Abstract
Introduction
The stability of hematological status indices is a key determinant of optimal sport performance. The capacity to monitor hematological behaviors of elite soccer players may better explain the stresses placed upon physiological systems and the potential decrements in performance and physical capacity. The primary aim of this investigation was to examine the post-seasonal hematological status of professional top-level soccer players in response to seasonal match-play and training demands, in terms of the training practices, intensity, and loadings that they experience before, during, and after each season.
Methods
Seventeen male elite European soccer players participated in the study (mean±SD: age 26.8±4.6 years, weight 78.1±5.7 kg, height 182.4±4.8 cm, body fat 9.8%±2.9%, and maximal aerobic capacity 56.5±4.2 mL kg−1 min−1). The season culminated in 74 competitive matches including domestic, Champions League, and UEFA Cup matches. Blood samples were collected between 9:00 and 10:30 am after an overnight fast (~10 hours), 72 hours post conclusion of the final match of the competitive season.
Results
Near-perfect correlations between white blood cells, neutrophils, the period of season, training availability, and total competitive minutes were found. When adjusting for all the confounding variables, a stability of the hematological profile was noticed. Only mean cell volume and mean cell hemoglobin values were associated with the requirement for elite European soccer teams to fulfill excessive competitive loadings. The reported lower mean cell volume and mean cell hemoglobin values may highlight the accumulative effects of seasonal training and match-play demands.
Conclusion
Regular blood testing could identify the need for both squad rotation and the implementation of interventions to assist in stabilizing transient hematological behaviors in order to optimize performance and sports output.
Keywords: soccer, hematology, biochemistry, training availability, match-play demands
Introduction
The demands of modern elite-level soccer require players to be able to compete in up to 50 matches per season (or even more) and participate in match-play approximately every 4 days over a 10-month competitive season.1 For top elite-level teams, periods of fixture congestion are common and materialize through the requirement to fulfill fixture commitments across domestic league championships and cups in conjunction with European club competitions, such as Champions League or UEFA Cup competitions. The accumulative physical demand for both domestic and continental pursuits routinely exposes players to more than one fixture per week. Subsequently, this can result in significant strain on various physiological, nervous, musculoskeletal, immune, and metabolic systems that can have the impact on performance.2–4
With insufficient time available for recovery and the increasing propensity for injury, the requirement for effective player management and monitoring strategies by technical and medical staff across intensified training and competitive schedules is unquestionably important.5 As such, monitoring tools that better explain underlying physiological justifications for underperformance and decrements in physical capacity outputs are therefore in demand.6 One such tool could be the potential utility offered through routine screening of collected blood samples.7
Literature has shown how strenuous exercise can facilitate multiple changes in blood parameters of both the components of the blood and those derived from other tissue, mainly muscle.8–10 Despite this, there remains limited research concerning the clinical and performance-related significance of hematological and biochemical screening of players within elite professional soccer players. Indeed, many previous investigations within this area have been conducted with amateur or sub-elite soccer players, due to the limited access to elite players. However, in this current investigation, the players used were not only deemed as elite professional soccer players but also competed within a major European final during the season assessed.
The stability of hematological parameters, specifically with reference to iron status, seems to be of paramount importance with respect to determining hemoglobin (Hb) production, maximal oxygen uptake (VO2max), and optimal physical performance capacity.11,12 However, following intensive training and competition, elite athletes have been found to be at an increased risk of developing iron deficits.13 In view of the functional consequences of low or depleted iron stores (i.e., measureable impairment of aerobic performance capacities),14 greater attention has been given to the prevalence of iron deficiency in team-based sports. In this regard, Landahl et al10 reported that 57% of 28 elite female soccer players were considered to have an iron deficiency, and 29% were considered anemic.
Supported by more recent findings within soccer,15–17 the underlying etiology proposed for the incidence of iron deficiency among athletes could be attributable to a number of mechanisms, e.g., gastrointestinal, genitourinary and menstrual blood loss, iron loss through sweat, or nutritional deficits.18 Importantly, however, hemolysis, being characterized by a rupturing of the red blood cell, resulting in the release of its Hb and associated iron into the surrounding plasma, has been considered as a major contributing factor to the high incidence of iron deficiency in endurance athletes.13,19,20 Such exercise-induced hemolytic blood-profile responses are commonly observed in athletes, especially after weight-bearing activities such as running, with positive correlations between the degree of hemolysis incurred and the biomechanical stress imparted on the foot during the heel-strike phase of running.19–21
As reported, the high-intensity physical activity accumulated over an extended period of time through intensive daily training sessions and competitive match-play can impose significant strains to various physiological systems, which might be reflected in changes in hematological parameter behaviors. To date, to the best of our knowledge, no study has highlighted the post-seasonal blood profiles of successful, elite European players in response to seasonal matchplay and training demands. As such, the primary aim of this investigation was to examine the association between post-seasonal hematological profiles of elite-level European players in correspondence to player training availability (TA) and seasonal minutes played.
