Abstract
We aimed at comparing the Durie–Salmon Plus (DS Plus) staging system based on Italian Myeloma criteria for PET USe (IMPeTUs) with other two staging systems in predicting prognosis of patients with all stages of newly diagnosed multiple myeloma (MM). A total of 33 MM patients were enrolled in this retrospective study. The variation between the DS Plus classification and Durie–Salmon staging system (DSS) or Revised International Staging System (RISS) classification was assessed. When staged by the DSS, patients in stage I and stage II did not reach the median overall survival (OS), and the median OS was 33 months for stage III (p=0.3621). When staged by the DS Plus, patients in stage I did not reach the median OS of stage I, and the median OS for stages II and III was 38 and nine months, respectively (p=0.0064). When staged by the RISS, patients in stage I did not reach the median OS, and the median OS was 33 and 16 months for stage II and stage III, respectively (p=0.0319). The concordances between two staging systems were 0.07 (DS Plus versus DSS) and 0.37 (DS Plus versus RISS), respectively. Multivariate analysis revealed that DS Plus stage III (HR: 11.539, p=0.021) and the Deauville score of bone marrow ≥4 (HR: 3.487, p=0.031) were independent prognostic factors associated with OS. Both the DS Plus based on IMPeTUs and RISS possessed a better potential in characterizing and stratifying MM patients compared with the DSS. Moreover, DS Plus stage III and the Deauville score of bone marrow ≥4 were reliable prognostic factors in newly diagnosed MM patients.
1. Introduction
As a clonal hematologic malignancy, multiple myeloma (MM) is characterized by bone marrow plasma cell infiltration and the presence of serum and urine monoclonal immunoglobulins. The prognosis of MM patients is highly variable; therefore, a reliable staging system is extremely important to optimize appropriate treatment as quickly as possible and avoid irreversible organ damage [1]. The most widely applied staging systems in MM patients include the International Staging System (ISS) and Durie–Salmon staging system (DSS) [2]. However, both of these two staging systems have some limitations. As a powerful and reproducible stage classification, the ISS simply segregates patients into three different groups based on the levels of β2-microglobulin and serum albumin. However, the ISS-derived outcome can be affected by serum albumin, which is a host factor and not disease-specific [3]. Moreover, the ISS relies solely on tumor biological parameters but does not integrate any medical imaging modalities. The DSS relies on a combination of clinical factors, such as the number of lytic bone lesions on a skeletal radiographic survey, serum calcium, level of hemoglobin, amount of M protein, and renal function [4]. However, the DSS has a poor reproducibility because its classification based on the extent and number of bone lesions found by X-ray is observer-dependent [5].
Recently, the Revised International Staging System (RISS) improves the prognostic value of the ISS by combining the variables in the ISS with the chromosomal abnormalities (CA) detected by interphase fluorescence in situ hybridization (t(14;16), t(4;14), and del17p) and serum lactate dehydrogenase (LDH) in those patients with newly diagnosed MM [6]. According to one recent study, the RISS can also be used to stratify patients with relapsed/refractory MM [7].
In 2006, the Durie–Salmon Plus (DS Plus) staging system integrated new imaging techniques, such as 18F-2-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) and magnetic resonance imaging (MRI), into a new generation of MM staging and offered the opportunity to precisely stage patients by anatomic and functional techniques [8]. 18F-FDG PET/CT provides prognostic information on symptomatic MM at the baseline and therapeutic follow-up, allowing the detection of extramedullary disease (EMD) [9]. However, no standard interpretation criteria have been proposed for the evaluation of 18F-FDG PET/CT scans in MM, preventing reproducibility of data.
A group of Italian nuclear medicine experts, medical physicists, and hematologists have defined new visual interpretation criteria (Italian Myeloma criteria for PET USe; IMPeTUs) to standardize the 18F-FDG PET/CT interpretation criteria and methods for extensive use in clinical practice for symptomatic MM patients. However, a larger series of patients should be adopted to define a visual cutoff for positivity [10].
It remains unclear whether the DS Plus based on IMPeTUs can be used to appropriately interpret 18F-FDG PET/CT imaging in MM patients. Therefore, we aimed at comparing the DS Plus based on IMPeTUs with other staging systems in predicting prognosis of patients with all stages of newly diagnosed MM in this study.
