Abstract
The cell envelope is the first line of defense between a bacterium and the world-at-large. Often, the initial steps that determine the outcome of chemical warfare, bacteriophage infections, and battles with other bacteria or the immune system greatly depend on the structure and composition of the bacterial cell surface. One of the most studied bacterial surface molecules is the glycolipid known as lipopolysaccharide (LPS), which is produced by most Gram-negative bacteria. Much of the initial attention LPS received in the early 1900s was owed to its ability to stimulate the immune system, for which the glycolipid was commonly known as endotoxin. It was later discovered that LPS also creates a permeability barrier at the cell surface and is a main contributor to the innate resistance that Gram-negative bacteria display against many antimicrobials. Not surprisingly, these important properties of LPS have driven a vast and still prolific body of literature for more than a hundred years. LPS research has also led to pioneering studies in bacterial envelope biogenesis and physiology, mostly using Escherichia coli and Salmonella as model systems. In this review, we will focus on the fundamental knowledge we have gained from studies of the complex structure of the LPS molecule and the biochemical pathways for its synthesis, as well as the transport of LPS across the bacterial envelope and its assembly at the cell surface.
INTRODUCTION
Gram-negative bacteria are characterized by an envelope that contains two membranes: an inner membrane (IM) that surrounds cytoplasmic components, and an outer membrane (OM) that separates the cell from its environment. These two membranes surround an aqueous cellular compartment termed the periplasm, which contains the peptidoglycan cell wall (Fig. 1A) (1). Thus, in Gram-negative bacteria, the OM serves as the first line of defense against environmental threats. Notably, in contrast to many biological membranes, the OM of most Gram-negative bacteria is not a phospholipid bilayer. Instead, it is a highly asymmetric bilayer that contains phospholipids in the inner leaflet and lipopolysaccharide (LPS) molecules in the outer leaflet (1–3). The glycolipid LPS is the focus of this review.
Figure 1.
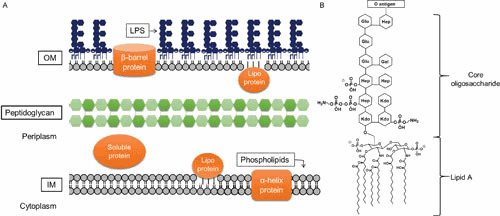
Architecture of the Gram-negative cell envelope. (A) Depiction of the Gram-negative cell envelope and its components. The inner membrane (IM) contains phospholipids, while the outer membrane (OM) contains phospholipids in the inner leaflet and lipopolysaccharide (LPS) in the outer leaflet. (B) Structure of prototypical LPS produced by E. coli (shown is the core structure associated with core type K-12).
LPS performs several functions in Gram-negative bacteria. The most fundamental function of LPS is to serve as a major structural component of the OM. Perhaps not surprisingly, LPS is an essential component of the cell envelope in most, although interestingly not all, Gram-negative bacteria (4). In addition, LPS molecules transform the OM into an effective permeability barrier against small, hydrophobic molecules that can otherwise cross phospholipid bilayers, making Gram-negative bacteria innately resistant to many antimicrobial compounds (5, 6). LPS can also play a crucial role in bacteria-host interactions by modulating responses by the host immune system.
Three main areas of LPS biology are covered in this review. We will first discuss the overall structure of LPS and its function from the bacterial and human point of view. We will then review LPS biosynthesis in Escherichia coli and Salmonella, and discuss how bacteria can regulate LPS synthesis and modify its chemical structure in response to environmental stressors. Last, in the 1970s, Mary Jane Osborn and collaborators posed a question that has dominated a great part of LPS biogenesis research in the past two decades: since LPS is synthesized in the IM but displayed at the cell surface, how is it transported across the cell envelope (7, 8)? Here, we will summarize the work that has uncovered a novel intermembrane transport system that solves the challenges of shuttling this complex glycolipid across diverse cellular compartments.
STRUCTURE AND FUNCTION OF LPS
Main Features of the Structure of LPS
LPS is a large glycolipid composed of three structural domains: lipid A, the core oligosaccharide, and the O antigen (Fig. 1B) (9). Lipid A, the hydrophobic portion of the molecule, is an acylated β-1′-6-linked glucosamine disaccharide that forms the outer leaflet of the OM (9). In E. coli and Salmonella, the glucosamines are phosphorylated at the 1 and 4′ positions and acylated at the 2, 3, 2′, and 3′ positions (9). Two additional secondary acyl chains are also typically present in the distal glucosamine so that mature lipid A is mostly hexa-acylated (9). The core oligosaccharide is a nonrepeating oligosaccharide that is linked to the glucosamines of lipid A (9, 10). The core structure usually contains 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) residues, heptoses, and various hexoses, which can be modified with phosphates and other substituents such as phosphoethanolamine (9–12). The O antigen is an extended polysaccharide that is attached to the core oligosaccharide. It is composed of a repeating oligosaccharide made of two to eight sugars (13–15).
The overall structure of LPS is conserved, but there are many variations that can occur at the species and strain level (9–12, 16, 17). Similarly, the lipid A structure is conserved at the species level; however, as described below, it can undergo regulated modifications in response to environmental conditions (12, 18–20). The core oligosaccharides vary among species and even between some strains of one species (9–12). However, the most diverse component of LPS is the O antigen (13, 17). Not only can the structure and composition of the O antigen differ within a species at the strain level, but, in addition, some Gram-negative bacteria do not synthesize this component of LPS (13, 21). In such cases, molecules composed of only lipid A and the core oligosaccharide are typically referred to as lipooligosaccharides, or LOS (9). Classically, LOS has been referred to as “rough” LPS, as opposed to “smooth” LPS, which includes the O antigen (9).
The Function of LPS
While the structure of LPS (or LOS) may vary among bacteria, in all cases, this glycolipid populates much of the cell surface and establishes a permeability barrier that protects the cell from the entry of toxic molecules such as antibiotics and bile salts (5, 22). Additionally, because LPS is the primary bacterial component encountered by the host immune system, LPS often plays a major role in bacterial pathogenicity (20, 23).
The barrier function of LPS stems in part from its strong amphipathic nature. As in other lipid bilayers, the acyl portion of lipid A provides hydrophobic character that inhibits the passage of hydrophilic molecules through the OM. However, in contrast to other bilayers, the core oligosaccharide and O antigen additionally provide extensive hydrophilic character to LPS that makes the OM particularly impermeable to hydrophobic compounds as well (5, 22). The effectiveness of the barrier posed by LPS is also heavily reliant on the ability of LPS to pack densely within the outer leaflet of the OM. This dense packing is mediated in part by hydrophobicity-driven association of the acyl chains of lipid A. Because lipid A molecules typically bear a large number (that is, 4 to 7) of saturated fatty acid moieties, the extensive interactions between these acyl chains result in low fluidity within the membrane bilayer (5). However, packing of LPS is complicated by the presence of negatively charged phosphate groups throughout its structure. Most salient and conserved are the phosphates of the 1 and 4′ positions of the glucosamines in lipid A, which lie at the exterior surface of the OM, but phosphates can also be found in the core oligosaccharide (9, 12). To prevent repulsion between these negatively charged phosphates, divalent cations such as Mg2+ intercalate between LPS molecules, forming polyionic interactions that greatly enhance LPS packing and, consequently, promote the barrier function of the OM (5, 22).
Because LPS decorates the surface of many bacterial pathogens, the host immune system has evolved to respond dramatically to its presence, making LPS a PAMP, or pathogen-associated molecular pattern (20, 23, 24). In fact, this response can be so dramatic as to prove toxic to the host. For this reason, LPS has been classically termed “endotoxin” in reference to the cell-associated (endo) toxicity observed for many Gram-negative organisms. Understandably, the immune system has evolved to respond primarily to the most conserved feature of LPS, the lipid A structure, for which host toll-like receptor 4 (TLR4) is the primary receptor (20, 23, 24). However, as mentioned above, there is considerable diversity in LPS structures, even within lipid A. A consequence of this diversity is that different LPS structures have varying ability to trigger the host immune response (20). Thus, while the classical, hexacylated, bisphosphorylated lipid A molecule produced by E. coli and Salmonella is highly immunogenic, other forms of lipid A are less so. Some forms of lipid A not only elicit no response themselves, but inhibit the host response to more immunogenic varieties (20, 25–27). In fact, production of less immunogenic lipid A is a strategy used by certain pathogens to evade the host immune response. For example, Yersinia pestis, the causative agent of the bubonic plague, modulates the acylation of its lipid A at mammalian body temperature to produce less immunogenic lipid A (28). Alternatively, some organisms evade the host immune response by masking the more conserved aspects of their LPS with a highly variable O antigen (23). Although the O antigen induces the production of antibodies, the length of the O-antigen chain prevents the antibody-mediated deposition of complement at the bacterial cell surface (13, 29, 30). Consequently, the O-antigen structure protects bacteria from lysis by complement. Possession of an O antigen has also been shown to contribute to pathogen evasion of phagocytosis by immune cells (13, 31).
We should also mention that the combination of the antigenicity and great structural diversity of O antigens has been exploited in the clinic to identify and classify pathogens. This application relies on the fact that the immune system produces specific antibodies that recognize one type of O antigen. Collections of O-antigen-specific antisera have been classically utilized to categorize Gram-negative organisms by serotype, that is, antigenicity in serological testing, of their O antigen (17).
LPS SYNTHESIS PATHWAY
Kdo2-Lipid A Biosynthesis: the Raetz Pathway
Lipid A was first identified as the lipid component that could be released from the rest of the LPS molecules by mild-acid hydrolysis (32–34). Historically, this degradation product was marked as one of the three structural components of LPS. However, it is worth noting that cells synthesize lipid A together with the Kdo moieties of the core oligosaccharide using a biosynthetic pathway that is the most conserved aspect of LPS synthesis (9). This pathway has been extensively characterized in E. coli and Salmonella (9, 35) and is referred to as the Raetz pathway because much of the research describing it was led by Christian Raetz and his team (Fig. 2).
Figure 2.
Lipid A biosynthesis pathway. Modifications to the preceding structure made by each enzyme in the pathway are marked in red, with the exception of the last step, where the modifications made by LpxL and LpxM are colored in red and blue, respectively. Donor molecules are not shown. At low temperatures, LpxP acts instead of LpxL to add a C16:1 palmitoleoyl group instead of a lauroyl group.
