Abstract
Objectives: In this study, we investigated the antimicrobial activity of resveratrol in combination with colistin, a last-resort agent for the treatment of severe infections caused by multidrug resistant Gram-negative pathogens.
Methods: The synergistic activity and the bactericidal activity of colistin in combination with resveratrol was investigated by checkerboard assays and time-kill assays, respectively. A total of 21 strains were investigated, including 16 strains of different species (Klebsiella pneumoniae, n = 6, Escherichia coli, n = 6; Citrobacter braakii, n = 1; Stenotrophomonas malthophilia, n = 1; Enterobacter cloaceae, n = 1; Acinetobacter baumannii, n = 1) with acquired colistin resistance, three colistin-susceptible K. pneumoniae precursors, and two strains of intrinsically colistin-resistant species (Serratia marcescens, n = 1; Proteus mirabilis, n = 1). Mechanisms of acquired colistin resistance included chromosomal mutations (i.e., mgrB, pmrAB) and plasmid genes (mcr-1, mcr-1.2).
Results: Resveratrol did not show any significant intrinsic antimicrobial activity. Overall, a relevant synergistic antimicrobial activity of resveratrol in combination with colistin was observed with all tested strains, except for the three colistin-susceptible K. pneumoniae strains, and for two mcr-1-positive E. coli strains. In time-kill assays, performed with 15 selected strains, the combination of colistin 2 mg/L plus resveratrol 128 mg/L was bactericidal with 11 strains, and bacteriostatic for the remaining ones.
Conclusions: Resveratrol was found to potentiate colistin activity against a wide panel of colistin-resistant strains, regardless of species and resistance mechanisms, which would deserve further investigation for potential clinical applications.
Keywords: colistin, resveratrol, colistin resistance, antibiotic resistance breakers, combination therapy
Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid compound found in numerous plants. Resveratrol has been investigated for potential therapeutic effects in various diseases (Chen et al., 2005; Albani et al., 2010; Sawda et al., 2010; Sun et al., 2010; Singh et al., 2014; Li et al., 2016, 2017) and has also shown the potential for antiviral (Abba et al., 2015; Lin et al., 2017a,b) and antibacterial activity against some pathogens, including Helicobacter pylori, Propionibacterium acnes, and Staphylococcus aureus (Mahady et al., 2003; Su et al., 2014; Taylor et al., 2014). Anti-oxydant activity and interaction with various molecular targets, including kinases, sirtuins, and cytokines, have been suggested as mechanisms responsible for resveratrol activity, although knowledge on this aspect remains limited (Kuršvietiene et al., 2016).
Polymyxins are old antibiotics that, until recently, were rarely used in the clinical setting except for the practice of Selective Digestive Decontamination (SDD), carried out in some ICU settings to reduce infections caused by microorganisms from oropharyngeal and gastrointestinal tracts (Abis et al., 2013; Bar-Yoseph et al., 2016; Rawson et al., 2016). Recently, due to the emergence of extremely drug resistant (XDR) strains of Gram-negative pathogens, such as carbapenem-resistant Enterobacteriaceae (CRE) and carbapenem-resistant Acinetobacter sp. (CRA), polymyxins have regained a major role as last-resort agents for these infections, and their consumption has remarkably increased (Falagas and Kasiakou, 2005; Kaye et al., 2016). Unfortunately, also polymyxin resistance has emerged and is now increasingly reported, especially among CRE and CRA (Cannatelli et al., 2013; Monaco et al., 2014; Granata and Petrosillo, 2017; Jeannot et al., 2017; Nowak et al., 2017), further narrowing the treatment options.
In this study, we have tested the in vitro activity of resveratrol, alone and in combination with colistin, against a collection of colistin-resistant (COL-R) Gram-negative pathogens of different species. Despite the lack of any significant intrinsic antimicrobial activity, resveratrol exhibited a strong synergistic effect with colistin against many COL-R strains of different species, including Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Stenotrophomonas maltophilia, Citrobacter braakii, and also enterobacterial species that are naturally resistant to polymyxins (e.g., Proteus mirabilis and Serratia marcescens).