2. Materials and Methods
2.1. Patients
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University with waiver of informed consent.
Patients with newly diagnosed monoclonal plasma cell disease who had available data of RISS stage and DSS stage were enrolled in this analysis between May 2007 and December 2017. All patients underwent whole-body 18F-FDG PET/CT for initial diagnosis of disease.
Subjects were selected for inclusion if they met the following criteria: (1) the diagnosis of MM confirmed by the presence of an M-component in serum and/or urine plus clonal plasma cells in the bone marrow and/or a documented clonal plasmacytoma, (2) digital image data available for retrospective analysis, and (3) the time interval between assessment of hematological and immunologic parameters and 18F-FDG PET/CT <3 weeks.
The following patients were excluded: (1) patients who had insufficient information on the RISS, DSS, and DS Plus, (2) MM patients who had additional diseases, and (3) patients who had received treatment before 18F-FDG PET/CT acquisition.
2.2. RISS, DSS, and DS Plus
Staging of 33 MM patients was conducted using the RISS, DSS, or DS Plus based on 18F-FDG PET/CT imaging and laboratory data (Table 1).
Table 1.
Criteria of RISS, DSS, and DS Plus staging systems.
| RISS [6] | DSS [4] | DS Plus [8] | |
|---|---|---|---|
| Stage I | ISS stage I (serum albumin ≥3.5 and serum β2-microglobulin <3.5), normal LDH levels, and no high-risk cytogenetic abnormalities | All of the following: hemoglobin value >10 g/dL; serum calcium value normal or ≤10.5 mg/dL; bone X-ray shows normal bone structure or solitary bone plasmacytoma only; and low M-component production rates (IgG < 5 g/dL, IgA < 3 g/dL, and Bence Jones protein < 4 g/24 h) | Stage IA, smoldering or indolent: single plasmacytoma and/or limited disease at imaging Stage IB: 0–4 focal lesions or mild diffuse disease |
|
| |||
| Stage II | Neither stage I nor stage III | Neither stage I nor stage III | 5–20 focal lesions or moderate diffuse disease |
|
| |||
| Stage III | ISS stage III (serum β2-microglobulin >5.5 mg/L) and either elevated LDH levels or high-risk cytogenetic abnormalities | One or more of the following: hemoglobin value <8.5 g/dL; serum calcium value <12 mg/dL; advanced lytic bone lesions; and high M-component production rates (IgG > 7 g/dL, IgA > 5 g/dL, or Bence Jones protein >12 g/24 h) | >20 focal lesions or severe diffuse disease |
|
| |||
| Subgroup A | — | Relatively normal renal function: serum creatinine value <2.0 mg/dL | Serum creatinine level <2.0 mg/dL and no EMD |
|
| |||
| Subgroup B | — | Abnormal renal function: serum creatinine value ≥2.0 mg/dL | Serum creatinine level >2.0 mg/dL and/or the presence of EMD |
2.3. PET/CT Acquisition
All patients underwent whole-body 18F-FDG PET/CT according to the standard protocol in the same center. The patients were instructed to fast for at least 6 h. The blood glucose level of all the patients was lower than 11 mmol/L. Next, 60 min after the injection of 18F-FDG (dose of 0.12 mCi/kg), imaging was started on a Discovery STE PET/CT scanner (General Electric Medical Systems, Milwaukee, WI, USA) with a CT of the whole body (140 kV, 120 mA, transaxial FOV 700 mm, pitch 1.75, rotation time 0.8 s, and slice thickness 3.75 mm). Subsequently, a whole-body emission scan in a 3D mode was performed from the base of the skull to the midfemur, 2-3 min per bed position. PET images were reconstructed by a standard iterative algorithm (ordered-subset expectation maximization), with the low-dose CT data utilized for attenuation correction and image fusion.
2.4. Image Analysis
18F-FDG PET images were blindly evaluated by two experienced nuclear medicine physicians in order to avoid any bias on image analysis. Image interpretation of 18F-FDG PET/CT was based on IMPeTUs. To make sure IMPeTUs was suitable for DS Plus, bone lesions with a Deauville score ≥4 on PET scan and diffuse lytic lesions ranging between 0.5 and 1 cm in size with a Deauville score ≥3 were all identified as positive bone lesions. For EMD, positive lesions were also defined as the Deauville score ≥4 on PET scan. If there was discordance between the two independent physicians for image analysis, a third investigator was advised to make a decision.
