).
Hypotonic Lysis Buffer: 50 mM PIPES, pH 7.4, 10 mM KCl, 5 mM MgCl2, 2 mM EDTA, 1 % NP-40, 4 mM DTT (add DTT fresh when ready to use). Store at 4 °C.
Bicinchoninic acid protein quantitation (BCA) kit or equivalent.
Fluorescent activity-based probe: 100× stock solution in dimethyl sulfoxide (DMSO). (This concentration will vary by probe. For AB50 and LE22, 100× = 100 μM.)
4× sample buffer: 40 % glycerol (v/v), 200 mM Tris-HCl, pH 6.8, 8 % SDS (w/v), 0.04 % bromophenol blue (w/v), 5 % beta-mercaptoethanol (v/v). Store aliquots at −20 °C or at room temperature for 1–2 weeks.
2.2. Components for Fluorescent SDS-PAGE
SDS-PAGE gel (15 % acrylamide is ideal for caspase separation).
Gel running apparatus.
Laemmli Running Buffer: 25 mM Tris base, 192 mM glycine, 0.1 % SDS. The pH should be ~8.3 with no adjustment required.
Fluorescent protein ladder (such as Precision Plus Protein™ Dual Color Standards from BioRad).
Flatbed laser scanner with appropriate filters, for example Typhoon from GE Healthcare or Odyssey from LiCOR.
2.3. Immunoprecipitation Components
Probe-labeled lysate.
Protein A/G agarose beads.
Caspase-specific antibodies.
Immunoprecipitation buffer (IP buffer): 1× PBS, pH 7.4, 0.5 % NP-40 (v/v), 1 mM EDTA. Store at room temperature for 1 year.
0.9 % sodium chloride in water.
4× sample buffer (see step 10 of Subheading 2.1).
SDS-PAGE components (see Subheading 2.2).
2.4. Components for In Vivo Detection of Caspases
Mice (chosen based on model).
Caspase activation agent (typically an apoptosis stimulant; chosen based on model).
Fluorescent activity-based probe, for example, AB50 or LE22.
Dimethyl sulfoxide (DMSO).
Phosphate buffered saline (PBS).
Isoflurane to anesthetize the mice.
Non-fluorescent (alfalfa-free) chow.
Beard trimmer.
Nair hair removal lotion or equivalent.
Insulin syringes.
Restrainer for tail vein injections.
Anesthesia vaporizer.
Fluorescent mouse imager such as the IVIS or FMT or BioFLECT or equivalent.
Dissection tools.
Muscle lysis buffer: 1×x PBS, pH 7.4, 1 % Triton X-100 (v/v), 0.1 % SDS (w,v), 0.5 % sodium deoxycholate (w,v). Store at 4 °C.
BCA kit or equivalent.
4× sample buffer (see step 10 of Subheading 2.1).
SDS-PAGE components (see Subheading 2.2).
3. Methods
3.1. Labeling of Active Caspases in Cell Lysates
This protocol describes the most basic assay for activity-based labeling of caspases in a complex proteome. Labeling cell lysates will provide a snapshot of the caspase activity at the time the cells are harvested. The timing and degree of caspase activation will depend on the method used to stimulate apoptosis. If this information is not readily available in the literature for the cell line used, a time course and/or dose curve may be performed to determine optimal conditions for caspase activation. This assay detects caspases at neutral pH, making it less likely to detect common off-targets of caspase ABPs such as legumain or cathepsins, which are active in the acidic conditions of the lysosome.
Seed cells in 6-well dishes at 80 % confluency (depending on the size of the cells, this will be about 1–1.5 × 106 cells).
The following day, refresh media and stimulate apoptosis/caspase activation by preferred method (see Notes 1 and 2).
Harvest the cells at the optimal time point. In cell death models, some of the cells may have already detached and will be floating in the media. Using cell scrapers or the broad end of a P200 pipette tip, dislodge the remaining cells.
Transfer the cells in media to a microfuge tube and spin using a tabletop centrifuge at 1000 × g for 1–2 min.
Aspirate media with care, so as not to disturb the cell pellet and then add 1 mL cold PBS to the cells. Pipette up and down 2–3 times to wash the pellet. Keep tubes on ice from this point to preserve caspase activity.
Centrifuge as in step 4 above and aspirate PBS. Then add 50 μL of hypotonic lysis buffer, resuspend pellet, and incubate on ice for 10 min to lyse the cells.
At this point, the sample may be snap frozen in liquid nitrogen and stored at −20 °C for later use, or be advanced to the next step.