Materials and methods
Bacterial strains
Bacterial strains investigated in this work are listed in Table 1. These included 18 COL-R strains of different species (A. baumannii, K. pneumoniae, E. coli, E. cloacae, S. maltophilia, C. braakii, P. mirabilis, and S. marcescens) and three colistin-susceptible (COL-S) K. pneumoniae that were precursors of three COL-R strains. For S. maltophilia, for which clinical breakpoints for colistin are not available, the definition as COL-R was arbitrarily based on the high-level colistin MIC (i.e., 32 mg/L) as compared with the colistin MIC distribution for the species (Sergio et al., 2017). For some strains, the mechanism of colistin resistance had been previously characterized (Table 1).
Table 1.
Results of checkerboard assays of colistin in combination with resveratrol.
| Strains | Source | Phenotype | ST | Reference | Mechanism of colistin resistance | Colistin MICs (mg/L) at different resveratrol concentrations (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resveratrol concentration (mg/L) | ||||||||||||
| 0 | 8 | 16 | 32 | 64 | 128 | 256 | ||||||
| E. coli LC711/14 | Urine | COL-R | ST59 | Cannatelli et al., 2017 | PmrB Leu10Pro | 8 | 4 | 2* | 1* | 0.25* | 0.25* | 0.125* |
| E. coli LC761/12 | Urine | COL-R | ST131 | This study | PmrA Asp82Asnc | 4 | 0.5* | 0.5* | 0.25* | 0.125* | 0.125* | 0.125* |
| E. coli FI-4451 | Urine | COL-R | ST117 | Cannatelli et al., 2016 | mcr-1 | 8 | 8 | 8 | 4 | 4 | 4 | 4 |
| E. coli FI-4531 | Urine | COL-R | ST648 | Cannatelli et al., 2016 | mcr-1 | 8 | 4 | 4 | 4 | 4 | 2* | 2* |
| E. coli FI-4592 | Urine | COL-R | ST804 | Cannatelli et al., 2016 | mcr-1 | 8 | 4 | 4 | 4 | 4 | 4 | 4 |
| E. coli LC902/14 | Urine | COL-R | ST602 | Cannatelli et al., 2016 | mcr-1 | 8 | 4 | 4 | 4 | 4 | 2* | 2* |
| C. braakii CA-26 | Food | COL-R | – | Sennati et al., 2017 | mcr-1 | 8 | 4 | 4 | 4 | 4 | 2* | 2* |
| S. maltophilia 157 | – | COL-R | n.d. | This study | n.d.d | 32 | 4 | 2* | 1* | 0.5* | 0.125* | 0.125* |
| E. cloacae CIP6085 | – | COL-R | n.d. | – | n.d.e | 128 | 128 | 32* | 8* | 0.5* | 0.25* | 0.125* |
| K. pneumoniae KKBO-1a | Blood | MDR/COL-S | ST258 | Cannatelli et al., 2013 | – | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| K. pneumoniae KKBO-4 | Blood | MDR/COL-R | ST258 | Cannatelli et al., 2013 | IS5-like at nt.75 of mgrB | 64 | 64 | 64 | 16* | 8* | 4* | 4* |
| K. pneumoniae KPB-1a | Blood | MDR/COL-S | ST512 | Cannatelli et al., 2014a | – | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| K. pneumoniae KPB-2 | Blood | MDR/COL-R | ST512 | Cannatelli et al., 2014a | PmrB Leu82Arg | 4 | 4 | 4 | 4 | 2 | 1* | 0.5* |
| K. pneumoniae KK207-1a | Blood | MDR/COL-S | ST258 | This study | – | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 |
| K. pneumoniae KK207-2 | Blood | MDR/COL-R | ST258 | Cannatelli et al., 2014b | IS5-like at nt.75 of mgrB | 64 | 64 | 64 | 64 | 4* | 1* | 0.5* |
| K. pneumoniae 6884 | Blood | MDR/COL-R | ST512 | Di Pilato et al., 2016 | mcr-1.2 | 8 | 8 | 8 | 8 | 4 | 1* | 1* |
| K. pneumoniae KPFanb | – | COL-R | ST674 | Cannatelli et al., 2015 | IS102-like at nt.69 of mgrB | 32 | 32 | 16 | 8* | 2* | 1* | 1* |
| K. pneumoniae KPGP1b | – | COL-R | ST16 | Cannatelli et al., 2015 | ISkpn14-like at nt.124 of mgrB | 32 | 32 | 32 | 32 | 2* | 1* | 1* |
| A. baumannii N50 | Respiratory tract | MDR/COL-R | ST2 | D'Andrea et al., 2009 | ISAva1 at nt. 531 of pmrB | 64 | 64 | 64 | 64 | 8* | 1* | 1* |
| S. marcescens CCUG1647T | – | COL-R | n.d. | – | Naturally resistant | >128 | >128 | >128 | >128 | 16* | 1* | 1* |
| P. mirabilis NO-051 | Cutaneous ulcer | COL-R | n.d. | D'Andrea et al., 2006 | Naturally resistant | >128 | >128 | >128 | >128 | >128 | >128 | 8* |
These strains were the COL-S precursors of COL-R K. pneumoniae KKBO-4, K. pneumoniae KPB-2, and K. pneumoniae KK207-2 strains, respectively. Resveratrol MICs were >512 mg/L for all tested strains.