2.5. Statistical Analysis
To assess the variation between DS Plus classification and DSS or ISS classification, an exact weighted kappa value was calculated with STATA 11 software package (StataCorp, College Station, TX, USA), which was expressed as a number between 0 and 1, with 0 representing complete nonconcordance and 1 representing complete agreement [11]. To better compare the three staging systems, DS Plus and DSS subgroups were combined within the main numerical grouping for analysis.
OS was determined from the date of the 18F-FDG PET/CT scan until the date of death or last follow-up. The OS was estimated by the Kaplan–Meier method. Comparisons among groups were made by a log-rank test. For multivariate analysis, variables that were considered clinically relevant or independently predictive of survival in univariate analysis were introduced into a Cox proportional-hazards model. Statistical analysis was performed by IBM SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Characteristics of Patients
A total of 59 MM patients met inclusion criteria for 18F-FDG PET/CT. Among them, 20 patients had received treatment before 18F-FDG PET/CT acquisition, one patient had myelodysplastic syndromes (MDS), one patient had gastric cancer, one patient had no β2-MG results, and three patients did not do the examination of cytogenetic abnormalities. According to our exclusion criteria, the abovementioned 26 patients were excluded from the present study. Finally, 33 consecutive patients (10 women, 23 men; mean age ± SD, 60 ± 10 years; range, 34–77 years) were retrospectively enrolled in this study. Table 2 lists detailed characteristics of patients.
Table 2.
Characteristics of patients.
| Number | Sex | Age | Myeloma type | IMPeTUs | DSS | DS Plus | RISS | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | IgG λ | BM(4)A, F4.S.SP.ExtraSP(5), L4, PM, EM.EN(4) | B | IIIB | III | None |
| 2 | M | 38 | IgG κ | BM(3), F4.SP.ExtraSP(3), L4, EM.N.EN(4) | IIIA | IIIB | III | Chemotherapy |
| 3 | M | 63 | Nonsecretory | BM(2), F4.SP.ExtraSP(4), L4, PM, EM.EN(3) | IIIB | IIB | III | None |
| 4 | M | 68 | Light chain κ | BM(2), F4.SP.ExtraSP(4), L4, PM, EM.EN(3) | IIIB | IIIB | III | Chemotherapy |
| 5 | F | 48 | IgG κ | BM(2), F4.SP.ExtraSP(4), L4, PM | IIIA | IIA | I | Chemotherapy + IMIDs |
| 6 | M | 55 | IgG κ | BM(2), F4.SP.ExtraSP(4), L1, PM | IIIA | IIA | I | Chemotherapy + IMIDs |
| 7 | M | 58 | Light chain λ | BM(2), F3.SP.ExtraSP(4), L3, PM | IIIA | IA | II | Chemotherapy |
| 8 | M | 63 | IgG λ | BM(2), F4.S.SP.ExtraSP(4), L4 | IIIA | IIIA | I | Chemotherapy |
| 9 | M | 58 | IgG κ | BM(3), F4.SP.ExtraSP(4), L4, PM, EM.N.EN(5) | IIIB | IIIB | III | Chemotherapy |
| 10 | F | 42 | IgG κ | BM(4), F1, L1 | IIIA | IA | II | Chemotherapy + ASCT |
| 11 | M | 60 | Light chain κ | BM(3), F4.SP.ExtraSP(4), L4, EM.EN(3) | IIIA | IIA | II | Chemotherapy + ASCT |