The process of lipid A synthesis begins in the cytoplasm with the precursor molecule N-acetylglucosamine linked to a nucleotide carrier (UDP-GlcNAc). This UDP-GlcNAc precursor is initially acylated by the enzyme LpxA to yield UDP-3-O-(acyl)-GlcNAc (36–38). LpxA is selective for the 14-carbon acyl group β-hydroxymyristate carried by the acyl carrier protein (ACP) (37). This selectivity is based on the LpxA active site functioning as a hydrocarbon ruler that most readily incorporates 14-carbon substrates (39, 40). The acylation of UDP-GlcNAc is unfavorable, however, and thus the first committed step of lipid A synthesis is the second reaction in the pathway, which is the irreversible deacetylation of UDP-3-O-(acyl)-GlcNAc to UDP-3-O-(acyl)-GlcN by the Zn2+-dependent metalloenzyme LpxC (41–43). Because LpxC catalyzes the first committed step in the synthesis of LPS, much of the regulation of this pathway, which will be discussed below, appears to center around this enzyme. Following the action of LpxC, UDP-3-O-(acyl)-GlcN is subsequently acylated a second time by LpxD to yield UDP-2,3-diacylglucosamine (44, 45). Like its earlier homologous counterpart LpxA, LpxD is selective for β-hydroxymyristate-ACP as a donor (44). In fact, it has been suggested that LpxD could, to some extent, be capable of substituting for LpxA in the first acylation step. However, since both enzymes are essential (44, 46, 47), it appears any cross-specificity between LpxA and LpxD is insufficient to support growth. After the second acylation by LpxD, LpxH removes the sugar nucleotide carrier from UDP-2,3-diacylglucosamine to generate 2,3-diacylglucosamine-1-phosphate, otherwise known as lipid X (48–50). Lipid X is subsequently added by LpxB to a molecule of UDP-2,3-diacylglucosamine (the product of the LpxD reaction) through a β-1′-6 linkage that releases the UDP nucleotide carrier. The resulting product is a tetraacylated glucosamine disaccharide that is inserted in the inner leaflet of the IM and is sometimes referred to as lipid A disaccharide (51, 52). Following this condensation step, lipid A disaccharide is phosphorylated at the 4′ position by the kinase LpxK, becoming the bisphosphorylated lipid IVA (53, 54). As noted above, while not strictly part of lipid A synthesis, the next step is the addition of two Kdo sugar groups of the core oligosaccharide to lipid IVA (55–57). This step is mediated by the enzyme WaaA, previously known as KdtA, which sequentially adds Kdo groups to lipid IVA from activated Kdo (CMP-Kdo) (56, 57). Finally, two additional acylation events catalyzed by the LpxL and LpxM acyltransferases occur in sequence (58–60). LpxL adds a lauroyl group to the hydroxyl of the 2′-hydroxymyristoyl group and, subsequently, LpxM transfers a myristoyl group to the hydroxyl of the 3′-hydroxymyristoyl group (58–60). Like their earlier counterparts LpxA and LpxD, LpxL and LpxM only utilize substrates carried by ACP (58–60). LpxM functions best after the lauroyl group has already been added by LpxL, but it is capable of functioning to some extent in the absence of LpxL activity (60). After the sequential action of LpxL and LpxM, mature, hexacylated lipid A, which also contains the first two Kdo residues of the inner core, is ready to serve as an acceptor for the sugar groups composing the core oligosaccharide.
An important note is the fact that, under normal laboratory growth conditions, about one-third of LPS molecules are modified by LpxT, which adds a second phosphate group to the 1-phosphate of lipid A, utilizing the pyrophosphate-undecaprenol (Und-PP) as the donor (discussed below in “Modifications of Lipid A” and shown in Fig. 6) (61, 62). This reaction is not part of the Raetz pathway and occurs after the core-lipid A molecule is flipped across the IM. In addition, as discussed below, LpxT activity is subject to regulation by environmental conditions.
Figure 6.
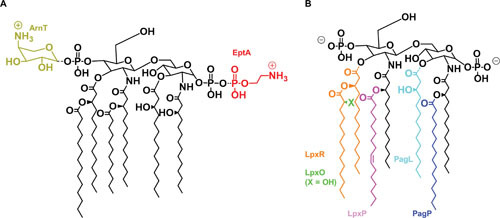
Modifications to the structure of lipid A. Shown are modifications made to lipid A described in the text, alongside the enzymes that mediate them in corresponding colors. (A) Modifications made to the glucosamine phosphates. While shown in their preferred positions, it is possible for either phosphate to be modified with either substituent. (B) Modifications made to the acyl groups. The X indicated for LpxO is a hydroxyl (-OH) when LpxO is active, and a hydrogen (-H) when it is not. When acyl chains are removed, the cleaved bond is shown as a dotted line. It is important to note that LpxP is part of the conserved Raetz pathway but only active at low temperatures, at which LpxP substitutes for LpxL to add a C16:1 palmitoleoyl group instead of a lauroyl group.
Biosynthesis of the Core Oligosaccharide
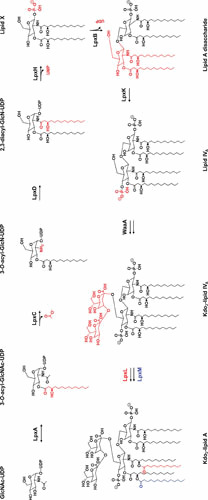
The core oligosaccharide can be subdivided into an inner core, which is proximal to lipid A, and an outer core, which becomes the attachment site for the O antigen (9). The inner core is generally well conserved and is composed of Kdo and l-glycero-d-manno-heptose (heptose) groups (9, 63, 64). The outer core constituents are less conserved, and will vary depending on the type of core oligosaccharide, but in general consist of a series of hexoses (9, 63, 64). For the sake of simplicity, we will focus on the synthesis pathway for the K-12 core type from E. coli (Fig. 3), although various types of core oligosaccharide structures of E. coli and Salmonella can be found in Fig. 4 (9, 11).
Figure 3.
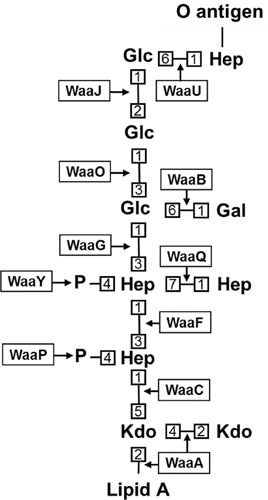
Structure and biosynthetic enzymes of the E. coli K-12 core oligosaccharide. Numbers represent bond positions between sugars. Note that nonstoichiometric modifications are not shown. All linkages are α-anomeric unless preceded by the β symbol, which specifies the β-anomeric state. Enzyme names are boxed, with arrows indicating the linkages they catalyze. It is worth noting that, while the O-antigen ligation site is indicated, E. coli K-12 does not typically produce O antigen because of an ancestral mutation that inactivates its synthesis.
Figure 4.
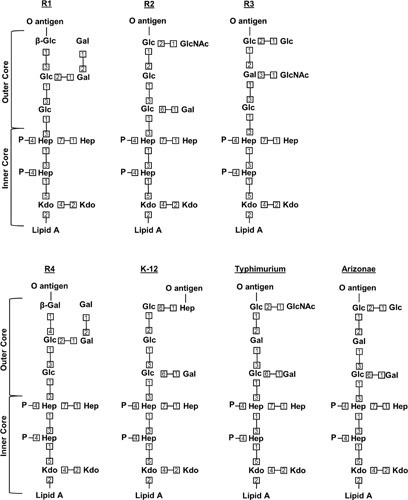
Structure of various core oligosaccharides. Shown are the known core types in E. coli (R1 to R4, K-12) and S. enterica serovar Typhimurium and S. enterica serovar Arizonae IIIA. Numbers represent bond positions between sugars. Note that nonstoichiometric modifications are not shown. All linkages are α-anomeric unless preceded by the β symbol, which specifies the β-anomeric state.
The first step of core oligosaccharide synthesis is the sequential addition by the WaaA enzyme of the first two Kdo groups to the glucosamines of lipid A, which, as discussed above, occurs before the final acylation steps that conclude lipid A synthesis (Fig. 2) (56–60). Next, following completion of lipid A synthesis by LpxL and LpxM, the inner core is extended with two heptose residues by the sequential action of WaaC and WaaF (11, 65). ADP-L-glycero-D-manno-heptose generally serves as the donor substrate for these inner core glycosylation reactions (65). After the addition of the heptoses by WaaC and WaaF, the final three steps that complete inner core synthesis must be catalyzed in order by the enzymes WaaP, WaaQ, and WaaY, respectively (66). WaaP is a kinase that phosphorylates the first heptose of the inner core, which was added by WaaC (66, 67). WaaQ then transfers an additional heptose to the second heptose of the inner core, which was added by WaaF. This third heptose added by WaaQ is then phosphorylated by the WaaY kinase (66). Interestingly, while loss of inner core phosphorylation inhibits outer core extension, the loss of the enzymes that extend the outer core also inhibits inner core phosphorylation, implying complexities in the synthesis pathway that have not been fully explored (68).
Synthesis of the outer core begins with the addition of a glucose group to the second heptose, not only in the K-12 core type, but in all E. coli and Salmonella LPS structures (11, 64). This addition is mediated by WaaG (and its homologs), which utilizes UDP-glucose as its donor substrate (11, 64). The glucose added by WaaG is acted on by the glycosyltransferases WaaO and WaaB, which independently add a glucose and a galactose group, respectively, from UDP-bound donors (11, 64). Additionally, it has been shown that WaaO activity is dependent on divalent cations (64). Next, the penultimate glucose residue is added by the enzyme WaaJ (alternatively known as WaaR), whose activity depends on that of WaaB (11, 69). The final step of core synthesis, the addition of a heptose group to the penultimate glucose, is mediated by the WaaU glycosyltransferase (10, 11). This final heptose group serves as the acceptor of the O antigen after this core-lipid A precursor is translocated to the outer leaflet of the IM.
Biosynthesis of the O Antigen
As might be expected for the outermost, and therefore most exposed, component of LPS, O-antigen structures are highly diverse, with roughly 200 different serogroups identified to date in E. coli alone (9, 17). Because of the great diversity of O antigens, the following discussion will focus on the more conserved aspects of O-antigen biosynthesis and its subsequent ligation to the core-lipid A molecule. We should also note that E. coli K-12 strains, which are often used in research, do not produce O antigen as the result of an ancestral mutation that inactivates its synthesis in that lineage (21).