These COL-R strains were selected in vitro, using two COL-S precursors (Cannatelli et al., 2015). Multidrug-resistant phenotypes (MDR) refer to strains resistant to carbapenems (imipenem and meropenem), ciprofloxacin, and amikacin. n.d. = not determined.
Colistin resistance in E. coli LC761/12 was putatively associate to PmrA Asp82Asn novel allelic variant, being absent all other known mechanisms of acquired colistin resistance in E. coli (data not shown).
The colistin resistance mechanism in this strain is unknown.
Enterobacter CIP6085 was found to be negative for mcr-1 and mcr-2 detection. The colistin resistance mechanism in this strain is unknown.
Combinations in which colistin/resveratrol combinations yielded a synergistic activity (FICI ≤ 0.5). The lower concentration of resveratrol needed for restoring susceptibility to colistin is shaded in gray.
Chemicals
Colistin sulfate and resveratrol were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France). Resveratrol (Thermo Fisher, Germany) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Saint Louis, USA) at a concentration of 20 mg/mL.
In vitro susceptibility testing, checkerboard assays, and time-kill assays
MICs of colistin and resveratrol were determined by reference broth microdilution (Clinical and Laboratory Standards Institute, 2015) using cation-adjusted Mueller-Hinton broth (MHB) (bioMérieux, Florence, Italy). Colistin MICs were interpreted accordingly to the EUCAST clinical breakpoints, version 8.0 (www.eucast.org). Checkerboard assays to test the antimicrobial activity of combinations of colistin plus resveratrol were carried out as described previously (Tascini et al., 2013), using MHB and 96-well microtiter plates (Sarsted, Nümbrecht, Germany). Each well was inoculated with 50 μl of a suspension of 5 × 105 CFU/mL of the test strain in a final volume of 100 μl. Inocula were prepared by direct suspension in MHB of bacteria grown overnight onto MH agar plates. Results were read after incubation at 35°C for 16–20 h and interpreted as follows: FICI ≤ 0.5, synergism; FICI > 4.0 antagonism; FICI 0.5–4 no interaction. Data were obtained in at least two independent experiments.
Time–kill assays were performed in duplicate, by inoculating 5 × 106 CFU of each strain into 2 mL of MHB in 24 Deep Well RB Block (Thermo Fisher Scientific, MA USA), at 35°C, under static condition (Clinical and Laboratory Standards Institute, 2015). CFU counts were determined at different time points, by plating appropriate dilutions onto LB Agar (Sezonov et al., 2007).
In time kill assays, the DMSO concentration remained always below 1% (v/v), as recommended by CLSI guidelines (Clinical and Laboratory Standards Institute, 2015). In MIC testing and checkerboard assays, the conditions with resveratrol concentrations of 256 and 512 mg/L contained DMSO concentrations higher than 1% (i.e., 1.36 and 2.72%, respectively). Appropriate controls to exclude any potential synergistic activity between colistin and DMSO were always included.
Results and discussion
Synergistic activity of colistin in combination with resveratrol in checkerboard assays
A collection of 21 strains of eight different Gram-negative species were tested for susceptibility to resveratrol, colistin, and combinations thereof. The collection included 15 COL-R strains of species that are naturally susceptible to colistin (C. braakii, E. coli, K. pneumoniae, E. cloacae, A. baumannii), two COL-R strains of naturally resistant species (S. marcescens and P. mirabilis), and one S. maltophilia strain with high colistin MIC (that was considered COL-R for the purpose of this work). It also included three COL-S strains of K. pneumoniae, which were the precursors of three of the COL-R strains (Table 1).