| 12 | M | 63 | IgG κ | BM(2), F2.ExtraSP(4), L1 | IIIA | IA | II | Chemotherapy |
| 13 | M | 52 | IgG λ | BM(3)A, F4.S.SP.ExtraSP(3), L2, EM.EN(3) | IIIA | IIIA | III | None |
| 14 | M | 67 | IgG λ | BM(4), F3.ExtraSP(4), L2 | IIIA | IIA | II | Chemotherapy + allo-BMT |
| 15 | F | 71 | Light chain κ | BM(5), F4.SP.ExtraSP(5), L3, PM, EM.N(5) | IIIA | IIIB | II | Chemotherapy + radiotherapy |
| 16 | F | 64 | IgG κ | BM(3), F4.S.SP.ExtraSP(3), L3 | IIIB | IIIB | III | Chemotherapy + IMIDs |
| 17 | F | 67 | IgG κ | BM(4)A, F4.SP.ExtraSP(4), L4 | IIIB | IIIB | III | None |
| 18 | M | 34 | IgG κ | BM(3), F4.SP.ExtraSP(5), L3, PM, EM.EN(4) | IIIA | IIB | II | None |
| 19 | M | 77 | IgA κ | BM(2)A, F3.SP(4), L1, PM, EM.N.EN(3) | IIIA | IIA | II | None |
| 20 | M | 72 | IgG κ | BM(3), F3.SP.ExtraSP(4), L2, PM, EM.N(2) | IA | IIA | II | Chemotherapy |
| 21 | M | 60 | IgG λ | BM(3), F4.SP.ExtraSP(5), L3, PM, EM.N(5) | IIIA | IIB | II | Chemotherapy + IMIDs |
| 22 | M | 73 | Light chain κ | BM(3), F4.SP.ExtraSP(5), L2, EM.EN(5) | IIIB | IIIB | III | Chemotherapy + IMIDs |
| 23 | M | 60 | IgD λ | BM(2), F2.SP(4), L2 | IIIA | IA | III | Chemotherapy + IMIDs |
| 24 | M | 59 | Light chain κ | BM(3), F3.SP.ExtraSP(5), L1, PM, EM.N(5) | IIA | IB | I | None |
| 25 | M | 64 | IgG κ | BM(2), F2.SP(4), L4, PM | IIB | IIB | II | Chemotherapy + IMIDs |
| 26 | F | 57 | IgG λ | BM(4)A, F4.SP.ExtraSP(4), L4, PM, EM.N(5)EN(4) | IIIA | IIIB | II | Chemotherapy |
| 27 | F | 76 | Light chain κ | BM(4)A, F4.SP.ExtraSP(5), L4, PM, EM.EN(5) | IIIA | IIIB | III | None |
| 28 | F | 58 | IgA λ | BM(4)A, F4.S.SP.ExtraSP(5), L4, EM.EN(5) | IIIA | IIIB | III | Chemotherapy + IMIDs |
| 29 | F | 47 | Light chain κ | BM(2), F2.SP(5), L2, PM, EM.EN(5) | IIIA | IB | II | Chemotherapy + radiotherapy |
| 30 | M | 69 | IgG λ | BM(2), F2.SP(2), L2, EM.EN(4) | IIIB | IB | II | Chemotherapy |
| 31 | M | 65 | IgG λ | BM(2), PM, EM.EN(4) | IIIA | IB | III | Chemotherapy + ASCT |
| 32 | F | 53 | IgD λ | BM(4)A, EM.EN(4) | IIIB | IB | II | Chemotherapy + IMIDs |
| 33 | M | 50 | IgG λ | BM(5)A, F4.S.SP.ExtraSP(5), L4, EM.EN(4) | IIIB | IIIB | III | Chemotherapy |
ASCT: autologous stem cell transplant; IMIDs: immunomodulatory drugs; BM: bone marrow; F: focal bone lesions; S: skull; Sp: spine; L: lytic lesion; Fr: fracture; EM: EMD; PM: paramedullary disease; N: nodal disease; EN: extranodal disease.
3.2. Staging Distribution
Table 3 and Figure 1 summarize a detailed classification in MM patients according to the DS Plus staging system compared with RISS or classic DSS classification.
Table 3.
Correlation of classification according to DS Plus, DSS, and RISS.
| DS Plus | DSS | Total | RISS | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage I | Stage II | Stage III | |||
| Stage I | 0 | 1 | 8 | 9 | 1 | 6 | 2 | 9 |
| Stage II | 1 | 1 | 8 | 10 | 2 | 7 | 1 | 10 |
| Stage III | 0 | 0 | 14 | 14 | 1 | 2 | 11 | 14 |
| Total | 1 | 2 | 30 | 33 | 4 | 15 | 14 | 33 |
Figure 1.