Methods
Subjects
Seventeen elite, male European professional soccer players who at the time of study were competing at the elite level of game and within the UEFA Champions League volunteered to take part in this study and, as such, were involved in this investigation. These players were also, at the time of the study, recognized as the most successful domestic team in their league. Players were advised not to make any substantial changes in their regular dietary intakes upon entering into the study. Players involved in the study had a mean±SD age of 26.8±4.6 years, stature of 182.4±4.8 cm, and body mass of 78.1±5.7 kg at the start of the monitored season (Table 1). In addition, the players’ percentage body fat prior to the season commencing was 9.8%±2.9%, and the group of players had a mean VO2 max of 56.5±4.2 mL kg−1 min−1. All participants had been playing professional soccer for 4 years or more, and all of them except one were competing for their respective international team. Procedures for the study were in accordance with the Helsinki Declaration and approved by the ethical committee of the University and research center involved. The nominated medical physician of the football club ensured the players were in appropriate health. Participants were informed that their data could be withdrawn from the study without penalty.
Table 1.
Characteristics of the players
| Parameter | Mean±SD |
|---|---|
| Age (years) | 26.8 ±4.6 |
| Body mass (kg) | 78.1±5.7 |
| Height (cm) | 182.4±4.8 |
| Body fat (%) | 9.8±2.9 |
| VO2max (mL·kg−1·min−1) | 56.5±4.2 |
| Sum of eight skinfolds (mm) | 58.2±7.3 |
| Training availability (%) | 89.4±2.8 |
| Total competitive minutes (min) | 2656.7±1714.9 |
Study design
In order to examine the relationship between competitive match-play volume and end-of-season hematological profiles among the participants, total match minutes across one whole season (July–May) were recorded along with a venous blood sample taken 72 hours following the last competitive match. This specific timeline was chosen, because, among the white blood cells (WBCs), neutrophils (Neuts), being the most abundant ones and the “first responders” in case of injury or infection, have an average life span of about 24 hours (range ~8 hours to ~3 days). Muscles need 48 hours or more to recover from exercise, even though there is not a one- size-fits-all timeline. Some research suggests that, because muscle soreness can peak 2 days post exercise, a minimum of 48 hours of rest should be considered optimal to allow recovery and prevent injury – at least among competitive athletes.22 Other experts suggest that resting up to 72 hours between workouts could be ideal for one’s body to recover. Finally, 2 meta-analyses determined that for optimal strength development, one to 2 rest days between sessions could be ideal for beginners training 3 days per week as well as for experienced exercisers training 2 days per week.23,24 Other factors that should be taken into account when determining adequate rest include age (older athletes may experience slower muscle recovery), training intensity, and duration of exercise, among others.
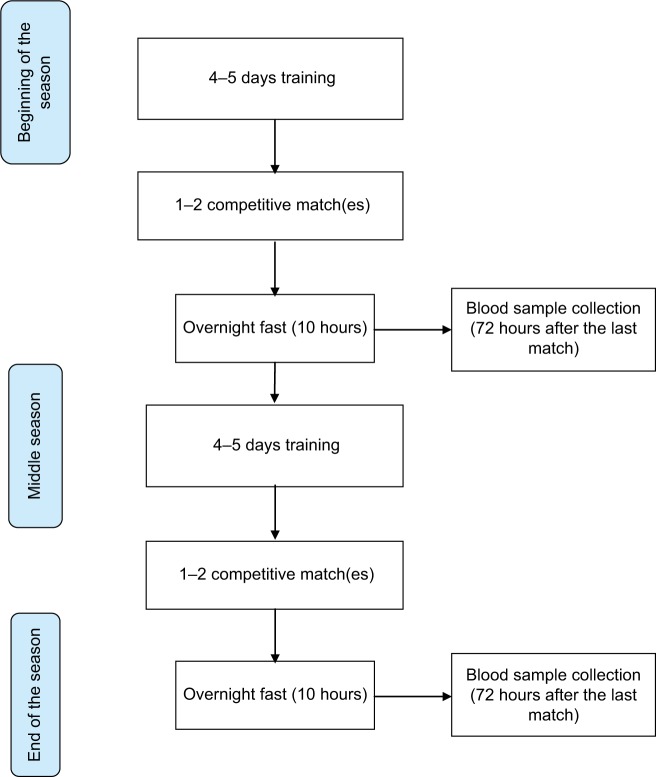
During the study, players were engaged within a controlled weekly training structure comprising between 4 and 5 training days, and between 1 and 2 competitive matches. Throughout the course of the season, a total of 74 competitive matches including friendly games (n=6), domestic league, and cup matches (n=49) in addition to a successful European campaign ending with a European Cup final (n=19), alongside friendly matches, were disputed. Venous blood samples and biochemical markers were collected between 9:00 and 10:30 am after an overnight fast (10 hours), 72 hours post last match of the competitive season (Figure 1).
Figure 1.
Flowchart of the current study.
Hematological collection and analysis
Blood samples were taken by the club’s official physician, with samples being collected within heparin vacutainer tubes, K-DETA-treated vacutainer tubes, and nonadditive serum vacutainer tubes (Greiner Bio-One, Kremsmunster, Austria). Both serum and plasma were immediately separated by centrifugation and multiple aliquots of each sample were stored at −80°C until analysis. Hematological parameters such as leukocytes (WBCs), erythrocytes (RBCs), Hb, hematocrit (Hct), platelets, mean cell volume (MCV), mean cell hemoglobin (MCH), and Neuts were assessed from the CellDyn 3700 (Abbott, Chicago, IL, USA).