The O antigen consists of a variable number of repeating oligosaccharide units, and as such can vary in size from molecule to molecule quite dramatically (9, 70). Additionally, rather than being synthesized directly on the core-lipid A molecule, the O antigen is fully synthesized independently from the rest of the LPS molecule. The O antigen is first built stepwise on a lipid carrier molecule, undecaprenyl phosphate (Und-P), and is then transferred to the core oligosaccharide of the nascent LPS molecule in the periplasmic face of the IM (71, 72). Despite the polymorphic nature of O antigens as a whole, the first step in their synthesis is well conserved, and consists of the transfer of a sugar monophosphate to the carrier molecule Und-P at the inner leaflet of the IM. The resulting sugar-pyrophosphate-undecaprenol (sugar-Und-PP) serves as an acceptor for additional glycosylation reactions (13). Aside from this conserved feature, the routes taken to complete the O antigen vary among different organisms and even strains, but generally fall into three categories: the so-called Wzy-dependent pathway, named for the polymerase that founded the group; the ABC-dependent pathway, which, as the name suggests, relies on an ATP-binding cassette (ABC) transporter to translocate the completed O antigen across the IM; and the synthase-dependent pathway, which is poorly characterized and has only been identified in a single species of Salmonella (9, 13). A summary of these different routes can be found in Fig. 5.
Figure 5.
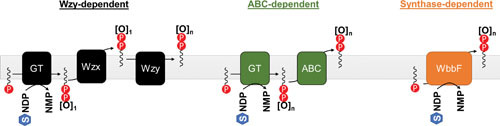
Summary of different O-antigen synthesis pathways. GT stands for glycosyltransferase, and for the purposes of illustration represents all GTs required to generate the O antigen. [O] represents a repeating unit of the O antigen, while the subscript represents the number of repeats present (n being an arbitrary integer). Individual sugar units are represented by “S” inside a hexagon, and are shown bound to an arbitrary nucleotide carrier NDP. The lipid carrier is Und-P.
The Wzy-dependent pathway entails the synthesis of single O units on Und-P, requiring initiation for each O-antigen subunit, and the subsequent flipping of these Und-P-linked O units to the periplasmic face of the IM by the Wzx flippase (9, 13, 73, 74). These O units are then polymerized on a single Und-P carrier molecule through the activity of Wzy. Polymer length is controlled by a partner protein, Wzz, and exhibits a modal distribution of polymer sizes (9, 13, 75–77). Recent structural studies using cryoelectron microscopy have proposed a new model for how Wzz controls polymer length through a synergistic interaction with Wzy (78). Specifically, this model proposes that association between Wzz and Wzy serves to trigger polymerization, with polymer length being controlled by both a molecular ruler mechanism based on Wzz’s polysaccharide-binding capacity, and a molecular stopwatch mechanism based on the time of association between Wzz and Wzy. Finally, the polymerized O antigen is ligated to the core-lipid A acceptor at the outer leaflet of the IM by the ligase WaaL, and the Und-PP carrier is recycled (9). It is worth noting that this biosynthetic strategy of generating O antigen by initiating the synthesis of each subunit on an Und-P molecule places substantial demand on the Und-P carrier pool (9). Indeed, interrupting O-antigen biosynthesis at certain steps in this pathway can lead to the sequestration of Und-P by O-antigen precursors, which causes severe growth defects because Und-P also functions in the synthesis of several envelope glycopolymers, including the essential peptidoglycan cell wall (79, 80).
In contrast to the Wzx-dependent pathway, the ABC-dependent pathway only requires a single initiation event per molecule of polymerized O antigen and performs the entirety of its polymerization in the cytoplasm (9, 13). Glycosyltransferases first polymerize the completed O antigen on a single Und-P carrier molecule in the inner leaflet of the IM utilizing nucleotide-activated sugar donors. The completely polymerized O-antigen-Und-PP molecule is subsequently flipped to the periplasmic face of the IM by an ABC transporter, where the O-antigen portion is then added to the core-lipid A molecule by the WaaL ligase (13, 81).
The synthase-dependent pathway is peculiar to Salmonella enterica serovar Borreze (rfbO:54) (13, 82). The mechanistic details for this mechanism of O-antigen synthesis are unclear, but the titular synthase (WbbF) of the pathway is presumed to simultaneously polymerize and translocate the O antigen across the IM (13, 82). As in the other two biosynthetic pathways, the resulting O-antigen-Und-PP molecule is then used as a donor by the WaaL ligase, which transfers the O-antigen polymer to the outer core oligosaccharide of nascent LPS molecules and releases the lipid carrier, which is then recycled.
REGULATION OF LPS BIOSYNTHESIS
The pathways for the biosynthesis of phospholipids and LPS share a common precursor, β-hydroxymyristate-ACP, the substrate of the FabZ and LpxA enzymes, respectively. As a result, proper balance in lipid biosynthesis, and thereby balanced growth of the IM and OM, requires regulation of LPS synthesis. As stated earlier, LpxC mediates the first committed step in LPS synthesis (9). Consequently, this step becomes a logical control point for the pathway, and indeed, much of our understanding of the regulation of LPS synthesis centers around LpxC. It was noted as early as 1996 that LpxC activity was upregulated when the early steps of LPS synthesis were inhibited, while many other enzymes of the pathway were nonresponsive (83). This response was later shown to be due to regulation of LpxC protein levels by the essential, AAA+ metalloprotease FtsH (for a recent review regarding FtsH, the reader is directed to reference 84) (85). Interestingly, FtsH has also been shown to degrade WaaA, which adds Kdo groups to lipid IVA (86). Proteolysis of LpxC by FtsH is dependent on a sequence present at the carboxyl terminus of LpxC, and is controlled by the cellular growth state and levels of the alarmone (p)ppGpp. Specifically, LpxC is stable during fast growth but degraded by FtsH during slow growth; this relationship is inverted with the loss of (p)ppGpp synthesis (87, 88). The precise signal(s) that directly controls FtsH proteolysis of LpxC has not been elucidated, although feedback through lipid A disaccharide has been proposed as a candidate (46). Nonetheless, a protein that regulates the FtsH-dependent proteolysis of LpxC has also been discovered. The bitopic IM protein LapB (formerly YciM) functions as a negative regulator of LpxC levels in an FtsH-dependent manner (89–91). LapB has also been reported to copurify with, in addition to FtsH and LPS, several LPS synthesis and transport proteins, including WaaC and the entirety of the LPS transport (Lpt) complex (described below). These copurification results imply that LapB may serve a larger role in coordinating disparate aspects of LPS biogenesis. However, the precise nature and mechanism of that role and the relevance of some of those interactions are not clear (91). Furthermore, additional proteolytic control of LpxC by an unknown protease has also been proposed (92). Clearly, more research is needed to understand regulation of LPS synthesis.
MODIFICATION OF LPS STRUCTURE
To better adapt to their varying environments, Gram-negative bacteria will often deviate from the LPS biosynthesis pathways described above. The most frequent modifications include changes in the number and type of acyl chains, as well as the number of phosphates in lipid A; the addition of covalent modifications to lipid A, generally at the 1- and 4′-phosphates, and the core oligosaccharide; and the conversion of the type of O antigen whenever the genomic locus responsible for O-antigen synthesis is exchanged through horizontal gene transfer (18–20). Additionally, due to the incorporation of nonstoichiometric modifications, LPS synthesized by a single strain is not entirely uniform under any growth condition. The most relevant modifications in E. coli and Salmonella are discussed in detail below, and those in the lipid A and core oligosaccharide regions are summarized in Figs. 6 and 7.
Figure 7.
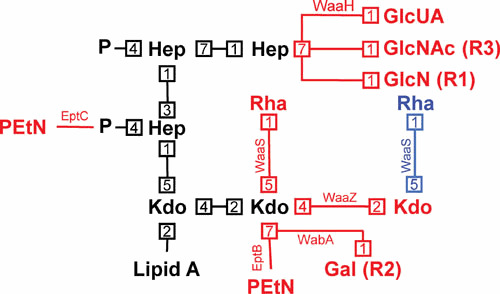
Modifications to the structure of the core oligosaccharide. Shown is the conserved inner core oligosaccharide (and its linkage to lipid A) in black, with potential modifications being indicated in red (although the alternate rhamnose modification by WaaS when the second Kdo is modified with PEtN by EptB is shown in blue). Numbers represent bond positions between sugars. Enzymes mediating modifications are next to each linkage, and, when not associated with E. coli K-12, core type associations are listed in parentheses beside the modification.
Modifications of Lipid A
Bacteria adapt to different temperatures by modulating the fluidity of their membranes through alterations of the type of acyl chains of their membrane lipids (93). LPS is no exception. Under low-temperature conditions (∼12°C), E. coli will express an alternate acyltransferase homologous to LpxL, termed LpxP, that adds a 16-carbon palmitoyl group in place of the 12-carbon lauroyl group normally added by LpxL (18, 94, 95). Presumably, this modification serves to offset the decrease in membrane fluidity resultant from lower temperatures (18). Like its counterpart LpxL, LpxP requires ACP-bound substrates for activity (94).
However, most of the changes to lipid A structure occur in response to the amount of cations and positively charged antimicrobials in the environment. A number of enzymes that catalyze those modifications are under control of the PhoQP two-component regulatory system (96, 97). In Salmonella, the PhoQP system is primarily implicated in responding to low levels of divalent cations, namely Mg2+, the presence of cationic antimicrobial peptides (CAMPs), and host interactions (97–99). When the kinase PhoQ is activated by these signals, it phosphorylates its cognate response regulator PhoP. Once phosphorylated, PhoP activates the transcription of several genes encoding LPS-modifying enzymes. Among them, PagP is an enzyme that resides in the OM and transfers a palmitoyl group to LPS, resulting in heptacylated LPS species that are implicated in CAMP resistance (18, 100, 101). In this transfer, PagP uses the 16-carbon acyl chains of phospholipids that are mislocalized to the outer leaflet of the OM as donors (18, 100, 101). Additionally controlled by PhoQP are LpxR and PagL (the latter is absent in E. coli), which also reside in the OM and modify the acyl chain content in lipid A. LpxR and PagL, respectively, mediate the removal of the 3′-acyl groups and the 3-hydroxymyristoyl group, which modulate the immunogenicity of lipid A (102, 103).