Spin at 20,000 × g in a tabletop centrifuge at 4 °C for ~15 min and then transfer supernatant to a fresh microfuge tube.
Quantify total protein concentration using a BCA kit or equivalent (see Note 3).
Aliquot 50–100 μg lysate into a fresh tube, bringing the volume to 20 μl with hypotonic lysis buffer.
Add 0.2 μl of a 100× DMSO stock of the activity-based probe. For AB50 or LE22, use 100 μM stock solution (see Notes 4–6).
Incubate the sample at 37 °C. Labeling time may vary by ABP. For LE22 and AB50, 30 min is the optimal labeling time.
Add 6.7 μl 4× sample buffer to quench the reaction. Samples may be snap frozen in liquid nitrogen and stored at −20 °C or directly analyzed by SDS-PAGE (see Subheading 3.3).
3.2. Labeling of Active Caspases in Intact/Dying Cells
This protocol depends on the ability of the ABP to freely penetrate cells and enter the cytoplasm as in the case of caspases. AB50 and LE22 are both permeable. One of the major caveats of caspase probes is their varied propensity to label the lysosomal proteases (cathepsins and/or legumain) at acidic pH due to similarities in structure and mechanism of catalysis [7, 8, 10]. If the probe enters the cell by endocytosis or macropinocytosis, it may pass through the lysosome before reaching the cytosol, labeling these off-targets in addition to caspases.
Seed cells in 6-well dishes to 80 % confluence (depending on the size of the cells, this will be about 1–1.5 × 106 cells).
The following day, aspirate medium and add 1 mL fresh medium. Stimulate apoptosis/caspase activation by preferred method (see Note 7).
The time at which the probe is added to the cells will vary by experiment and probe. To obtain a more cumulative picture of the caspases that become activated over time, the probe can be added as soon as the apoptosis stimulus is added and incubated throughout the course of the experiment (see Note 8). To obtain a snapshot of the particular caspases that are active at a given time point after stimulation, add the probe during the desired window. To do so, add 1 μl of a 1000× DMSO stock solution of ABP directly to the media and mix well. (Use 1 μl of 1 mM stock to make a final concentration of 1 μM. The final DMSO concentration should be no more than 0.2 %.) (see Notes 9 and 10).
Harvest the cells at the optimal time point after probe addition (shorter incubation for snapshots, longer for cumulative pictures) by detaching them from the dish. This can be achieved using cell scrapers or the broad end of a P200 pipette tip. Collect the cells and media and centrifuge at 1000 × g for 1–2 min. Then carefully aspirate the media and wash once with cold PBS, making sure to resuspend the pellet. Remove the PBS.
Resuspend the pellet in 50 μl hypotonic lysis buffer and incubate on ice for ten minutes. At this point, the sample can be snap frozen in liquid nitrogen and stored at −20 °C, or the protocol may be continued.
Centrifuge at 20,000 × g in a bench top centrifuge at 4 °C for 15 min. Transfer the supernatant to a fresh microfuge tube. Reserve 5 μl for protein quantification by BCA or equivalent. To the remaining 45 μl add 15 μl 4× sample buffer. The sample may then be frozen at −20 °C, or boiled and loaded onto a gel according to Subheading 3.3.
3.3. Detection of Probe-Labeled Caspases by Fluorescent SDS-PAGE
This protocol enables detection of the caspases that are labeled with probe in Subheadings 3.1, 3.2, 3.4, and 3.5.
Prepare 15 % acrylamide gel according to standard protocols [11].
Boil protein samples at 95 °C for 5–10 min and load samples, along with a fluorescent protein ladder on the gel.
Run gel according to standard protocol [11]. Stop just after the dye front runs off (see Note 11).
To visualize the active caspases, scan the gel on a Typhoon or Odyssey scanner (or equivalent) at the wavelength appropriate for the fluorophore used (see Notes 12–16).
3.4. Immunoprecipitations of Probe-Labeled Lysates to Confirm ABP Targets
Once ABP-labeled species have been visualized by SDS-PAGE, it may be necessary to confirm their identity. This can usually be achieved by pre-treating samples with selective inhibitors to compete for probe labeling; however, in the case of caspases, truly selective inhibitors do not exist. They tend to react broadly with members of the caspase family as well as lysosomal cysteine proteases like legumain and cathepsins. For this reason, immunoprecipitation may be the ideal way to validate probe-labeled species. If enough material remains, the sample used for SDS-PAGE analysis may be used directly in the immunoprecipitation assay.
Prepare probe-labeled lysate according to Subheadings 3.1, 3.2 or 3.5.