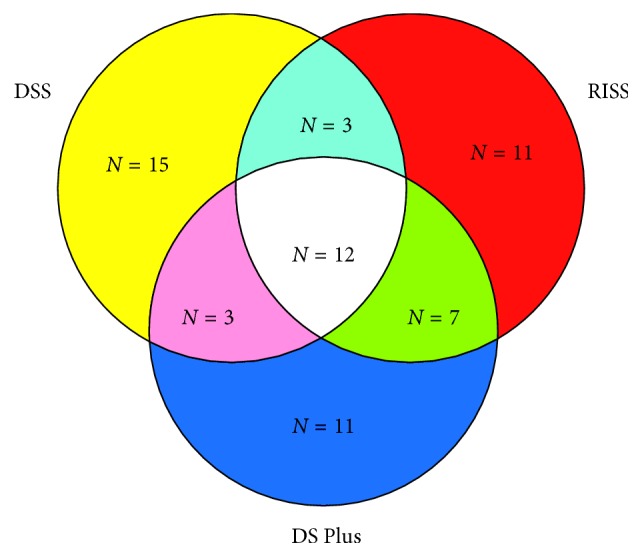
Details of classifications. Different colors represent different staging systems. Red color indicates that when staged by the RISS, the stages of nine patients were different from those staged by the DS Plus and DSS. Blue color indicates that when staged by the DS Plus, the stages of 11 patients were different from those staged by the RISS and DSS. Yellow color indicates that when staged by the DSS, the stages of 15 patients were different from those staged by the RISS and DS Plus. Light blue color indicates that the stages of 3 patients were the same when staged by the DSS and RISS. Green color indicates that the stages of 7 patients were the same when staged by the DS Plus and RISS. Pink color indicates that the stages of 3 patients were the same when staged by the DS Plus and DSS. The total number of patients was 33. Among the 33 MM patients, 12 patients were staged the same according to the three various staging systems (N = number of patients).
The comparison of the DS Plus and DSS showed that 45.45% of patients had concordant stages across systems. The patient with DSS stage I MM was upstaged according to the DS Plus staging system. Of two patients with DSS stage II MM, lesions in one patient were staged the same, while lesions in the other patients were downstaged according to the DS Plus staging system. Of 30 patients with DSS stage III MM, lesions in 14 (46.67%) patients were staged the same, while lesions in 16 (53.33%) patients were downstaged according to the DS Plus staging system. Agreement between the DS Plus and DSS stages calculated using the weighted kappa statistic was 0.07 (95% CI, −0.07 to 0.22; p=0.167), indicating no concordance between DS Plus and DSS stages. Examples are given in Figure 2.
Figure 2.
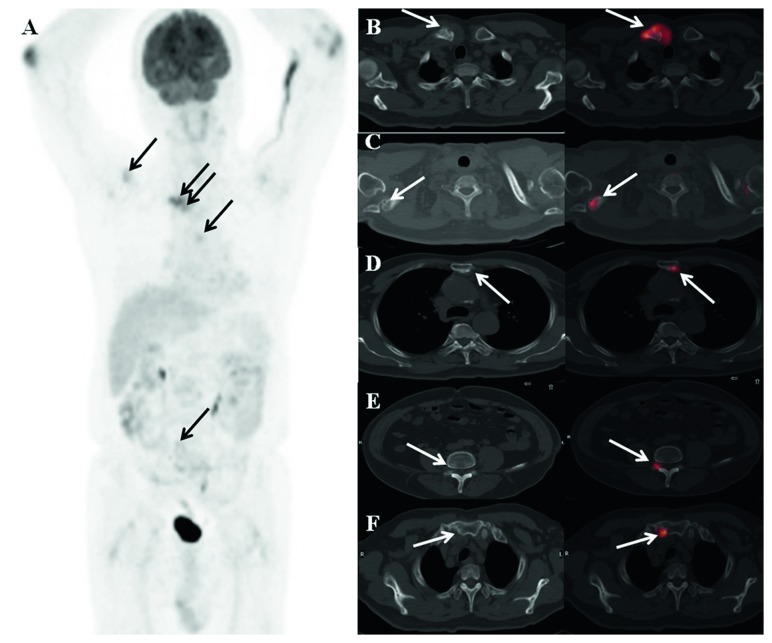
18F-FDG PET/CT imaging in a 72-year-old man with MM: (a) MIP image; (b–f) transaxial CT and fused images. In this patient, the descriptive criteria (IMPeTUs) were BM(3), F3.SP.ExtraSP(4), L2, PM, EM.N(2), where BM3 indicates that bone marrow uptake is <liver but >mediastinum, F3 indicates 4 to 10 lesions (arrows), SP indicates the spine, ExtraSP indicates outside the spine with (4) indicating reference lesion uptake >liver uptake + 10%, L2 indicates that 1 to 3 lesions were also lytic, PM indicates a bone lesion in surrounding soft tissues with bone cortical interruption, EM indicates at least one extramedullary lesion, and N indicates that the extramedullary lesion was nodal disease with (2) indicating extramedullary lesion uptake ≤mediastinum. This patient was classified as IIA according to DS Plus.