The PhoQP system also controls modification to the glucosamine disaccharide portion of lipid A, albeit indirectly through another two-component regulatory system. These modifications are directly regulated by the PmrBA (alternatively BasSR for E. coli) two-component system. The PmrBA system is positively regulated by PhoQP through the adapter protein PmrD, which protects the phosphorylated response regulator PmrA (BasR) from dephosphorylation (104–107). In addition, the sensor kinase PmrB (BasS) directly responds to acidic pH and elevated concentrations of certain metals such as Fe3+, Al3+, and Zn2+ (12, 107–109). When phosphorylated, PmrA upregulates the covalent modification of lipid A with 4-amino-4-deoxy-L-arabinose (L-Ara4N) and phosphoethanolamine (PEtN) (18). L-Ara4N modification is mediated by the enzyme ArnT, which transfers L-Ara4N from Und-P to lipid A primarily at the 4′-phosphate, although it can also act on the 1-phosphate (110, 111). PEtN modification is mediated by the enzyme EptA, which transfers PEtN from phosphatidylethanolamine to lipid A primarily at the 1-phosphate, although it can also act on the 4′-phosphate (112, 113). Activation of PmrBA also inhibits modification of lipid A by LpxT, the kinase that adds a second phosphate group to the 1-phosphate from Und-PP to ∼33% of LPS molecules under standard laboratory growth conditions (61, 62). Both the addition of positively charged moieties to lipid A by ArnT and EptA and the loss of the negatively charged modification catalyzed by LpxT are associated with increased resistance to CAMPs, presumably due to the masking of negative charges (i.e., phosphates) in LPS to which CAMPs bind (62, 107).
In addition, Salmonella can alter lipid A through the action of LpxO, which is absent in E. coli. LpxO is not regulated by PhoQP and mediates the oxygen-dependent hydroxylation of the secondary 3′-acyl group immediately after the carboxyl group in the cytoplasm (114, 115).
It is worth noting that, while these are the primary well-characterized modifications to lipid A performed by Salmonella and E. coli species, other species use similar strategies with alternate modifying groups. For example, certain isolates of Vibrio cholerae modify the acyl chains of their lipid A with amino acid moieties (116, 117). Additionally, some Bordetella isolates modify the phosphates of their lipid A with glucosamine (118, 119). Both of these modifications promote CAMP resistance, much like many of their Salmonella and E. coli counterparts.
Modifications to the Core Oligosaccharide
Given that the outer core is relatively variable, well-characterized modifications tend to be confined to the inner core region. Modifications to the core oligosaccharide include the addition of various sugar groups as well as other moieties, such as PEtN. What types of modifications occur will also depend to some extent on the core type. The best characterized modifications will be discussed in detail below, and are also summarized in Fig. 7.
Starting from the lipid A-proximal sugars and working up the sugar chain of the core, the second Kdo residue can be modified with additional Kdo, PEtN, rhamnose, or galactose groups (11, 12). Kdo addition is mediated by the WaaZ transferase. Modification of the second Kdo with PEtN is mediated by EptB and confers resistance to polymyxin B and high levels of Ca2+ (12, 120–122). Rhamnose addition is mediated by WaaS, and can occur to either the second Kdo or the third Kdo added by WaaZ, but both activities are dependent on prior addition of the third Kdo (12, 123, 124). When PEtN is present on the second Kdo group, WaaS can only act on the third Kdo (added by WaaZ), whereas it otherwise acts on the second Kdo (12, 124). At least in E. coli K-12, these modifications are associated with induction of the envelope stress response effector σE (for a review on the function and induction of σE, the reader is directed to reference 125), as well as with the truncation of the outer core caused by downregulation of WaaJ (WaaR), which leads to the loss of the terminal glucose and heptose groups (12, 124). In addition, EptB is negatively regulated by PhoQP, but induced by high levels of Ca2+ (12, 120, 121). Last, addition of galactose to the second Kdo group is mediated by WabA, which is absent in E. coli K-12 and was instead characterized in E. coli with the R2 core type (11).
The first heptose of the inner core may be modified with PEtN, specifically at the phosphate added by WaaP, which confers resistance to CAMPs (126). This addition is mediated by EptC, which is induced by PhoBR, a two-component system responsive to phosphate-limiting conditions (126–128). The third heptose of the inner core (added by WaaQ) may be modified with several different glucose derivatives, including GlcNAc, GlcN, and glucuronic acid (GlcUA) (11, 126). The former two modifications were characterized in E. coli with the R3 and R1 core types, respectively, whereas the latter is found in E. coli K-12 (11, 126). Modification of the third heptose with GlcUA is mediated by the enzyme WaaH, which like EptC, is induced by the PhoBR system (12, 126). Consistent with its response to phosphate-limiting conditions, the GlcUA addition mediated by WaaH proceeds more efficiently in the absence of the phosphate group added by WaaY (12, 126).
O-Antigen Modification
While modifications to basic O-antigen structures have been reported, they will not be discussed in detail here due to the sheer breadth of O-antigen synthesis (13, 129). More striking than minor additions or replacements of individual sugars is that the O antigen may be replaced entirely. In some cases, a serotype of O antigen is replaced by another through the genetic exchange of part or the entire biosynthetic locus resulting from horizontal gene transfer (16, 130). In addition, the O antigen can be replaced altogether by a different type of polysaccharide. Specifically, in E. coli K-12, it has been shown that induction of capsular colanic acid synthesis results in replacement of the O antigen in LPS with colanic acid repeats in a WaaL-dependent manner (131, 132). A similar phenomenon has been observed with the enterobacterial common antigen (ECA) (133, 134). Curiously, a missense mutation in waaL that broadens the substrate specificity of the ligase led to the discovery that this WaaL variant can modify the LPS core with the peptidoglycan-building block by utilizing lipid II as a donor as well (135).
LPS TRANSPORT
Overview
In 1972, Mary Jane Osborn and collaborators published seminal studies demonstrating that LPS is synthesized at the IM and, therefore, must be transported across the envelope to the OM. Furthermore, the authors demonstrated that this intermembrane transport is unidirectional (7, 8). That body of work opened a new area of research that eventually led to the discovery of the factors required for LPS transport during the 1990s and 2000s. We now know that, as described in the sections above, the core-lipid A and Und-P-linked O-antigen components of the LPS structure are independently synthesized at the cytoplasmic face of the IM. Using different transporters, these two subunits are separately flipped to the periplasmic leaflet of the IM. Once there, the O antigen can be ligated onto the core oligosaccharide moiety by the WaaL ligase. The resulting newly synthesized LPS molecules must then be extracted from the IM, cross the aqueous periplasm, and traverse the OM to ultimately be assembled at the cell surface. We describe our current knowledge of these steps in LPS transport in the next sections.
Crossing the IM: MsbA
Transport of the core-lipid A molecule from the inner leaflet to the outer leaflet of the IM is mediated by a homodimer of MsbA, a flippase that belongs to the ABC transporter superfamily (Fig. 8) (33, 136–138). MsbA was first characterized as a multicopy suppressor of the loss of LpxL (previously known as HtrB) activity, and was named accordingly (multicopy suppressor of htrB A) (136). It would not be until years later that the flippase activity of MsbA was demonstrated, both by the accumulation of LPS in the cytoplasmic side of the IM upon MsbA depletion and the in vitro reconstitution of functional MsbA (139, 140). Recent structural studies of MsbA have put forth a model in which LPS directly enters a largely hydrophobic cavity within the MsbA homodimer (141, 142). Interestingly, this cavity is localized in the outer leaflet of the IM, indicating that LPS traverses the IM before being flipped (141, 142). Interactions between this cavity and the lipid A moiety of LPS, together with ATP hydrolysis by MsbA, cause conformational changes in MsbA that lead to the exposure of this cavity to the aqueous periplasmic environment to drive LPS into the outer leaflet of the IM in concert with the closing of the cavity (141). These studies suggest that interaction between positively charged residues within the cavity and the phosphates of lipid A serve as recognition determinants for LPS by MsbA (141, 142). Moreover, the placement of these interactions has been proposed to favor accommodation of shorter acyl chains within the cavity, which may serve to discriminate against flipping of phospholipids (142). It has also been revealed that MsbA’s substrate specificity likely drives the requirement for Kdo2-lipid A as the minimal LPS structure. Flipping of LPS by MsbA is most efficient when the glycolipid contains the late-stage acyl chains, which are added by LpxL and LpxM after the addition of the first two Kdo residues (143–145). The requirement for Kdo2-lipid A as the minimal LPS structure is thus primarily a reflection of the preference of MsbA for hexacylated substrates (142).
Figure 8.
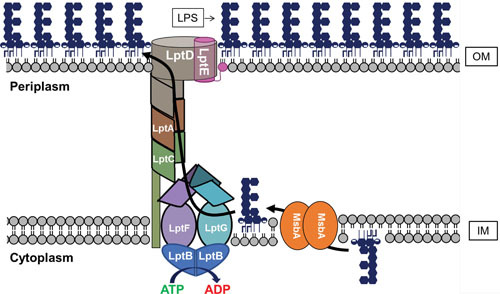
Transport of LPS across the cell envelope. Shown are representations of MsbA, which mediates the transport of core-lipid A across the IM, and the Lpt complex (LptB2FGCADE), which mediates LPS extraction from the IM and its transport through the periplasm and OM. As described in the text, the O antigen can be synthesized on Und-P and transported across the IM by different pathways (Fig. 5). If made, the O antigen is ligated to core-lipid A in the periplasmic leaflet of the IM by WaaL (not shown).