MICs of resveratrol were >512 mg/L for all tested strains, showing the lack of any intrinsic antimicrobial activity of resveratrol alone against these Gram-negative pathogens.
Checkerboard assays revealed a clear dose-dependent synergistic activity of resveratrol with colistin vs. COL-R strains of different species, including intrinsically resistant species such as S. marcescens and P. mirabilis. Synergism was not observed with two of the four COL-R E. coli strains carrying the mcr-1 determinant and with the COL-S K. pneumoniae precursors of three COL-R strains (Table 1).
When synergism was evident, resveratrol was able to decrease colistin MICs to values equal or lower than the susceptibility breakpoint (i.e., 2 mg/L) in most cases, at concentrations variable from 8 to 128 mg/L. In particular, this was the case with four of the six COL-R E. coli, with five of the six COL-R K. pneumoniae, with the COL-R C. braakii, with the COL-R strains of A. baumanni, E. cloacae and S. maltophilia, and with the type strain of S. marcescens. The synergistic effect was observed in presence of different colistin resistance mechanisms and with strains of different clonal lineages, including representatives of known high risk clones (e.g., ST131 and ST59 for E. coli, or ST512 and ST258 for K. pneumoniae; Table 1).
Synergistic activity of resveratrol and colistin in time-kill assays
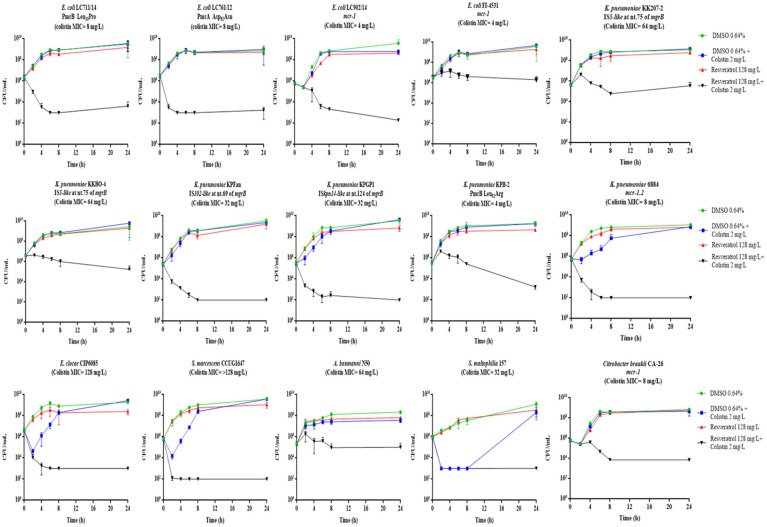
In order to investigate if colistin in combination with resveratrol had a bactericidal effect, time-kill assays were carried out with the 15 COL-R strains for which checkerboard assays had showed a synergistic effect. The colistin concentration used in time-kill experiments corresponded to the clinical breakpoint for susceptibility (2 mg/L), or to 0.5 × MIC and 1 × MIC, while the resveratrol concentration used was 128 mg/L, which was able to inhibit the growth in most cases when combined with colistin at 2 mg/L (Table 1).
The time-kill assays showed a bactericidal activity (i.e., a reduction ≥3 log10 of the initial bacterial inoculum) of resveratrol 128 mg/L in combination with colistin 2 mg/L with 11 of the 15 COL-R strains tested, while with four strains (E. coli FI-4531, K. pneumoniae KKBO-4, K. pneumoniae KK207-2, A. baumannii N50) the combination exerted a static effect (Figure 1). Overall, with these strains when colistin was used at 0.5 X MIC and 1 X MIC in combination with resveratrol 128 mg/L, a bactericidal effect was observed, except for E. coli FI-4531, in which this combinations seem to be less effectives (Figure 2).
Figure 1.
Time-kill assays with colistin 2 mg/L in combination with resveratrol 128 mg/L. Data are mean values from the results of two independent experiments, and the error bars represent standard deviations. The lowest number of CFU (detection limit) that can be detected by the method used is 1E+02 CFU/mL.
Figure 2.