Statistical analysis
Results were expressed as mean±SD. Preliminary assumption testing was conducted to check for normality, linearity, and multicollinearity, performing the D’Agostino-Pearson omnibus test. The relationships between total match-play (TMP) and TA and hematological tests were analyzed using Pearson’s product-moment correlation. These correlational analyses were not performed on the entire dataset, but on a subset of 7 elite athletes for which a complete longitudinal follow-up was available for the entire duration of the season. In particular, each hematological parameter was divided per its baseline. According to Hopkins,25 the magnitude of correlation coefficients was considered as trivial (r<0.1), small (0.1<r<0.3), moderate (0.3<r<0.5), large (0.5<r<0.7), very large (0.7<r<0.9), nearly perfect (0.9<r<1), and perfect (r=1). Coefficients of determination (R2) were used to interpret the meaningfulness of the relationships. Mixed linear models with repeated measures were carried out for each hematological parameter and adjusted for confounding variables.
Statistical analyses were performed using SPSS v23.0 software statistical package (SPSS Inc., Chicago, IL, USA), and statistical significance was set at p<0.05.
Results
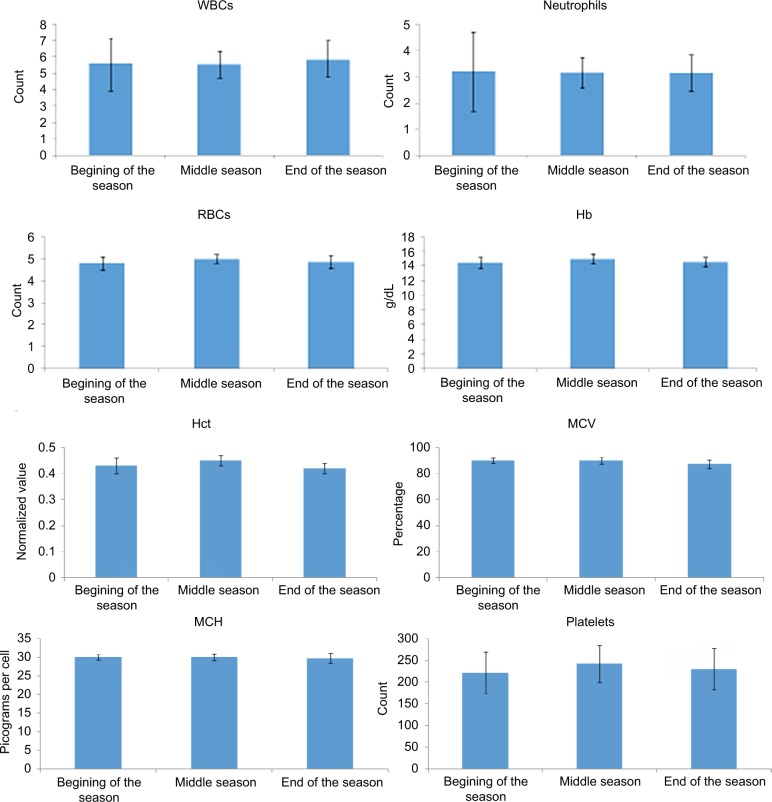
Data for hematological variables, broken down for the period of the season, are displayed in Table 2 and illustrated in Figure 2. In the correlational analysis, Hct ratio showed a statistically borderline correlation with TA (r=−0.71, R2=0.50, p=0.0729), while Neuts ratio significantly correlated with total competitive minutes (TCMs; r=0.91, R2=0.82, p=0.0047) and with TA (r=0.90, R2=0.80, p=0.0059). Also the WBC ratio significantly correlated with TCM (r=0.91, R2=0.83, p=0.0045) and with TA (r=0.91, R2=0.83, p=0.0041). According to Hopkins,25 the magnitude of these correlations was near perfect (Table 3 and Figures 3 and 4).
Table 2.
Hematological parameters broken down for the period of season
| Parameter | WBCs | Neutrophils | RBCs | Hb | Hct | MCV | MCH | Platelets | Iron (ferritin) |
|---|---|---|---|---|---|---|---|---|---|
| Beginning of the season | 5.58±1.65 (n=7) |
3.20±1.50 (n=7) |
4.81±0.36 (n=7) |
14.46±0.79 (n=7) |
0.43±0.03 (n=7) |
89.97±2.00 (n=7) |
30.10±0.75 (n=7) |
221.57±48.50 (n=7) |
47.00 (n=1) |
| Middle season | 5.55±0.85 (n=7) |
3.16±0.63 (n=7) |
5.00±0.29 (n=7) |
15.01±0.74 (n=7) |
0.45±0.02 (n=7) |
90.11±2.68 (n=7) |
30.04±0.88 (n=7) |
242.29±43.19 (n=7) |
91.00±36.77 (n=2) |
| End of the season | 5.88±1.10 (n=13) |
3.14±0.77 (n=13) |
4.87±0.34 (n=13) |
14.55±0.63 (n=13) |
0.42±0.02 (n=13) |
87.32±3.34 (n=13) |
29.79±1.30 (n=13) |
230.46±47.07 (n=13) |
57.92±30.79 (n=13) |
Note: Data presented as mean ± SD.