From the IM to the OM: the Lpt System
After its translocation across the IM by MsbA, and possibly undergoing the addition of the O antigen and other chemical modifications (e.g., by the addition of L-Ara4N and PEtN) at the outer leaflet of the IM, LPS must be transported across the periplasm and OM. This transport necessitates the extraction of LPS from the IM, the sheltering of its acyl moieties as it traverses the aqueous periplasm, and, finally, its translocation across the OM to arrive at its final destination in the outer leaflet of the OM. All these processes are mediated by a protein complex, termed the Lpt complex (Fig. 8) (1). The Lpt complex is composed of seven different proteins that span all compartments of the cell: LptB in the cytoplasm; LptF, LptG, and LptC in the IM; LptA in the periplasm; and LptD and LptE in the OM (1, 146). A dimer of LptB proteins together with LptF and LptG form the ABC transporter LptB2FG. LptB constitutes the nucleotide-binding domains (NBDs) that bind and hydrolyze cytoplasmic ATP to power the transport of LPS from the IM to the OM (147, 148). Recent structural studies have revealed that LptF and LptG, the transmembrane domains of the LptB2FG ABC transporter, form a cavity that is predicted to accommodate LPS during its extraction from the IM (149–151). Whether or not the bitopic IM protein LptC is involved in the extraction process is still somewhat unclear, although it is worth noting that its single transmembrane domain is dispensable for function (152). While the precise mechanism of extraction of LPS from the IM remains to be elucidated, it is known that, eventually, LPS makes its way to the periplasmic domain of LptC (146, 148). The periplasmic domain of LptC consists of a series of antiparallel β-strands arranged to form a β-jellyroll domain that contains a hydrophobic groove that is thought to shelter the acyl chains of LPS during its transit across the periplasm (153, 154). LptA and the periplasmic domain of LptD, LptF, and LptG possess similar β-jellyroll folds. Moreover, the β-jellyroll domains of LptC, LptA, and LptD have been shown to associate in a head-to-tail manner to extend the hydrophobic groove across the periplasm (154–156). These domains in LptCAD are thus thought to form a bridge extending across the periplasm through which LPS can travel to reach the OM. In support of this model, LPS has been covalently cross-linked to each individual member of this bridge, and the in vitro reconstitution of LPS transport requires the formation of a bridge composed of the Lpt periplasmic components (148, 157, 158). It is worth noting that, in addition to its ability to associate with LptC and LptD at either end of the bridge, LptA can also form homo-oligomeric complexes (154, 159). As such, it is possible that more than one LptA protein may be incorporated into the Lpt complex. However, the ability of an LptA variant deficient in homo-oligomerization to complement the deletion of the native lptA gene in E. coli may suggest that one subunit of LptA per complex is sufficient under normal growth conditions (160). Nevertheless, a recent study has demonstrated that, in Salmonella, the width of the periplasm (i.e., the distance between the IM and OM) can be modulated by altering the length of Lpp, the OM lipoprotein that covalently tethers the OM to the peptidoglycan layer (161). It is therefore tempting to suggest that the number of LptA proteins per Lpt bridge might change depending on the width of the periplasm.
On arrival at the OM, LPS must be specifically inserted into the outer leaflet, a process mediated by LptD and LptE (146). The membrane-associated portion of LptD is a large, crenellated β-barrel, in which hydrogen bonding between strands is disrupted along the first and last β-strands, creating a crenellation, or small gap (146, 156). LptE is a lipoprotein that resides in the lumen of the barrel portion of LptD (162). Together, LptD and LptE are proposed to form the OM translocon responsible for the insertion of LPS into the outer leaflet of the OM. Based on structural studies, it has been proposed that when LPS arrives at the periplasmic β-jellyroll domain of LptD, its acyl chains are directly deposited into the hydrophobic environment of the OM through a gap that exists between this periplasmic domain and the β-barrel domain of LptD; the crenellation of LptD may then provide for a means by which the barrel might open enough to allow lateral passage of the hydrophilic portion of LPS through the lumen of LptD and out into the OM (156). The role of LptE is unclear, but its high affinity for LPS suggests that it is more than simply a plug for the LptD barrel (163, 164). It has also been demonstrated that LptE plays a critical role in the proper assembly of LptD in the OM (162, 165).
Together, these data have led to the so-called “PEZ Model” of Lpt-mediated LPS transport (146). This model likens LPS to the originally Austrian PEZ candies, and the Lpt transport machinery to the mechanical PEZ candy dispenser invented by Oskar Uxa. Such a dispenser works by way of a spring-loaded platform at the base of the dispenser, which pushes a stack of PEZ candies up through the central channel of the dispenser, so that a candy is always present at the exit of the dispenser and ready to be taken and consumed by the user. Likewise, this model predicts that LPS travels as a stream of molecules, and that the driving force of LPS transport by the Lpt machinery is derived from the LptB2FG transporter at the base of the periplasmic channel formed by LptCAD. Accordingly, the LptB2FG transporter acts much like the spring-loaded platform of a PEZ dispenser by providing constant pressure to the base of the traveling LPS stream by constantly loading new LPS molecules into the channel. Thus, in this model, the channel formed by LptCAD is largely passive in transport, simply providing a compatible route by which LPS molecules might traverse the aqueous periplasm prior to their assembly at the cell surface through the action of the LptDE translocon, which, like the top of the PEZ dispenser, opens up to deliver its cargo. Importantly, the recent in vitro reconstitution of LPS transport supports the PEZ model for LPS transport (146, 158). This great technical achievement has demonstrated that transport of LPS can occur in an ATP-dependent manner between IM-like (i.e., containing LptB2FGC) and OM-like (containing LptDE) proteoliposomes only when they are physically connected by soluble LptA.
CONCLUDING REMARKS
Although much is known about the complex structure, synthesis, regulation, and transport of LPS, fundamental questions remain unanswered. In particular, the mechanism of transport for this complex glycolipid, and the regulation of LPS biosynthesis, as well, are highly active fields of investigation. Additionally, as the means for culturing different organisms and characterizing LPS modifications become more sophisticated and available, the various strategies used by Gram-negative bacteria to adapt their cell surfaces to their environment through nonstoichiometric modification of LPS are coming under heavy scrutiny.
In addition, the rather curious fact that LPS is essential in most, but not all, Gram-negative bacteria has recently received considerable attention. Efforts to elucidate why that is have primarily centered around those organisms in which LPS is not essential, which include Neisseria meningitidis, Acinetobacter baumannii, and Moraxella catarrhalis (35, 166–168). The best studied of these cases is that of A. baumannii, for which LPS essentiality has been linked to the activity of the peptidoglycan cell wall polymerase PBP1A (169). However, the precise mechanisms underlying the essentiality of LPS for A. baumannii, as well as other organisms, remain to be clarified. For a more in-depth review of the topic, the reader is directed to reference 170.
LPS biogenesis has also received attention as a potential drug target to treat infections caused by Gram-negative bacteria. Inhibitors of LPS biogenesis could kill organisms in which the glycolipid is essential, but they could also render all LPS producers highly permeable to other antibiotics. Several compounds that inhibit LPS biosynthesis and transport have been developed, and some are undergoing clinical trials, but no compound has been approved yet for clinical use (171–174). We can only hope that enhancing our understanding of LPS biogenesis will, in turn, bolster our ability to produce clinically relevant inhibitors, as well as facilitate our understanding of bacterial lifestyles as a whole.
ACKNOWLEDGMENTS
The authors would like to thank other members of the Ruiz laboratory (Sujeet Kumar, Emily Lundstedt, Rebecca Davis, and Brent Simpson) for assistance in editing this manuscript. This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01 GM100951 (to N.R.).
REFERENCES
- 1.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. 10.1101/cshperspect.a000414. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos MP, Robert V, Tommassen J. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu Rev Microbiol 61:191–214. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Kamio Y, Nikaido H. 1976. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 15:2561–2570. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Zhang G, Meredith TC, Kahne D. 2013. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr Opin Microbiol 16:779–785. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galloway SM, Raetz CR. 1990. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem 265:6394–6402. [PubMed] [PubMed] [Google Scholar]
- 7.Osborn MJ, Gander JE, Parisi E. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem 247:3973–3986. [PubMed] [PubMed] [Google Scholar]
- 8.Osborn MJ, Gander JE, Parisi E, Carson J. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem 247:3962–3972. [PubMed] [PubMed] [Google Scholar]
- 9.Raetz CR, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrichs DE, Yethon JA, Whitfield C. 1998. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol Microbiol 30:221–232. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Whitfield C, Kaniuk N, Frirdich E. 2003. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J Endotoxin Res 9:244–249. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Klein G, Raina S. 2015. Regulated control of the assembly and diversity of LPS by noncoding sRNAs. BioMed Res Int 2015:153561. 10.1155/2015/153561. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalynych S, Morona R, Cygler M. 2014. Progress in understanding the assembly process of bacterial O-antigen. FEMS Microbiol Rev 38:1048–1065. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Hong Y, Liu MA, Reeves PR. 2017. Progress in our understanding of wzx flippase for translocation of bacterial membrane lipid-linked oligosaccharide. J Bacteriol 200:e00154-17. 10.1128/JB.00154-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Wang Q, Reeves PR. 2010. The variation of O antigens in gram-negative bacteria. Subcell Biochem 53:123–152. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. 2014. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol Rev 38:56–89. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Iguchi A. 2016. A complete view of the Escherichia coli O-antigen biosynthesis gene cluster and the development of molecular-based O-serogrouping methods. Nippon Saikingaku Zasshi 71:209–215. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem 76:295–329. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado RF, Sá-Correia I, Valvano MA. 2016. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev 40:480–493. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott AJ, Oyler BL, Goodlett DR, Ernst RK. 2017. Lipid A structural modifications in extreme conditions and identification of unique modifying enzymes to define the Toll-like receptor 4 structure-activity relationship. Biochim Biophys Acta 1862:1439–1450. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson G, Neal B, Liu D, Hobbs M, Packer NH, Batley M, Redmond JW, Lindquist L, Reeves P. 1994. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol 176:4144–4156. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter TS, Parkin J, Khalid S. 2016. The free energy of small solute permeation through the Escherichia coli outer membrane has a distinctly asymmetric profile. J Phys Chem Lett 7:3446–3451. [PubMed] [DOI] [PubMed] [Google Scholar]