Time-kill assays with colistin at 0.5 or 1 X MIC in combination with resveratrol 128 mg/L, in the strains in which colistin 2 mg/L plus resveratrol 128 mg/L yielded a bacteriostatic effect. Data are mean values from the results of two independent experiments, and the error bars represent standard deviations. The lowest number of CFU (detection limit) that can be detected by the method used is 1E+02 CFU/mL.
Altogether, the results were consistent with data obtained in checkerboard assays (Table 1). In fact, all strains exhibiting a colistin MIC <1 mg/L or >2 mg/L in combination with resveratrol 128 mg/L, showed a bactericidal or bacteriostatic effect in time-kill curves, respectively. On the other hand, a variable effect was observed with strains for which, in the presence of resveratrol 128 mg/L, colistin MIC was lowered to 2 or 1 mg/L (Figure 1).
Considering the diversity of species tested, (C. braakii, E. coli, K. pneumoniae, E. cloacae, S. marcescens, A. baumannii, S. maltophilia) expressing different colistin resistance mechanisms, the “cidal/static” activity of colistin 2 mg/L in combination with resveratrol 128 mg/L did not appear to be dependent on species or the resistance mechanism.
The absence of any synergistic activity with COL-S strains could suggest a likely resveratrol interaction with the lipid A modification systems that are responsible for colistin resistance in COL-R strains. However, the mechanism of synergism observed between resveratrol and colistin with COL-R strains remains unknown and will be the subjects of further investigations.
Concluding remarks
MDR and XDR Gram-negative bacteria (e.g., CRE and CRA) have been increasingly reported worldwide (Cannatelli et al., 2013; Monaco et al., 2014), and are listed among resistant pathogens with the highest priority for research and development of new antibiotics by the WHO (WHO, 2017).
Colistin remains one of the few antibiotics active against these pathogens, and represents a drug of last resort for the treatment of CRE and CRA severe infections (Falagas and Kasiakou, 2005; Kaye et al., 2016). It is also used for the Selective Digestive Decontamination (SDD) in combination with other agents (i.e., tobramycin, amphotericin B) (Abis et al., 2013; Rawson et al., 2016), and is increasingly administered for the management of chronic lung colonization by Pseudomonas aeruginosa in cystic fibrosis (Sherrard et al., 2014). In this perspective, the increasingly dissemination of colistin resistance in these pathogens represents a matter of public health concern (Cannatelli et al., 2013; Monaco et al., 2014; Granata and Petrosillo, 2017; Jeannot et al., 2017; Nowak et al., 2017).
This worrisome scenario has forced the scientific community to evaluate new therapeutic approaches to face the antibiotic resistance crisis. One promising strategy is offered by non-antibiotic drugs which overcome the resistance mechanism (Antibiotic Resistance Breakers; ARB) when combined with failing antibiotics (Brown, 2015). A well-proven example of such approach is represented by the new beta-lactamase inhibitors (i.e., avibactam, vaborbactam; Giani et al., 2016). Nonetheless, considering the multitude of resistance determinants and their rapid evolution potential, additional solutions must be implemented. Among the diverse approaches investigated over the last years, some natural compounds (i.e., resveratrol, quercetin, curcumin, pterostilbene), which have shown anti-bacterial properties (Su et al., 2014; Taylor et al., 2014; Hwang and Lim, 2015; Kuršvietiene et al., 2016; Zhou et al., 2018), could also be of interest.
In this work we provided the first in vitro demonstration that resveratrol can act as an ARB, potentiating colistin activity against a collection of COL-R Gram-negative pathogens, covering a wide panel of species and colistin resistance mechanisms. A limitation of this study was that the synergism between resveratrol and colistin was not tested with COL-R strains of P. aeruginosa, which at the time of the study were not available in our collection.
The potential to exploit this feature in vivo could depend on the resveratrol concentrations achievable in vivo, at different body sites. A number of pre-clinical and clinical studies have previously investigated resveratrol administered orally (Tomé-Carneiro et al., 2013), intravenously (Tomé-Carneiro et al., 2013), or by inhalation (Varricchio et al., 2014), but current knowledge on resveratrol pharmacokinetics in humans remain limited. When given orally, resveratrol is absorbed but readily metabolized, leading to a rather low bioavailability (Walle et al., 2004; Boocock et al., 2007; Tomé-Carneiro et al., 2013), while the non-absorbed fraction can be transformed by components of the gut microbiota (Tomé-Carneiro et al., 2013). Several resveratrol-derived metabolites have been identified in human and animals following oral or parenteral administration, including trans- and/or cis- forms of mono- and diglucuronides, mono- and disulfates, sulfoglucuronides, and dihydroresveratrol metabolities, which undergo renal and fecal excretion (Walle et al., 2004; Boocock et al., 2007; Tomé-Carneiro et al., 2013).