Abbreviations: Hb, hemoglobin; Hct, hematocrit; MCH, mean cell hemoglobin; MCV, mean cell volume; RBCs, red blood cells; WBCs, white blood cells.
Figure 2.
Variation of investigated parameters during season.
Abbreviations: Hb, hemoglobin; Hct, hematocrit; MCH, mean cell hemoglobin; MCV, mean cell volume; RBCs, red blood cells; WBCs, white blood cells.
Table 3.
Pearson's correlation between hematological parameters and total competitive minutes and training availability
| Parameter | Total competitive minutes | Training availability |
|---|---|---|
| Hb ratio | −0.64 (0.1190) | −0.62 (0.1416) |
| Hct ratio | −0.48 (0.2730) | −0.71 (0.0729) |
| MCH ratio | −0.65 (0.1142) | −0.38 (0.4068) |
| MCV ratio | −0.17 (0.7220) | −0.17 (0.7176) |
| Neuts ratio | 0.91 (0.0047) | 0.90 (0.0059) |
| Plts ratio | −0.24 (0.5981) | 0.04 (0.9279) |
| RBC ratio | −0.58 (0.1764) | −0.59 (0.1670) |
| WBC ratio | 0.91 (0.0045) | 0.91 (0.0041) |
Notes: The correlational analyses were performed in a subset of 7 elite athletes, for which a complete longitudinal follow-up was available.
Abbreviations: Hb, hemoglobin; Hct, hematocrit; MCH, mean cell hemoglobin; MCV, mean cell volume; Neuts, neutrophils; Plts, platelets; RBCs, red blood cells; WBCs, white blood cells.
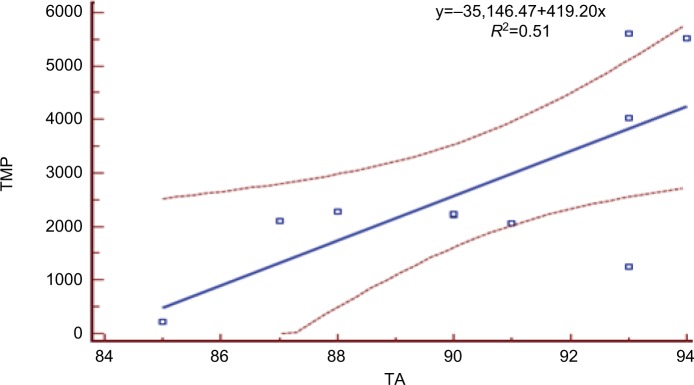
Figure 3.
Relationship between TA and TMP. Abbreviations: TA, training activity; TMP, total match-play.
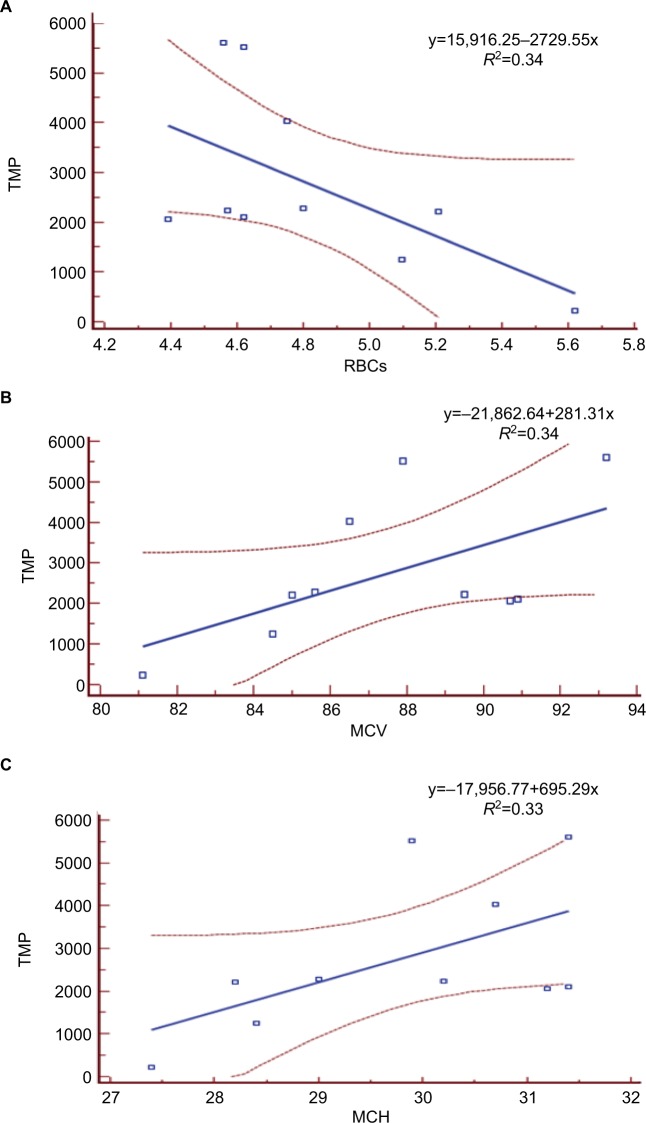
Figure 4.
Large relationship between TMP and RBCs (A), MCV (B), and mean MCH (C).
Abbreviations: TMP, total match-play; MCH, mean cell hemoglobin; MCV, mean cell volume; RBCs, red blood cells.