- 23.Needham BD, Trent MS. 2013. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 11:467–481. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sassi N, Paul C, Martin A, Bettaieb A, Jeannin JF. 2010. Lipid A-induced responses in vivo. Adv Exp Med Biol 667:69–80. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. 2013. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci USA 110:1464–1469. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wang Z, Chen J, Ernst RK, Wang X. 2013. Influence of lipid A acylation pattern on membrane permeability and innate immune stimulation. Mar Drugs 11:3197–3208. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coats SR, Pham TTT, Bainbridge BW, Reife RA, Darveau RP. 2005. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol 175:4490–4498. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 7:1066–1073. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Goebel EM, Wolfe DN, Elder K, Stibitz S, Harvill ET. 2008. O antigen protects Bordetella parapertussis from complement. Infect Immun 76:1774–1780. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray GL, Attridge SR, Morona R. 2006. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J Bacteriol 188:2735–2739. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saldías MS, Ortega X, Valvano MA. 2009. Burkholderia cenocepacia O antigen lipopolysaccharide prevents phagocytosis by macrophages and adhesion to epithelial cells. J Med Microbiol 58:1542–1548. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Westphal O, Luderitz O. 1954. Chemische Erforschung Von Lipopolysacchariden Gramnegativer Bakterien. Angew Chem 66:407–417. [Google Scholar]
- 33.Zhou Z, White KA, Polissi A, Georgopoulos C, Raetz CR. 1998. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J Biol Chem 273:12466–12475. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z, Lin S, Cotter RJ, Raetz CR. 1999. Lipid A modifications characteristic of Salmonella typhimurium are induced by NH4VO3 in Escherichia coli K12. Detection of 4-amino-4-deoxy-L-arabinose, phosphoethanolamine and palmitate. J Biol Chem 274:18503–18514. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Putker F, Bos MP, Tommassen J. 2015. Transport of lipopolysaccharide to the Gram-negative bacterial cell surface. FEMS Microbiol Rev 39:985–1002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Anderson MS, Bulawa CE, Raetz CR. 1985. The biosynthesis of gram-negative endotoxin. Formation of lipid A precursors from UDP-GlcNAc in extracts of Escherichia coli. J Biol Chem 260:15536–15541. [PubMed] [PubMed] [Google Scholar]
- 37.Anderson MS, Raetz CR. 1987. Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem 262:5159–5169. [PubMed] [PubMed] [Google Scholar]
- 38.Anderson MS, Bull HG, Galloway SM, Kelly TM, Mohan S, Radika K, Raetz CR. 1993. UDP-N-acetylglucosamine acyltransferase of Escherichia coli. The first step of endotoxin biosynthesis is thermodynamically unfavorable. J Biol Chem 268:19858–19865. [PubMed] [PubMed] [Google Scholar]
- 39.Wyckoff TJO, Lin S, Cotter RJ, Dotson GD, Raetz CRH. 1998. Hydrocarbon rulers in UDP-N-acetylglucosamine acyltransferases. J Biol Chem 273:32369–32372. [PubMed] [DOI] [PubMed] [Google Scholar]
- 40.Wyckoff TJ, Raetz CR. 1999. The active site of Escherichia coli UDP-N-acetylglucosamine acyltransferase. Chemical modification and site-directed mutagenesis. J Biol Chem 274:27047–27055. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Anderson MS, Robertson AD, Macher I, Raetz CR. 1988. Biosynthesis of lipid A in Escherichia coli: identification of UDP-3-O-[(R)-3-hydroxymyristoyl]-alpha-D-glucosamine as a precursor of UDP-N2,O3-bis[(R)-3-hydroxymyristoyl]-alpha-D-glucosamine. Biochemistry 27:1908–1917. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Young K, Silver LL, Bramhill D, Cameron P, Eveland SS, Raetz CR, Hyland SA, Anderson MS. 1995. The envA permeability/cell division gene of Escherichia coli encodes the second enzyme of lipid A biosynthesis. UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase. J Biol Chem 270:30384–30391. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Jackman JE, Raetz CRH, Fierke CA. 1999. UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase of Escherichia coli is a zinc metalloenzyme. Biochemistry 38:1902–1911. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Kelly TM, Stachula SA, Raetz CR, Anderson MS. 1993. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase. The third step of endotoxin biosynthesis. J Biol Chem 268:19866–19874. [PubMed] [PubMed] [Google Scholar]
- 45.Bartling CM, Raetz CR. 2008. Steady-state kinetics and mechanism of LpxD, the N-acyltransferase of lipid A biosynthesis. Biochemistry 47:5290–5302. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emiola A, George J, Andrews SS. 2015. A complete pathway model for lipid a biosynthesis in Escherichia coli. PLoS One 10:e0121216. 10.1371/journal.pone.0121216. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray BL, Painter G, Raetz CR. 1984. The biosynthesis of gram-negative endotoxin. Formation of lipid A disaccharides from monosaccharide precursors in extracts of Escherichia coli. J Biol Chem 259:4852–4859. [PubMed] [PubMed] [Google Scholar]
- 49.Babinski KJ, Kanjilal SJ, Raetz CR. 2002. Accumulation of the lipid A precursor UDP-2,3-diacylglucosamine in an Escherichia coli mutant lacking the lpxH gene. J Biol Chem 277:25947–25956. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Babinski KJ, Ribeiro AA, Raetz CR. 2002. The Escherichia coli gene encoding the UDP-2,3-diacylglucosamine pyrophosphatase of lipid A biosynthesis. J Biol Chem 277:25937–25946. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Radika K, Raetz CR. 1988. Purification and properties of lipid A disaccharide synthase of Escherichia coli. J Biol Chem 263:14859–14867. [PubMed] [PubMed] [Google Scholar]
- 52.Crowell DN, Anderson MS, Raetz CR. 1986. Molecular cloning of the genes for lipid A disaccharide synthase and UDP-N-acetylglucosamine acyltransferase in Escherichia coli. J Bacteriol 168:152–159. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray BL, Raetz CRH. 1987. The biosynthesis of gram-negative endotoxin. A novel kinase in Escherichia coli membranes that incorporates the 4′-phosphate of lipid A. J Biol Chem 262:1122–1128. [PubMed] [PubMed] [Google Scholar]
- 54.Garrett TA, Que NL, Raetz CR. 1998. Accumulation of a lipid A precursor lacking the 4′-phosphate following inactivation of the Escherichia coli lpxK gene. J Biol Chem 273:12457–12465. [PubMed] [DOI] [PubMed] [Google Scholar]
- 55.Clementz T, Raetz CR. 1991. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem 266:9687–9696. [PubMed] [PubMed] [Google Scholar]
- 56.Belunis CJ, Raetz CR. 1992. Biosynthesis of endotoxins. Purification and catalytic properties of 3-deoxy-D-manno-octulosonic acid transferase from Escherichia coli. J Biol Chem 267:9988–9997. [PubMed] [PubMed] [Google Scholar]
- 57.Brozek KA, Hosaka K, Robertson AD, Raetz CRH. 1989. Biosynthesis of lipopolysaccharide in Escherichia coli. Cytoplasmic enzymes that attach 3-deoxy-D-manno-octulosonic acid to lipid A. J Biol Chem 264:6956–6966. [PubMed] [PubMed] [Google Scholar]
- 58.Brozek KA, Raetz CR. 1990. Biosynthesis of lipid A in Escherichia coli. Acyl carrier protein-dependent incorporation of laurate and myristate. J Biol Chem 265:15410–15417. [PubMed] [PubMed] [Google Scholar]
- 59.Clementz T, Bednarski JJ, Raetz CR. 1996. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J Biol Chem 271:12095–12102. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Clementz T, Zhou Z, Raetz CR. 1997. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J Biol Chem 272:10353–10360. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Touzé T, Tran AX, Hankins JV, Mengin-Lecreulx D, Trent MS. 2008. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol Microbiol 67:264–277. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrera CM, Hankins JV, Trent MS. 2010. Activation of PmrA inhibits LpxT-dependent phosphorylation of lipid A promoting resistance to antimicrobial peptides. Mol Microbiol 76:1444–1460. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amor K, Heinrichs DE, Frirdich E, Ziebell K, Johnson RP, Whitfield C. 2000. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect Immun 68:1116–1124. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qian J, Garrett TA, Raetz CR. 2014. In vitro assembly of the outer core of the lipopolysaccharide from Escherichia coli K-12 and Salmonella typhimurium. Biochemistry 53:1250–1262. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gronow S, Brabetz W, Brade H. 2000. Comparative functional characterization in vitro of heptosyltransferase I (WaaC) and II (WaaF) from Escherichia coli. Eur J Biochem 267:6602–6611. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Yethon JA, Heinrichs DE, Monteiro MA, Perry MB, Whitfield C. 1998. Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J Biol Chem 273:26310–26316. [PubMed] [DOI] [PubMed] [Google Scholar]
- 67.Yethon JA, Whitfield C. 2001. Purification and characterization of WaaP from Escherichia coli, a lipopolysaccharide kinase essential for outer membrane stability. J Biol Chem 276:5498–5504. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Yethon JA, Vinogradov E, Perry MB, Whitfield C. 2000. Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J Bacteriol 182:5620–5623. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pradel E, Parker CT, Schnaitman CA. 1992. Structures of the rfaB, rfaI, rfaJ, and rfaS genes of Escherichia coli K-12 and their roles in assembly of the lipopolysaccharide core. J Bacteriol 174:4736–4745. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jann B, Reske K, Jann K. 1975. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Eur J Biochem 60:239–246. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.McGrath BC, Osborn MJ. 1991. Localization of the terminal steps of O-antigen synthesis in Salmonella typhimurium. J Bacteriol 173:649–654. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68. [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Liu D, Cole RA, Reeves PR. 1996. An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flippase. J Bacteriol 178:2102–2107. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldman MF, Marolda CL, Monteiro MA, Perry MB, Parodi AJ, Valvano MA. 1999. The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J Biol Chem 274:35129–35138. [PubMed] [DOI] [PubMed] [Google Scholar]