Consider that the activity of specific circulating resveratrol metabolites is still under debate, and the concentration of unchanged resveratrol in human urine, feaces and plasma has still not been clearly determined, the use of colistin/resveratrol combinations for Selective Digestive Decontamination or treatment of urinary and systemic infections would deserve further investigation.
The most promising setting to exploit the synergism between resveratrol and colistin would be that of respiratory tract infections, where the administration of inhaled formulations of resveratrol might overcome the issues related to low bioavailability and metabolism of this compound. Being resveratrol also administered by nebulization in humans (Varricchio et al., 2014), the potential efficacy of colistin/resveratrol inhaled formulations could deserve further attention, especially in cases of chronic lung colonization by difficult-to-treat Gram-negatives, such as in cystic fibrosis, chronic obstructive pulmonary disease or bronchiectasis not related to cystic fibrosis.
Present results represent a proof of principle for further studies aimed at evaluating the potential role of resveratrol as colistin ARB in other in vitro (e.g., biofilm susceptibility testing) and in vivo models (e.g., Selective decontamination of the digestive tract).
- “The content is object of Italian Patent Application No. 102017000025738 filed on 08.03.2017”
- “The content is object of International Patent Application No. PCT/EP2018/055595 filed on 22.05.2018.”
Author contributions
AC, LP and GR: study design, data analysis or interpretation, manuscript preparation. AC, SP, and OC: experimental studies, statistical analysis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MB and handling Editor declared their shared affiliation.
References
- Abba Y., Hassim H., Hamzah H., Noordin M. M. (2015). Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015:184241. 10.1155/2015/184241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abis G. S., Stockmann H. B., van Egmond M., Bonjer H. J., Vandenbroucke-Grauls C. M., Oosterling S. J. (2013). Selective decontamination of the digestive tract in gastrointestinal surgery: useful in infection prevention? A systematic review. J. Gastrointest. Surg. 17, 2172–2178. 10.1007/s11605-013-2379-y [DOI] [PubMed] [Google Scholar]
- Albani D., Polito L., Forloni G. (2010). Sirtuins as novel targets for Alzheimer's disease and other neurodegenerative disorders: experimental and genetic evidence. J. Alzheimers Dis. 19, 11–26. 10.3233/JAD-2010-1215 [DOI] [PubMed] [Google Scholar]
- Bar-Yoseph H., Hussein K., Braun E., Paul M. (2016). Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J. Antimicrob. Chemother. 71, 2729–2739. 10.1093/jac/dkw221 [DOI] [PubMed] [Google Scholar]
- Boocock D. J., Faust G. E., Patel K. R., Schinas A. M., Brown V. A., Ducharme M. P., et al. (2007). Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 16, 1246–1252. 10.1158/1055-9965.EPI-07-0022 [DOI] [PubMed] [Google Scholar]
- Brown D. (2015). Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 14, 821–832. 10.1038/nrd4675 [DOI] [PubMed] [Google Scholar]
- Cannatelli A., D'Andrea M. M., Giani T., Di Pilato V., Arena F., Ambretti S., et al. (2013). In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob. Agents Chemother. 57, 5521–5526. 10.1128/AAC.01480-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannatelli A., Di Pilato V., Giani T., Arena F., Ambretti S., Gaibani P., et al. (2014a). In vivo evolution to colistin resistance by PmrB sensor kinase mutation in KPC-producing Klebsiella pneumoniae is associated with low-dosage colistin treatment. Antimicrob. Agents Chemother. 58, 4399–4403. 10.1128/AAC.02555-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannatelli A., Giani T., Aiezza N., Di Pilato V., Principe L., Luzzaro F., et al. (2017). An allelic variant of the PmrB sensor kinase responsible for colistin resistance in an Escherichia coli strain of clinical origin. Sci. Rep. 7:5071. 10.1038/s41598-017-05167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannatelli A., Giani T., Antonelli A., Principe L., Luzzaro F., Rossolini G. M. (2016). First detection of the mcr-1 colistin resistance gene in Escherichia coli in Italy. Antimicrob. Agents Chemother. 60, 3257–3258. 