When performing the mixed linear models and correcting for all the confounding parameters, no statistically significant changes in hematological variables could be detected (Tables 4 and 5). Only MCV and MCH showed an association with the period of the season (F=14.844, p=0.004) and the TA (F=5.033, p=0.038), and with the TCM (F=6.05, p=0.027), respectively.
Table 4.
Results of the mixed linear model for the hematological variables
| Parameter | WBCs | Neutrophils | RBCs | Hb | Hct | MCV | MCH | Platelets | Iron (ferritin) |
|---|---|---|---|---|---|---|---|---|---|
| Season | 0.143 (0.868) | 0.057 (0.945) | 0.850 (0.453) | 1.003 (0.388) | 2.545 (0.123) | 14.844 (0.004) | 0.029 (0.971) | 0.910 (0.432) | 1.943 (–) |
| Training availability | 0.120 (0.733) | 0.595 (0.450) | 0.758 (0.395) | 0.292 (0.613) | 0.583 (0.459) | 5.033 (0.038) | 0.170 (0.684) | 0.101 (0.754) | 0.163 (0.696) |
| Total competitive minutes | 0.686 (0.418) | 0.004 (0.948) | 1.339 (0.267) | 0.404 (0.568) | 0.250 (0.630) | 6.142 (0.027) | 6.054 (0.027) | 0.268 (0.613) | 0.213 (0.656) |
Note: Statistically significant p-values are in bold.
Abbreviations: Hb, hemoglobin; Hct, hematocrit; MCH, mean cell hemoglobin; MCV, mean cell volume; RBCs, red blood cells; WBCs, white blood cells.
Table 5.
Estimates of fixed effects for the mixed linear model for the hematological variables
| Parameters | Beta coefficient | Standard error | T | Statistical significance | 95% CI
|
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| WBCs | ||||||
| Intercept | 3.288048 | 10.396695 | 0.316 | 0.755 | −18.467500 | 25.043595 |
| Beginning of season | −0.275196 | 0.608224 | −0.452 | 0.656 | −1.542881 | 0.992488 |
| Middle season | −0.261381 | 0.605842 | −0.431 | 0.671 | −1.522188 | 0.999426 |
| Training availability | 0.039345 | 0.113695 | 0.346 | 0.733 | −0.198464 | 0.277154 |
| Total competitive minutes | 0.000147 | 0.000177 | 0.828 | 0.418 | −0.000225 | 0.000518 |
| Neutrophils | ||||||
| Intercept | −2.823579 | 8.566063 | −0.330 | 0.745 | −20.734147 | 15.086989 |
| Beginning of season | 0.157597 | 0.501155 | 0.314 | 0.756 | −0.886135 | 1.201329 |
| Middle season | 0.113347 | 0.499203 | 0.227 | 0.823 | −0.924758 | 1.151452 |
| Training availability | 0.072288 | 0.093676 | 0.772 | 0.450 | −0.123494 | 0.268069 |
| Total competitive minutes | −9.663211E−006 | 0.000146 | −0.066 | 0.948 | −0.000316 | 0.000296 |
| RBCs | ||||||
| Intercept | 6.501730 | 2.819656 | 2.306 | 0.033 | 0.581553 | 12.421907 |
| Beginning of season | −0.056734 | 0.119732 | −0.474 | 0.643 | −0.314216 | 0.200747 |
| Middle season | 0.096939 | 0.105778 | 0.916 | 0.381 | −0.138648 | 0.332526 |
| Training availability | −0.026316 | 0.030220 | −0.871 | 0.395 | −0.089649 | 0.037017 |
| Total competitive minutes | −6.002246E–005 | 5.187141E−005 | − 1.157 | 0.267 | −0.000171 | 5.132495E−005 |
| Hb | ||||||
| Intercept | 16.597486 | 5.933597 | 2.797 | 0.049 | 0.108809 | 33.086164 |
| Beginning of season | −0.109683 | 0.347073 | −0.316 | 0.755 | −0.831605 | 0.612238 |
| Middle season | 0.402701 | 0.345693 | 1.165 | 0.267 | −0.350184 | 1.155585 |
| Training availability | −0.035038 | 0.064887 | −0.540 | 0.613 | −0.204093 | 0.134018 |
| Total competitive minutes | −6.439110E−005 | 0.000101 | −0.636 | 0.568 | −0.000377 | 0.000248 |
| Hct | ||||||
| Intercept | 0.516487 | 0.183023 | 2.822 | 0.017 | 0.113901 | 0.919073 |
| Beginning of season | 0.004463 | 0.010600 | 0.421 | 0.680 | −0.018092 | 0.027018 |
| Middle season | 0.023291 | 0.010517 | 2.215 | 0.056 | −0.000780 | 0.047362 |
| Training availability | −0.001526 | 0.001999 | −0.763 | 0.459 | −0.005847 | 0.002794 |
| Total competitive minutes | −1.564083E−006 | 3.128220E−006 | −0.500 | 0.630 | −8.683788E−006 | 5.555622E−006 |
| MCV | ||||||
| Intercept | 135.060165 | 19.781443 | 6.828 | 0.000 | 93.613063 | 176.507266 |
| Beginning of season | 0.930559 | 0.508537 | 1.830 | 0.111 | −0.279074 | 2.140193 |
| Middle season | 2.285914 | 0.423842 | 5.393 | 0.001 | 1.278020 | 3.293809 |
| Training availability | −0.463429 | 0.206566 | −2.243 | 0.038 | −0.899105 | −0.027752 |
| Total competitive minutes | 0.001173 | 0.000473 | 2.478 | 0.027 | 0.000152 | 0.002193 |
| MCH | ||||||
| Intercept | 35.427764 | 8.036454 | 4.408 | 0.000 | 18.658874 | 52.196655 |
| Beginning of season | 0.060636 | 0.289176 | 0.210 | 0.837 | −0.569915 | 0.691188 |
| Middle season | 0.049348 | 0.246676 | 0.200 | 0.845 | −0.499990 | 0.598686 |
| Training availability | −0.035342 | 0.085652 | −0.413 | 0.684 | −0.213883 | 0.143199 |
| Total competitive minutes | 0.000384 | 0.000156 | 2.461 | 0.027 | 5.078341E−005 | 0.000717 |
| Platelets | ||||||
| Intercept | 346.068027 | 428.806613 | 0.807 | 0.430 | −551.140616 | 1243.276671 |
| Beginning of season | −22.255563 | 16.542635 | −1.345 | 0.203 | −58.210998 | 13.699872 |
| Middle season | −9.315878 | 14.286984 | −0.652 | 0.529 | −1.270483 | 22.638727 |
| Training availability | −1.454554 | 4.580926 | −0.318 | 0.754 | −11.026065 | 8.116956 |
| Total competitive minutes | 0.004205 | 0.008122 | 0.518 | 0.613 | −0.013202 | 0.021612 |