- 75.Nath P, Morona R. 2015. Mutational analysis of the major periplasmic loops of Shigella flexneri Wzy: identification of the residues affecting O antigen modal chain length control, and Wzz-dependent polymerization activity. Microbiology 161:774–785. [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Nath P, Morona R. 2015. Detection of Wzy/Wzz interaction in Shigella flexneri. Microbiology 161:1797–1805. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Papadopoulos M, Tran EN, Murray GL, Morona R. 2016. Conserved transmembrane glycine residues in the Shigella flexneri polysaccharide co-polymerase protein WzzB influence protein-protein interactions. Microbiology 162:921–929. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Collins RF, Kargas V, Clarke BR, Siebert CA, Clare DK, Bond PJ, Whitfield C, Ford RC. 2017. Full-length, oligomeric Structure of Wzz determined by cryoelectron microscopy reveals insights into membrane-bound states. Structure 25:806–815.e3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 79.Jorgenson MA, Young KD. 2016. Interrupting biosynthesis of O antigen or the lipopolysaccharide core produces morphological defects in Escherichia coli by sequestering undecaprenyl phosphate. J Bacteriol 198:3070–3079. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorgenson MA, Kannan S, Laubacher ME, Young KD. 2016. Dead-end intermediates in the enterobacterial common antigen pathway induce morphological defects in Escherichia coli by competing for undecaprenyl phosphate. Mol Microbiol 100:1–14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenfield LK, Whitfield C. 2012. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr Res 356:12–24. [PubMed] [DOI] [PubMed] [Google Scholar]
- 82.Keenleyside WJ, Whitfield C. 1996. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J Biol Chem 271:28581–28592. [DOI] [PubMed] [Google Scholar]
- 83.Sorensen PG, Lutkenhaus J, Young K, Eveland SS, Anderson MS, Raetz CR. 1996. Regulation of UDP-3-O-[R-3-hydroxymyristoyl]-N-acetylglucosamine deacetylase in Escherichia coli. The second enzymatic step of lipid a biosynthesis. J Biol Chem 271:25898–25905. [PubMed] [DOI] [PubMed] [Google Scholar]
- 84.Bittner LM, Arends J, Narberhaus F. 2017. When, how and why? Regulated proteolysis by the essential FtsH protease in Escherichia coli. Biol Chem 398:625–635. [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Ogura T, Inoue K, Tatsuta T, Suzaki T, Karata K, Young K, Su L-H, Fierke CA, Jackman JE, Raetz CRH, Coleman J, Tomoyasu T, Matsuzawa H. 1999. Balanced biosynthesis of major membrane components through regulated degradation of the committed enzyme of lipid A biosynthesis by the AAA protease FtsH (HflB) in Escherichia coli. Mol Microbiol 31:833–844. [PubMed] [DOI] [PubMed] [Google Scholar]
- 86.Katz C, Ron EZ. 2008. Dual role of FtsH in regulating lipopolysaccharide biosynthesis in Escherichia coli. J Bacteriol 190:7117–7122. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Führer F, Langklotz S, Narberhaus F. 2006. The C-terminal end of LpxC is required for degradation by the FtsH protease. Mol Microbiol 59:1025–1036. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Schäkermann M, Langklotz S, Narberhaus F. 2013. FtsH-mediated coordination of lipopolysaccharide biosynthesis in Escherichia coli correlates with the growth rate and the alarmone (p)ppGpp. J Bacteriol 195:1912–1919. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahalakshmi S, Sunayana MR, SaiSree L, Reddy M. 2014. yciM is an essential gene required for regulation of lipopolysaccharide synthesis in Escherichia coli. Mol Microbiol 91:145–157. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Nicolaes V, El Hajjaji H, Davis RM, Van der Henst C, Depuydt M, Leverrier P, Aertsen A, Haufroid V, Ollagnier de Choudens S, De Bolle X, Ruiz N, Collet JF. 2014. Insights into the function of YciM, a heat shock membrane protein required to maintain envelope integrity in Escherichia coli. J Bacteriol 196:300–309. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein G, Kobylak N, Lindner B, Stupak A, Raina S. 2014. Assembly of lipopolysaccharide in Escherichia coli requires the essential LapB heat shock protein. J Biol Chem 289:14829–14853. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Emiola A, Andrews SS, Heller C, George J. 2016. Crosstalk between the lipopolysaccharide and phospholipid pathways during outer membrane biogenesis in Escherichia coli. Proc Natl Acad Sci USA 113:3108–3113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Mendoza D. 2014. Temperature sensing by membranes. Annu Rev Microbiol 68:101–116. [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Carty SM, Sreekumar KR, Raetz CRH. 1999. Effect of cold shock on lipid A biosynthesis in Escherichia coli. Induction At 12 degrees C of an acyltransferase specific for palmitoleoyl-acyl carrier protein. J Biol Chem 274:9677–9685. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Vorachek-Warren MK, Carty SM, Lin S, Cotter RJ, Raetz CRH. 2002. An Escherichia coli mutant lacking the cold shock-induced palmitoleoyltransferase of lipid A biosynthesis: absence of unsaturated acyl chains and antibiotic hypersensitivity at 12 degrees C. J Biol Chem 277:14186–14193. [PubMed] [DOI] [PubMed] [Google Scholar]
- 96.Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, Hackett M, Miller SI. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189–198. [DOI] [PubMed] [Google Scholar]
- 97.Dalebroux ZD, Miller SI. 2014. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr Opin Microbiol 17:106–113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato A, Groisman EA, Howard Hughes Medical I, Howard Hughes Medical Institute. 2008. The PhoQ/PhoP regulatory network of Salmonella enterica. Adv Exp Med Biol 631:7–21. [PubMed] [DOI] [PubMed] [Google Scholar]
- 99.MacRitchie DM, Buelow DR, Price NL, Raivio TL. 2008. Two-component signaling and gram negative envelope stress response systems. Adv Exp Med Biol 631:80–110. [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CR. 2000. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J 19:5071–5080. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia W, El Zoeiby A, Petruzziello TN, Jayabalasingham B, Seyedirashti S, Bishop RE. 2004. Lipid trafficking controls endotoxin acylation in outer membranes of Escherichia coli. J Biol Chem 279:44966–44975. [PubMed] [DOI] [PubMed] [Google Scholar]
- 102.Trent MS, Pabich W, Raetz CRH, Miller SI. 2001. A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J Biol Chem 276:9083–9092. [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Reynolds CM, Ribeiro AA, McGrath SC, Cotter RJ, Raetz CR, Trent MS. 2006. An outer membrane enzyme encoded by Salmonella typhimurium lpxR that removes the 3′-acyloxyacyl moiety of lipid A. J Biol Chem 281:21974–21987. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winfield MD, Groisman EA. 2004. Phenotypic differences between Salmonella and Escherichia coli resulting from the disparate regulation of homologous genes. Proc Natl Acad Sci USA 101:17162–17167. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubin EJ, Herrera CM, Crofts AA, Trent MS. 2015. PmrD is required for modifications to Escherichia coli endotoxin that promote antimicrobial resistance. Antimicrob Agents Chemother 59:2051–2061. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gunn JS. 2008. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol 16:284–290. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Chen HD, Groisman EA. 2013. The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Annu Rev Microbiol 67:83–112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perez JC, Groisman EA. 2007. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol Microbiol 63:283–293. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wösten MMSM, Kox LFF, Chamnongpol S, Soncini FC, Groisman EA. 2000. A signal transduction system that responds to extracellular iron. Cell 103:113–125. [DOI] [PubMed] [Google Scholar]
- 110.Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. 2001. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem 276:43122–43131. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Trent MS, Ribeiro AA, Doerrler WT, Lin S, Cotter RJ, Raetz CR. 2001. Accumulation of a polyisoprene-linked amino sugar in polymyxin-resistant Salmonella typhimurium and Escherichia coli: structural characterization and transfer to lipid A in the periplasm. J Biol Chem 276:43132–43144. [PubMed] [DOI] [PubMed] [Google Scholar]
- 112.Lee H, Hsu FF, Turk J, Groisman EA. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J Bacteriol 186:4124–4133. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gibbons HS, Kalb SR, Cotter RJ, Raetz CR. 2005. Role of Mg2+ and pH in the modification of Salmonella lipid A after endocytosis by macrophage tumour cells. Mol Microbiol 55:425–440. [PubMed] [DOI] [PubMed] [Google Scholar]
- 114.Gibbons HS, Lin S, Cotter RJ, Raetz CR. 2000. Oxygen requirement for the biosynthesis of the S-2-hydroxymyristate moiety in Salmonella typhimurium lipid A. Function of LpxO, A new Fe2+/alpha-ketoglutarate-dependent dioxygenase homologue. J Biol Chem 275:32940–32949. [PubMed] [DOI] [PubMed] [Google Scholar]
- 115.Gibbons HS, Reynolds CM, Guan Z, Raetz CRH. 2008. An inner membrane dioxygenase that generates the 2-hydroxymyristate moiety of Salmonella lipid A. Biochemistry 47:2814–2825. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. 2012. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria. Proc Natl Acad Sci USA 109:8722–8727. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henderson JC, Fage CD, Cannon JR, Brodbelt JS, Keatinge-Clay AT, Trent MS. 2014. Antimicrobial peptide resistance of Vibrio cholerae results from an LPS modification pathway related to nonribosomal peptide synthetases. ACS Chem Biol 9:2382–2392. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rolin O, Muse SJ, Safi C, Elahi S, Gerdts V, Hittle LE, Ernst RK, Harvill ET, Preston A. 2014. Enzymatic modification of lipid A by ArnT protects Bordetella bronchiseptica against cationic peptides and is required for transmission. Infect Immun 82:491–499. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marr N, Tirsoaga A, Blanot D, Fernandez R, Caroff M. 2008. Glucosamine found as a substituent of both phosphate groups in Bordetella lipid A backbones: role of a BvgAS-activated ArnT ortholog. J Bacteriol 190:4281–4290. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanipes MI, Lin S, Cotter RJ, Raetz CRH. 2001. Ca2+-induced phosphoethanolamine transfer to the outer 3-deoxy-D-manno-octulosonic acid moiety of Escherichia coli lipopolysaccharide. A novel membrane enzyme dependent upon phosphatidylethanolamine. J Biol Chem 276:1156–1163. [PubMed] [DOI] [PubMed] [Google Scholar]
- 121.Reynolds CM, Kalb SR, Cotter RJ, Raetz CR. 2005. A phosphoethanolamine transferase specific for the outer 3-deoxy-D-manno-octulosonic acid residue of Escherichia coli lipopolysaccharide. Identification of the eptB gene and Ca2+ hypersensitivity of an eptB deletion mutant. J Biol Chem 280:21202–21211. [PubMed] [DOI] [PubMed] [Google Scholar]