10.1128/AAC.00246-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannatelli A., Giani T., D'Andrea M. M., Di Pilato V., Arena F., Conte V., et al. (2014b). MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob. Agents Chemother. 58, 5696–5703. 10.1128/AAC.03110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannatelli A., Santos-Lopez A., Giani T., Gonzalez-Zorn B., Rossolini G. M., et al. (2015). Polymyxin resistance caused by mgrB inactivation is not associated with significant biological cost in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 59, 2898–2900. 10.1128/AAC.04998-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Jang J. H., Li M. H., Surh Y. J. (2005). Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys Res. Commun. 331, 993–1000. 10.1016/j.bbrc.2005.03.237 [DOI] [PubMed] [Google Scholar]
- D'Andrea M. M., Giani T., D'Arezzo S., Capone A., Petrosillo N., Visca P., et al. (2009). Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 53, 3528–3533. 10.1128/AAC.00178-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea M. M., Nucleo E., Luzzaro F., Giani T., Migliavacca R., Vailati F., et al. (2006). CMY-16, a novel acquired AmpC-type beta-lactamase of the CMY/LAT lineage in multifocal monophyletic isolates of Proteus mirabilis from northern Italy. Antimicrob. Agents Chemother. 50, 618–624. 10.1128/AAC.50.2.618-624.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pilato V., Arena F., Tascini C., Cannatelli A, Henrici De Angelis L., Fortunato S., et al. (2016). mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob. Agents Chemother. 60, 5612–5615. 10.1128/AAC.01075-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M. E., Kasiakou S. K. (2005). Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40, 1333–1341. 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- Giani T., Cannatelli A., Di Pilato V., Testa R., Nichols W. W., Rossolini G. M. (2016). Inhibitory activity of avibactam against selected β-lactamases expressed in an isogenic Escherichia coli strain. Diagn. Microbiol. Infect. Dis. 86, 83–85. 10.1016/j.diagmicrobio.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Granata G., Petrosillo N. (2017). Resistance to colistin in Klebsiella pneumoniae: a 4.0 strain? Infect. Dis. Rep. 9:7104. 10.4081/idr.2017.7104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D., Lim Y. H. (2015). Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 5:10029. 10.1038/srep10029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannot K., Bolard A., Plésiat P. (2017). Resistance to polymyxins in gram-negative organisms. Int. J. Antimicrob. Agents. 49, 526–535. 10.1016/j.ijantimicag.2016.11.029 [DOI] [PubMed] [Google Scholar]
- Kaye K. S., Pogue J. M., Tran T. B., Nation R. L., Li J. (2016). Agents of last resort: polymyxin resistance. Infect. Dis. Clin. North Am. 30, 391–414. 10.1016/j.idc.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Kuršvietiene L., Stanevičiene I., Mongirdiene A., Bernatoniene J. (2016). Multiplicity of effects and health benefits of resveratrol. Medicina 52, 148–155. 10.1016/j.medici.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Li W., Cao L., Chen X., Lei J., Ma Q. (2016). Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol. Rep. 35, 1718–1726. 10.3892/or.2015.4504 [DOI] [PubMed] [Google Scholar]
- Li Y. R., Li S., Lin C. C. (2017). Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 44, 69–82. 10.1002/biof.1400 [DOI] [PubMed] [Google Scholar]
- Lin S. C., Chen M. C., Li S., Lin C. C., Wang T. T. (2017a). Antiviral activity of nobiletin against chikungunya virus in vitro. Antivir. Ther. 22, 689–697. 10.3851/IMP3167 [DOI] [PubMed] [Google Scholar]
- Lin S. C., Ho C. T., Chuo W. H., Li S., Wang T. T., Lin C. C. (2017b). Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 17:144. 10.1186/s12879-017-2253-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahady G. B., Pendland S. L., Chadwick L. R. (2003). Resveratrol and red wine extracts inhibit the growth of CagA+ strains of Helicobacter pylori in vitro. Am. J. Gastroenterol. 98, 1440–1441. 10.1111/j.1572-0241.2003.07513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco M., Giani T., Raffone M., Arena F., Garcia-Fernandez A., Pollini S., et al. (2014). Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 19:20939. 10.2807/1560-7917.ES2014.19.42.20939 [DOI] [PubMed] [Google Scholar]