| Iron (ferritin) | ||||||
| Intercept | 249.997884 | 372.593594 | 0.671 | 0.521 | −604.848882 | 1104.844650 |
| Beginning of season | −13.312091 | 36.168483 | −0.368 | 0.721 | −94.672858 | 68.048676 |
| Middle season | 39.229576 | 20.400136 | 1.923 | 0.226 | −73.726373 | 152.185524 |
| Training availability | −1.629333 | 4.032828 | −0.404 | 0.696 | −10.833910 | 7.575245 |
| Total competitive minutes | −0.003170 | 0.006866 | −0.462 | 0.656 | −0.018792 | 0.012453 |
Abbreviations: Hb, hemoglobin; Hct, hematocrit; MCH, mean cell hemoglobin; MCV, mean cell volume; RBCs, red blood cells; WBCs, white blood cells.
Discussion
In this study, relatively inferior values were observed for the hematological variables at the end of the playing season in comparison with the reference standards.17 Indicators of Hb and Hct are the key variables suggested to be directly related to the volume and intensity of training.26,27 Furthermore, other studies report greater values of RBCs.27–29
Correlational analyses showed near-perfect correlations among Neuts and WBCs in players with greater match-play minutes accrued across the playing season versus players with less matches playing time. It is clear that the high number of games played and the TMP developed by the players in this study may have significantly impacted the hematological parameters. In fact, Neuts fight infections and provide an important defense system against microorganisms. In this regard, an increased amount of training and an increased number of competitions involved within may be detrimental to the immune functionality of soccer players due to the variance within the exercise intensity, duration, and sheer volume within elite professional soccer.30 Therefore, short exposure to exercise may promote benefits and an adequate response of the immune function within soccer players; adversely, the heavy exertion of repetitive high-intensity competitive demands may be detrimental to health and well-being. In general, these reduced levels are a product of extreme or heavy training schedules during the competition season as has been reported in such sports as the triathlon, cycling, and ultra-endurance.8,12,31
However, when performing the mixed linear models and adjusting for confounding variables, the results of this research related to the hematological parameters revealed a stability of hematological profile amongst elite players. Only MCV and MCH showed an association with the period of the season, TCM, and TA. Therefore, the stability of the hematological parameters depends on the management of the players’ workload (volume and intensity). Thus, physical exercise/training plays a fundamental role in the immune system, health, and physical performance of athletes.32
Based on this information, it has been reported that low and moderate training (intensity and volume) can improve the functioning of the immune system, reducing the incidence of infections in athletes.33 Furthermore, lifestyle and nutrition play a relevant role in the immune function of athletes,32 as inadequate nutritional intake of iron, copper, folic acid, vitamin B12 and B6 may trigger the decrease in red blood cells. These limit proper training and performance during competition.
In essence, previous research has suggested that most of the hematological variables (e.g., Hb and MCV) were higher at the beginning of the competition season when compared with end-of-season data.17 However, with respect to the data from the players of the present study, the MCV and MCH were lower than previously reported values in the literature.7,17 Therefore, according to previous research, the testing and monitoring of Hem and Bio profiles within elite-level athletes is a vital tool to ensure adequate health and well-being status through sustained periods of intense and volume of training.27,34
Based on the results of the current investigation, it may be suggested that a longitudinal study should be conducted to observe the changes across training phases in combination with competitive game loadings. The results of such a study may further confirm the findings of the current pilot investigation, since the procedures used and the variables evaluated can possibly reflect the training practices, intensity, and loadings that elite soccer clubs implement before, during, and after each season. Furthermore, it may be useful to study these parameters and compare them across playing positions as a source of interindividual variability35,36 to highlight specific positional workloads and how hematological variance may be apparent. This type of analysis and classification would provide valuable and relevant information not only for researchers of other team and multidisciplinary sports clubs but also for practitioners to apply rest, recovery, and nutritional interventions more specific for positional roles during periods or seasons of congested competitive fixtures. Additionally, future research could ascertain the effect of a mid-season break on the hematological changes within elite professional soccer and subsequent performance markers.