The comparison of the DS Plus and RISS showed that 57.58% of patients had concordant stages across systems. Among four patients with RISS stage I MM, one, two, and one were classified as stages I, II, and III based on the DS Plus staging system, respectively. Among 15 patients with RISS stage II MM, six, seven, and two were classified as stages I, II, and III based on the DS Plus staging system, respectively. Among 14 patients with RISS stage III MM, two, one, and 11 were classified as stages I, II, and III based on the DS Plus staging system, respectively. Agreement between the DS Plus and RISS stages calculated using the weighted kappa statistic was 0.37 (95% CI, 0.12–0.62; p < 0.01), indicating fair concordance between DS Plus and RISS stages. Examples are given in Figure 3.
Figure 3.
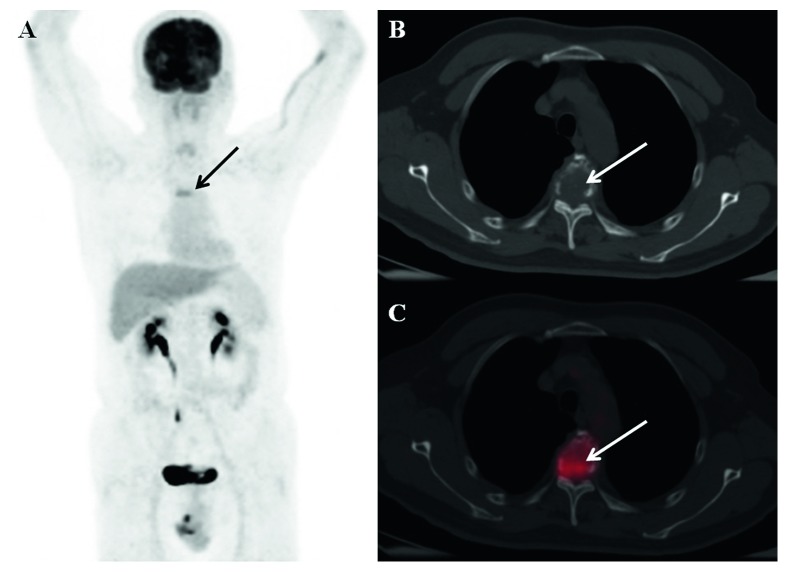
18F-FDG PET/CT imaging in a 60-year-old man with MM: (a) MIP image; (b-c) transaxial CT and fused images. In this patient, the descriptive criteria (IMPeTUs) were BM(2), F2.SP(4), L2. 18F-FDG PET/CT showed increased 18F-FDG uptake in the T4 spine (arrows). His disease stage was IA by DS Plus. The serum β2-microglobulin level was 6.08 mg/L, and the LDH level was 310 U/L. The patient was classified as III according to the RISS.