- 122.Moon K, Gottesman S. 2009. A PhoQ/P-regulated small RNA regulates sensitivity of Escherichia coli to antimicrobial peptides. Mol Microbiol 74:1314–1330. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frirdich E, Lindner B, Holst O, Whitfield C. 2003. Overexpression of the waaZ gene leads to modification of the structure of the inner core region of Escherichia coli lipopolysaccharide, truncation of the outer core, and reduction of the amount of O polysaccharide on the cell surface. J Bacteriol 185:1659–1671. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klein G, Lindner B, Brade H, Raina S. 2011. Molecular basis of lipopolysaccharide heterogeneity in Escherichia coli: envelope stress-responsive regulators control the incorporation of glycoforms with a third 3-deoxy-α-D-manno-oct-2-ulosonic acid and rhamnose. J Biol Chem 286:42787–42807. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grabowicz M, Silhavy TJ. 2017. Envelope stress responses: an interconnected safety net. Trends Biochem Sci 42:232–242. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klein G, Müller-Loennies S, Lindner B, Kobylak N, Brade H, Raina S. 2013. Molecular and structural basis of inner core lipopolysaccharide alterations in Escherichia coli: incorporation of glucuronic acid and phosphoethanolamine in the heptose region. J Biol Chem 288:8111–8127. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wanner BL. 1993. Gene regulation by phosphate in enteric bacteria. J Cell Biochem 51:47–54. [PubMed] [DOI] [PubMed] [Google Scholar]
- 128.Baek JH, Lee SY. 2006. Novel gene members in the Pho regulon of Escherichia coli. FEMS Microbiol Lett 264:104–109. [PubMed] [DOI] [PubMed] [Google Scholar]
- 129.Perepelov AV, Chen T, Senchenkova SN, Filatov AV, Song J, Shashkov AS, Liu B, Knirel YA. 2018. Structure and genetics of the O-specific polysaccharide of Escherichia coli O27. Carbohydr Res 456:1–4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 130.Iguchi A, Iyoda S, Kikuchi T, Ogura Y, Katsura K, Ohnishi M, Hayashi T, Thomson NR. 2015. A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Res 22:101–107. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meredith TC, Mamat U, Kaczynski Z, Lindner B, Holst O, Woodard RW. 2007. Modification of lipopolysaccharide with colanic acid (M-antigen) repeats in Escherichia coli. J Biol Chem 282:7790–7798. [PubMed] [DOI] [PubMed] [Google Scholar]
- 132.Clarke DJ. 2010. The Rcs phosphorelay: more than just a two-component pathway. Future Microbiol 5:1173–1184. [PubMed] [DOI] [PubMed] [Google Scholar]
- 133.Pinta E, Li Z, Batzilla J, Pajunen M, Kasanen T, Rabsztyn K, Rakin A, Skurnik M. 2012. Identification of three oligo-/polysaccharide-specific ligases in Yersinia enterocolitica. Mol Microbiol 83:125–136. [PubMed] [DOI] [PubMed] [Google Scholar]
- 134.Gozdziewicz TK, Lugowski C, Lukasiewicz J. 2014. First evidence for a covalent linkage between enterobacterial common antigen and lipopolysaccharide in Shigella sonnei phase II ECALPS. J Biol Chem 289:2745–2754. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grabowicz M, Andres D, Lebar MD, Malojčić G, Kahne D, Silhavy TJ. 2014. A mutant Escherichia coli that attaches peptidoglycan to lipopolysaccharide and displays cell wall on its surface. eLife 3:e05334. 10.7554/eLife.05334. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karow M, Georgopoulos C. 1993. The essential Escherichia coli msbA gene, a multicopy suppressor of null mutations in the htrB gene, is related to the universally conserved family of ATP-dependent translocators. Mol Microbiol 7:69–79. [PubMed] [DOI] [PubMed] [Google Scholar]
- 137.Doerrler WT, Raetz CR. 2002. ATPase activity of the MsbA lipid flippase of Escherichia coli. J Biol Chem 277:36697–36705. [PubMed] [DOI] [PubMed] [Google Scholar]
- 138.Ward A, Reyes CL, Yu J, Roth CB, Chang G. 2007. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc Natl Acad Sci USA 104:19005–19010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doerrler WT, Gibbons HS, Raetz CR. 2004. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J Biol Chem 279:45102–45109. [PubMed] [DOI] [PubMed] [Google Scholar]
- 140.Eckford PD, Sharom FJ. 2010. The reconstituted Escherichia coli MsbA protein displays lipid flippase activity. Biochem J 429:195–203. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mi W, Li Y, Yoon SH, Ernst RK, Walz T, Liao M. 2017. Structural basis of MsbA-mediated lipopolysaccharide transport. Nature 549:233–237. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ho H, Miu A, Alexander MK, Garcia NK, Oh A, Zilberleyb I, Reichelt M, Austin CD, Tam C, Shriver S, Hu H, Labadie SS, Liang J, Wang L, Wang J, Lu Y, Purkey HE, Quinn J, Franke Y, Clark K, Beresini MH, Tan MW, Sellers BD, Maurer T, Koehler MFT, Wecksler AT, Kiefer JR, Verma V, Xu Y, Nishiyama M, Payandeh J, Koth CM. 2018. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 557:196–201. [PubMed] [DOI] [PubMed] [Google Scholar]
- 143.Reynolds CM, Raetz CR. 2009. Replacement of lipopolysaccharide with free lipid A molecules in Escherichia coli mutants lacking all core sugars. Biochemistry 48:9627–9640. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Klein G, Lindner B, Brabetz W, Brade H, Raina S. 2009. Escherichia coli K-12 suppressor-free mutants lacking early glycosyltransferases and late acyltransferases: minimal lipopolysaccharide structure and induction of envelope stress response. J Biol Chem 284:15369–15389. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meredith TC, Aggarwal P, Mamat U, Lindner B, Woodard RW. 2006. Redefining the requisite lipopolysaccharide structure in Escherichia coli. ACS Chem Biol 1:33–42. [PubMed] [DOI] [PubMed] [Google Scholar]
- 146.Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. 2016. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol 14:337–345. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sherman DJ, Lazarus MB, Murphy L, Liu C, Walker S, Ruiz N, Kahne D. 2014. Decoupling catalytic activity from biological function of the ATPase that powers lipopolysaccharide transport. Proc Natl Acad Sci USA 111:4982–4987. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Okuda S, Freinkman E, Kahne D. 2012. Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. coli. Science 338:1214–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luo Q, Yang X, Yu S, Shi H, Wang K, Xiao L, Zhu G, Sun C, Li T, Li D, Zhang X, Zhou M, Huang Y. 2017. Structural basis for lipopolysaccharide extraction by ABC transporter LptB2FG. Nat Struct Mol Biol 24:469–474. [PubMed] [DOI] [PubMed] [Google Scholar]
- 150.Dong H, Zhang Z, Tang X, Paterson NG, Dong C. 2017. Structural and functional insights into the lipopolysaccharide ABC transporter LptB2FG. Nat Commun 8:222. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ruiz N, Gronenberg LS, Kahne D, Silhavy TJ. 2008. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc Natl Acad Sci USA 105:5537–5542. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Villa R, Martorana AM, Okuda S, Gourlay LJ, Nardini M, Sperandeo P, Dehò G, Bolognesi M, Kahne D, Polissi A. 2013. The Escherichia coli Lpt transenvelope protein complex for lipopolysaccharide export is assembled via conserved structurally homologous domains. J Bacteriol 195:1100–1108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tran AX, Dong C, Whitfield C. 2010. Structure and functional analysis of LptC, a conserved membrane protein involved in the lipopolysaccharide export pathway in Escherichia coli. J Biol Chem 285:33529–33539. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suits MD, Sperandeo P, Dehò G, Polissi A, Jia Z. 2008. Novel structure of the conserved gram-negative lipopolysaccharide transport protein A and mutagenesis analysis. J Mol Biol 380:476–488. [PubMed] [DOI] [PubMed] [Google Scholar]
- 155.Freinkman E, Okuda S, Ruiz N, Kahne D. 2012. Regulated assembly of the transenvelope protein complex required for lipopolysaccharide export. Biochemistry 51:4800–4806. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dong H, Xiang Q, Gu Y, Wang Z, Paterson NG, Stansfeld PJ, He C, Zhang Y, Wang W, Dong C. 2014. Structural basis for outer membrane lipopolysaccharide insertion. Nature 511:52–56. [PubMed] [DOI] [PubMed] [Google Scholar]
- 157.Li X, Gu Y, Dong H, Wang W, Dong C. 2015. Trapped lipopolysaccharide and LptD intermediates reveal lipopolysaccharide translocation steps across the Escherichia coli outer membrane. Sci Rep 5:11883. 10.1038/srep11883. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sherman DJ, Xie R, Taylor RJ, George AH, Okuda S, Foster PJ, Needleman DJ, Kahne D. 2018. Lipopolysaccharide is transported to the cell surface by a membrane-to-membrane protein bridge. Science 359:798–801. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Merten JA, Schultz KM, Klug CS. 2012. Concentration-dependent oligomerization and oligomeric arrangement of LptA. Protein Sci 21:211–218. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Laguri C, Sperandeo P, Pounot K, Ayala I, Silipo A, Bougault CM, Molinaro A, Polissi A, Simorre JP. 2017. Interaction of lipopolysaccharides at intermolecular sites of the periplasmic Lpt transport assembly. Sci Rep 7:9715. 10.1038/s41598-017-10136-0. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cohen EJ, Ferreira JL, Ladinsky MS, Beeby M, Hughes KT. 2017. Nanoscale-length control of the flagellar driveshaft requires hitting the tethered outer membrane. Science 356:197–200. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Freinkman E, Chng SS, Kahne D. 2011. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc Natl Acad Sci USA 108:2486–2491. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Malojčić G, Andres D, Grabowicz M, George AH, Ruiz N, Silhavy TJ, Kahne D. 2014. LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface. Proc Natl Acad Sci USA 111:9467–9472. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chng SS, Ruiz N, Chimalakonda G, Silhavy TJ, Kahne D. 2010. Characterization of the two-protein complex in Escherichia coli responsible for lipopolysaccharide assembly at the outer membrane. Proc Natl Acad Sci USA 107:5363–5368. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chimalakonda G, Ruiz N, Chng SS, Garner RA, Kahne D, Silhavy TJ. 2011. Lipoprotein LptE is required for the assembly of LptD by the beta-barrel assembly machine in the outer membrane of Escherichia coli. Proc Natl Acad Sci USA 108:2492–2497. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449–450. [PubMed] [DOI] [PubMed] [Google Scholar]
- 167.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peng D, Hong W, Choudhury BP, Carlson RW, Gu XX. 2005. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun 73:7569–7577. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boll JM, Crofts AA, Peters K, Cattoir V, Vollmer W, Davies BW, Trent MS. 2016. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc Natl Acad Sci USA 113:E6228–E6237. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Powers MJ, Trent MS. 2018. Expanding the paradigm for the outer membrane: Acinetobacter baumannii in the absence of endotoxin. Mol Microbiol 107:47–56. 10.1111/mmi.13872. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Erwin AL. 2016. Antibacterial drug discovery targeting the lipopolysaccharide biosynthetic enzyme LpxC. Cold Spring Harb Perspect Med 6:a025304. 10.1101/cshperspect.a025304. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Werneburg M, Zerbe K, Juhas M, Bigler L, Stalder U, Kaech A, Ziegler U, Obrecht D, Eberl L, Robinson JA. 2012. Inhibition of lipopolysaccharide transport to the outer membrane in Pseudomonas aeruginosa by peptidomimetic antibiotics. ChemBioChem 13:1767–1775. [PubMed] [DOI] [PubMed] [Google Scholar]
- 173.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RLA, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Käch A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. 2010. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327:1010–1013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 174.Sperandeo P, Martorana AM, Polissi A. 2017. Lipopolysaccharide biogenesis and transport at the outer membrane of Gram-negative bacteria. Biochim Biophys Acta 1862:1451–1460. [PubMed] [DOI] [PubMed] [Google Scholar]