Study limitations
Ensuring large subject participation within elite-level soccer research is extremely difficult. The ability to meet subject inclusion criteria is often challenging due to numerous external influences such as suspensions, team selections, injury, and illness. The lack of a baseline for the entire dataset of athletes forced us to perform a correlational analysis on a subset.
Therefore, with respect to this investigation, future research should look to increase sample size across various seasonal testing time points and draw from baseline data for the variables measured. In line with developing a clear relationship between training and competitive match-play and suppressed hematological markers, future research would benefit from the implementation of studies’ design incorporating nutritional- or periodization-based interventions.
Conclusion
To conclude, this investigation has revealed that the highcompetitive fixture period across the studied season has highlighted MCH and MCV values among elite professional soccer players being associated with the period of the season, TA, and TCM. In addition, near-perfect correlations have also been revealed among WBCs and Neuts in players with greater minutes accrued across the playing season versus players with less playing time. This highlights the need for players, conditioners, and medical personnel within elite- level professional soccer players to ensure hematological parameters are monitored throughout the season to guarantee specific training, loading, and nutritional interventions are implemented in order to maximize playing performance. Furthermore, rotation of squad players may be more apparent within high-achieving teams required to play increased number of fixtures.
Acknowledgments
The authors would like to thank the soccer players for their participation and professionalism throughout the duration of the study. Thanks are also due to the sport science and medical staff.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Strudwick AJ. Contemporary issues in the physical preparation of elite players. In: Williams Mark., editor. Science and Soccer: Developing Elite Performers. London: Routledge; 2012. pp. 335–356. [Google Scholar]
- 2.Carling C, Le Gall F, Dupont G. Are physical performance and injury risk in a professional soccer team in match-play affected over a prolonged period of fixture congestion? Int JSports Med. 2012;33:36–42. doi: 10.1055/s-0031-1283190. [DOI] [PubMed] [Google Scholar]
- 3.Dellal A, Lago-Penas C, Rey E, Chamari K, Orhant E. The effects of a congested fixture period on physical performance, technical activity and injury rate during matches in a professional soccer team. Br J Sports Med. 2015;49:390–394. doi: 10.1136/bjsports-2012-091290. [DOI] [PubMed] [Google Scholar]
- 4.Owen AL, Wong DP, Dunlop G, et al. High intensity training and salivary immunoglobulin a responses in professional top-level soccer players: effect of training intensity. J Strength Cond Res. 2016;30:2460–2469. doi: 10.1519/JSC.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 5.Dupont G, Nedelec M, McCall A, McCormack D, Berthoin S, Wislff U. Effect of 2 soccer matches in a week on physical performance and injury rate. Am J Sports Med. 2010;38:1752–1758. doi: 10.1177/0363546510361236. [DOI] [PubMed] [Google Scholar]
- 6.Meister S, Faude O, Ammann T, Schnittker R, Meyer T. Indicators for high physical strain and overload in elite football players. Scand J Med Sci Sports. 2013;23:156–163. doi: 10.1111/j.1600-0838.2011.01354.x. [DOI] [PubMed] [Google Scholar]
- 7.Baralic I, Andjelkovic M, Djordjevic B, et al. Effect of Astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evid Based Complement Alternat Med. 2015;2015:783761. doi: 10.1155/2015/783761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallon KE, Sivyer G, Sivyer K, Dare A. Changes in haematological parameters and iron metabolism associated with a 1600 kilometre ultramarathon. Br J Sports Med. 1999;33:27–31. doi: 10.1136/bjsm.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heisterberg MF, Fahrenkrug J, Andersen JL. Multiple blood samples in elite soccer players. Is it worthwhile? J Sports Sci. 2014;32:1324–1327. doi: 10.1080/02640414.2014.898859. [DOI] [PubMed] [Google Scholar]
- 10.Landahl G, Adolfsson P, Borjesson M, Mannheimer C, Rödjer S. Iron deficiency and anemia: a common problem in female elite soccer players. Int J Sport Nutr Exerc Metab. 2005;15:689–694. doi: 10.1123/ijsnem.15.6.689. [DOI] [PubMed] [Google Scholar]
- 11.Escanero JF, Villanueva J, Rojo A, Herrera A, del Diego C, Guerra M. Iron stores in professional athletes throughout the sports season. Physiol Behav. 1997;62:811–814. doi: 10.1016/s0031-9384(97)00242-4. [DOI] [PubMed] [Google Scholar]
- 12.Schumacher YO, Schmid A, Grathwohl D, Bültermann D, Berg A. Hematological indices and iron status in athletes of various sports and performances. Med Sci Sports Exerc. 2002;34:869–875. doi: 10.1097/00005768-200205000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Tan D, Dawson B, Peeling P. Hemolytic effects of a football-specific training session in elite female players. Int J Sports Physiol Perform. 2012;7:271–276. doi: 10.1123/ijspp.7.3.271. [DOI] [PubMed] [Google Scholar]