After boiling in sample buffer, reserve 50 μg of probe-labeled protein lysate to load as an input sample for SDS PAGE analysis. Store at −20 °C.
For each immunoprecipitation, aliquot 100+ μg probe-labeled lysate into microfuge tubes (see Note 17).
Add 500 μl IP buffer and 5–10 μl antibody and incubate on ice for 10 min (see Note 18).
Aliquot a 40-μl slurry of protein A/G agarose beads to a new microfuge tube. Add 500 μl IP buffer to wash beads and centrifuge at high speed on a tabletop centrifuge for 30 s. Remove buffer, add 50 μl fresh IP buffer, and transfer beads to the tube containing the protein + antibody. Rock tube overnight at 4 °C.
The next morning, transfer supernatant to a 2-mL microfuge tube. To precipitate and concentrate the proteins in the supernatant, add enough acetone to fill the tube and freeze at −80 °C for at least 2 h. Then spin the tube at high speed at 4 °C for 15 min. Aspirate the acetone, taking great care not to disturb the protein pellet. Allow the pellet to dry completely before dissolving it in 30 μl of 1× sample buffer.
While the supernatant is precipitating, wash the agarose beads four times with IP buffer and then once with 0.9 % sodium chloride. After the last wash, remove all of the remaining supernatant (see Note 19). Then add 30 μl 2× sample buffer and boil the beads to elute the immunoprecipitated proteins.
Analyze Immunoprecipitation by SDS-PAGE as described in Subheading 3.3. Load the input sample first, followed by the pull down and the supernatant (see Notes 20 and 21).
3.5. In Vivo Detection of Caspase Activity
Protocol details for in vivo experiments will largely depend on the mouse model and probes used, but these are guidelines to follow that will help increase the success rate of noninvasive imaging experiments. Since light does not penetrate tissue particularly well, the most successful noninvasive imaging experiments will involve superficial tissues, for example, subcutaneous tumors. Deeper tissues may need to be excised and imaged ex vivo. Models involving at least a threefold induction of protease activity will have the highest chance of obtaining enough contrast for imaging. If whole animal fluorescence imaging is to be performed, optimal results will be obtained if mice are fed alfalfa-free chow for 4–7 days prior to imaging (see Note 22). Ensure that all animal experiments are approved by the appropriate Institutional Animal Care and Use Committee (IACUC) and that government regulations regarding the care and use of laboratory animals are followed.
Thin the hair using a small beard trimmer and use hair removal lotion to dissolve any remaining hair. Make sure to completely wash off the lotion, since extended exposure can burn the mice (see Note 23).
Induce caspase activity by administering the appropriate stimulant (see Notes 24 and 25). In parallel, treat negative control mice with the vehicle in which the drug was administered.
At the peak of caspase activation, administer the activity-based probe intravenously by tail vein injection. The probe will then circulate throughout the mouse and bind to active caspases. Free, unbound probe will clear from the body primarily through the renal system (see Note 26). It is also a good idea to include a “no-probe” control to distinguish probe fluorescence from autofluorescence.
Anesthetize the mouse using isoflurane, and image it using the appropriate filters on a whole animal-imaging machine (IVIS, FMT, or BioFLECT) (see Note 27).
Kill the mouse and remove the tissue of interest. Place the tissue on a sheet of black paper and image it ex vivo using the same filters on an IVIS or FMT machine.
Next, a biochemical analysis of the labeled caspases should be performed. At this stage, the tissue may be snap frozen or lysed directly.
Place the tissue in a microfuge tube. Add an appropriate volume of muscle lysis buffer and homogenize the tissue (see Notes 28 and 29). Spin the homogenates at 20,000 × g in a benchtop centrifuge for 15 min, and then transfer the supernatant to a fresh tube.
Determine the protein concentration using a BCA kit or equivalent and solubilize the protein with 4× sample buffer.
Analyze the probe-labeled lysate (50–100 μg total protein) by fluorescent SDS-PAGE described in Subheading 3.3.
4. Notes
The timing will vary depending on the cell line and stimulant used.
“No-treatment” controls are also important to include.
Remember to account for DTT in the buffer by making protein standards in hypotonic lysis buffer at the same dilution as the samples.
For LE22 or AB50, the optimal concentration for lysate labeling is 1 μM. Probe concentration varies by molecule; a dose curve will aid in optimizing if this information is not already available.
It is highly recommended to run a “no-probe” control in parallel to account for any background/autofluorescent bands that may appear in the gel.