- Nowak J., Zander E., Stefanik D., Higgins P. G., Roca I., Vila J., et al. (2017). High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 72, 3277–3282. 10.1093/jac/dkx322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T. M., Moore L. S., Hatcher J. C., Donaldson H., Holmes A. H. (2016). Plasmid-mediated colistin resistance mechanisms: is it time to revise our approach to selective digestive decontamination? Lancet Infect. Dis. 16, 149–150. 10.1016/S1473-3099(15)00539-3 [DOI] [PubMed] [Google Scholar]
- Sawda C., Moussa C., Turner R. S. (2010). Resveratrol for Alzheimer's disease. Ann. NY. Acad. Sci. 1403, 142–149. 10.1111/nyas.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennati S., Di Pilato V., Riccobono E., Di Maggio T., Villagran A. L., Pallecchi L., et al. (2017). Citrobacter braakii carrying plasmid-borne mcr-1 colistin resistance gene from ready-to-eat food from a market in the Chaco region of Bolivia. J. Antimicrob. Chemother. 72, 2127–2129. 10.1093/jac/dkx078 [DOI] [PubMed] [Google Scholar]
- Sergio F., Pallecchi L., Landini G., Di Maggio T., Cariani L., Blasi F., et al. (2017). Antimicrobial activity of N-acetylcysteine against Stenotrophomonas maltophilia and Burkholderia cepacia complex grown in planktonic phase and in biofilm, in Conference: ERS International Congress (Vienna: ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezonov G., Joseleau-Petit D., D'Ari R. (2007). Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 189, 8746–8749. 10.1128/JB.01368-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard L. J., Tunney M. M., Elborn J. S. (2014). Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. Lancet 384, 703–713. 10.1016/S0140-6736(14)61137-5 [DOI] [PubMed] [Google Scholar]
- Singh B., Shoulson R., Chatterjee A., Ronghe A., Bhat N. K., Dim D. C., et al. (2014). Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 35, 1872–1880. 10.1093/carcin/bgu120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2015). Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically-10th Edn: Approved Standard M07-A10, NCCLS. Wayne, PA. [Google Scholar]
- Su Y., Ma L., Wen Y., Wang H., Zhang S. (2014). Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules 19, 12630–12639. 10.3390/molecules190812630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. Y., Wang Q., Simonyi A., Sun G. Y. (2010). Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 41, 375–383. 10.1007/s12035-010-8111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tascini C., Tagliaferri E., Giani T., Leonildi A., Flammini S., Casini B., et al. (2013). Synergistic activity of colistin plus rifampin against colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 57, 3990–3993. 10.1128/AAC.00179-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. J., Yu Y., Champer J., Kim J. (2014). Resveratrol demonstrates antimicrobial effects against Propionibacterium acnes in vitro. Dermatol. Ther. 4, 249–257. 10.1007/s13555-014-0063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomé-Carneiro J., Larrosa M., González-Sarrías A., Tomás-Barberán F. A., García-Conesa M. T., Espín J. C. (2013). Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 19, 6064–6093. 10.2174/13816128113199990407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio A. M., Capasso M., Della Volpe A., Malafronte L., Mansi N., Varricchio A., et al. (2014). Resveratrol plus carboxymethyl-β-glucan in children with recurrent respiratory infections: a preliminary and real-life experience. Ital. J. Pediatr. 40:93. 10.1186/s13052-014-0093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T., Hsieh F., DeLegge M. H., Oatis J. E., Jr, Walle U. K. (2004). High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 32, 1377–1382. 10.1124/dmd.104.000885 [DOI] [PubMed] [Google Scholar]
- WHO (2017) Essential Medicines and Health Products: Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics Available online at: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria.
- Zhou Y., Liu S., Wang T., Li H., Tang S., Wang J., et al. (2018). Pterostilbene, a potential MCR-1 inhibitor that enhances the efficacy of polymyxin B. Antimicrob. Agents Chemother. 16:AAC.02146-17 10.1128/AAC.02146-17 [DOI] [PMC free article] [PubMed] [Google Scholar]