- 14.Banfi G, Dolci A, Freschi M, Verdini C. Immature reticulocyte fraction (IRF) monitored in elite athletes during a whole season. Clin Lab Haematol. 2005;27:213–214. doi: 10.1111/j.1365-2257.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 15.Karakoc Y, Duzova H, Polat A, Emre MH, Arabaci I. Effects of training period on haemorheological variables in regularly trained footballers. Br J Sports Med. 2005;39:e4. doi: 10.1136/bjsm.2003.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer T, Meister S. Routine blood parameters in elite soccer players. Int J Sports Med. 2011;32:875–881. doi: 10.1055/s-0031-1280776. [DOI] [PubMed] [Google Scholar]
- 17.Ostojic SM, Ahmetovic Z. Weekly training volume and hematological status in female top-level athletes of different sports. J Sports Med Phys Fitness. 2008;48:398–403. [PubMed] [Google Scholar]
- 18.Shaskey DJ, Green GA. Sports haematology. Sports Med. 2000;29:27–38. doi: 10.2165/00007256-200029010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Peeling P, Dawson B, Goodman C, et al. Training surface and intensity: inflammation, hemolysis, and hepcidin expression. Med Sci Sports Exerc. 2009;41:1138–1145. doi: 10.1249/MSS.0b013e318192ce58. [DOI] [PubMed] [Google Scholar]
- 20.Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, Smith JA. Footstrike is the major cause of hemolysis during running. J Appl Physiol. 1985;94:38–42. doi: 10.1152/japplphysiol.00631.2001. [DOI] [PubMed] [Google Scholar]
- 21.Hardin EC, Hamill J. The influence of midsole cushioning on mechanical and hematological responses during a prolonged downhill run. Res Q Exerc Sport. 2002;73:125–133. doi: 10.1080/02701367.2002.10609001. [DOI] [PubMed] [Google Scholar]
- 22.Burt DG, Twist C. The effects of exercise-induced muscle damage on cycling time-trial performance. J Strength Cond Res. 2011;25:2185–2192. doi: 10.1519/JSC.0b013e3181e86148. [DOI] [PubMed] [Google Scholar]
- 23.Rhea MR, Alvar BA, Burkett LN, Ball SD. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc. 2003;35:456–464. doi: 10.1249/01.MSS.0000053727.63505.D4. [DOI] [PubMed] [Google Scholar]
- 24.Peterson MD, Rhea MR, Alvar BA. Maximizing strength development in athletes: a meta-analysis to determine the dose-response relationship. J Strength Cond Res. 2004;18:377–382. doi: 10.1519/R-12842.1. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins WG. A scale of magnitudes for effect statistics. 2009. [Accessed February 21, 2018]. Available from: http://www.sportsci.org/resource/stats/index.html.
- 26.Martinez AC, Camara FJ, Vicente GV. Status and metabolism of iron in elite sportsmen during a period of professional competition. Biol Trace Elem Res. 2002;89:205–213. doi: 10.1385/bter:89:3:205. [DOI] [PubMed] [Google Scholar]
- 27.Santi Maria T, Arruda M, Portella D, et al. Haematological parameters of elite soccer players during the competitive period. JEP Online. 2013;16:68–76. [Google Scholar]
- 28.Banfi G, Del Fabbro M, Lippi G. Relation between serum creatinine and body mass index in elite athletes of different sport disciplines. Br J Sports Med. 2006;40:675–678. doi: 10.1136/bjsm.2006.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colombini A, Machado M, Lombardi G, Lanteri P, Banfi G. Modifications of biochemical parameters related to protein metabolism and renal function in male soccer players after a match. J Sports Med Phys Fitness. 2014;54:658–664. [PubMed] [Google Scholar]
- 30.Bangsbo J, Norregaard L, Thorso F. Activity profile of competition soccer. Can J Sport Sci. 1991;16:110–116. [PubMed] [Google Scholar]
- 31.Rietjens GJ, Kuipers H, Hartgens F, Keizer HA. Red blood cell profile of elite olympic distance triathletes. A three-year follow-up. Int J Sports Med. 2002;3:391–396. doi: 10.1055/s-2002-33736. [DOI] [PubMed] [Google Scholar]
- 32.Nunes LA, Grotto HZ, Brenzikofer R, et al. Hematological and biochemical markers of iron status in a male, young, physically active population. Biomed Res Int. 2014;2014:349182. doi: 10.1155/2014/349182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleeson M. Immune function in sport and exercise. JAppl Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Salvagno GL, Danese E, et al. Variation of red blood cell distribution width and mean platelet volume after moderate endurance exercise. Adv Hematol. 2014;2014:192173. doi: 10.1155/2014/192173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolaidis PT, Ziv G, Arnon M, et al. Physical Characteristics and physiological attributes of football goalkeepers – the importance of individual data. J Human Sport Exercise. 2015;10:602–614. [Google Scholar]
- 36.Nikolaidis PT, Ziv G, Lidor R, et al. Intra-individual variability in soccer players of different age groups playing different positions. J Human Kinetics. 2014;40:1–13. doi: 10.2478/hukin-2014-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]