A protease inhibitor may be used to validate the selectivity of the probe. Add it before the probe at the recommended concentration. The length of the pre-incubation time will vary by inhibitor.
“No-treatment” controls are also important to include.
If the time course is longer than ~4 h, fresh probe may need to be added, depending on probe stability and the concentration used.
1 μM is optimal for LE22 and AB50.
Various protease inhibitors may be used to validate the selectivity of the ABP. The inhibitor should be added before the addition of the probe. The pre-incubation time and dose will vary by inhibitor.
Ensure that the caspases (16–21 KDa) do not run off the gel.
For LE22 and AB50, the Cy5 filter set should be used.
The gel can be left in the glass plates during scanning. The scan depth must be changed from “platen” to +3 mm. If the gel is removed from the plates for the scan, rinse it with water to avoid tears and do not touch the gel with ungloved fingers (this causes autofluorescence). Use the platen setting.
The photomultiplier tube (PMT/sensitivity) setting should be adjusted such that the scan is not overexposed or may be increased to boost signal.
Mature, active caspases should appear in the 16–21 kDa range.
At this point, the proteins on the gel may be transferred to a membrane and blotted according to standard western protocols. This is a good way to compare protease activity with total expression.
This amount may vary depending on the intensity of the labeling. If weak, use more.
This antibody should be carefully chosen such that it binds selectively to the protease of interest. It is critical that the antibody be raised towards the subunit to which the probe binds. In the case of caspases, the probes bind to the active site cysteine, which is found within the large subunit.
An insulin syringe is helpful to remove the liquid without aspirating the beads.
Sometimes it is helpful to leave a blank lane in between each sample.
Bands in the pull down lane confirm that the labeled protease corresponds to the target of the immunoprecipitating antibody. When immunoprecipitation is complete, this band should be absent from the “supernatant” sample.
Normal chow causes autofluorescence in the abdominal region.
This step is only required for whole animal imaging experiments, as fluorescent light cannot not pass through the hair.
Stimulant, dosage, and the time it takes to produce active caspases are dependent on the model and must be optimized.
For LE22 or AB50, inject 20 nmol of probe (10 % DMSO/PBS, 100 μl volume).
For LE22 and AB50, maximal caspase labeling occurs after 1 h.
For LE22 and AB50, use the Cy5 filter.
Generally, add 10 μl buffer for every 1 mg of tissue.
Sonication is a good way to disrupt the tissue; however, Dounce homogenizers, bead beaters, or other means of mechanical disruption may be effective, depending on the tissue.
References
- 1.Li J, Yuan J (2008) Caspases in apoptosis and beyond. Oncogene 27(48):6194–6206 [DOI] [PubMed] [Google Scholar]
- 2.McIlwain DR, Berger T, Mak TW (2015) Caspase functions in cell death and disease. Cold Spring Harbor Perspect Biol 7(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edgington LE, Verdoes M, Bogyo M (2011)Functional imaging of proteases: recent advances in the design and application of substrate-based and activity-based probes. Curr Opin Chem Biol 15(6):798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deu E, Verdoes M, Bogyo M (2012) New approaches for dissecting protease functions to improve probe development and drug discovery. Nat Struct Mol Biol 19(1):9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanman LE, Bogyo M (2014) Activity-based profiling of proteases. Annu Rev Biochem 83:249–273 [DOI] [PubMed] [Google Scholar]
- 6.Serim S, Haedke U, Verhelst SH (2012) Activity-based probes for the study of proteases: recent advances and developments. ChemMedChem 7(7):1146–1159 [DOI] [PubMed] [Google Scholar]
- 7.Edgington LE, Berger AB, Blum G, Albrow VE, Paulick MG, Lineberry N, Bogyo M (2009) Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat Med 15(8):967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgington LE, van Raam BJ, Verdoes M, Wierschem C, Salvesen GS, Bogyo M (2012) An optimized activity-based probe for the study of caspase-6 activation. Chem Biol 19(3):340–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgington LE, Bogyo M (2013) In vivo imaging and biochemical characterization of protease function using fluorescent activity-based probes. Curr Protoc Chem Biol 5(1):25–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgington LE, Verdoes M, Ortega A, Withana NP, Lee J, Syed S, Bachmann MH, Blum G, Bogyo M (2013) Functional imaging of legumain in cancer using a new quenched activity-based probe. J Am Chem Soc 135(1): 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher SR (2012) One-dimensional SDS gel electrophoresis of proteins Curr Protoc Mol Biol Chapter 10:Unit 10 12A [DOI] [PubMed] [Google Scholar]