3.3. Survival according to the Three Staging Systems
When staged by the DSS, patients in stage I and stage II did not reach the median overall survival (OS), and the median OS was 33 months for stage III (p=0.3621; Figure 4(a)). When staged by the RISS, patients in stage I did not reach the median OS of RISS stage I (the median follow-up duration of the stage I patients was 59 months), and the median OS was 33 and 16 months for stage II and stage III, respectively (p=0.0319; Figure 4(b)). When staged by the DS Plus, patients in stage I did not reach the median OS of stage I, and the median OS for stages II and III was 38 and nine months, respectively (p=0.0064; Figure 4(c)). For the RISS, the 5-year OS rate was 100%, 45.02%, and 12.5% for MM patients in stages I, II, and III, respectively. For the DSS, the 5-year OS rate was 100%, 100%, and 28.29% for MM patients in stages I, II, and III, respectively. For the DS Plus, the 5-year OS rate was 85.71%, 45.71%, and 17.14% for MM patients in stages I, II, and III, respectively.
Figure 4.
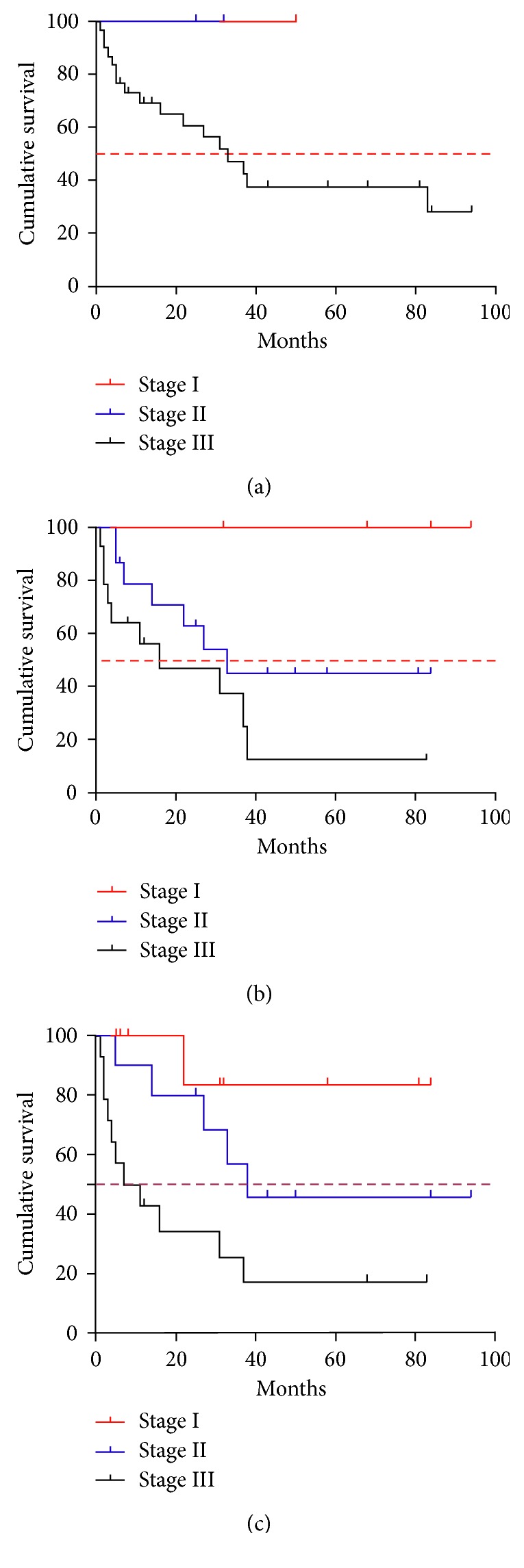
OS of the 33 MM patients according to the DSS (a), RISS (b), and DS Plus (c).
The p value was statistically significant for both DS Plus and RISS, while no statistical significance was noted for the DSS.
3.4. Prognostic Factors
According to the univariate analysis, other factors, such as the presence of EMD (p=0.015), subgroup B of DS Plus (p=0.012), and the Deauville score of bone marrow ≥4 (p=0.013), were significantly associated with shorter OS (Table 4).
Table 4.
Results of univariate analysis of the factors that influence the OS.
| Characteristics | n=33 (%) | Median OS (months) | p value |
|---|---|---|---|
| Sex | |||
| Male | 23 (69.7) | 37 | 0.721 |
| Female | 10 (30.3) | 22 | |
|
| |||
| Age | |||
| <60 | 15 (45.5) | 31 | 0.915 |
| ≥60 | 18 (54.5) | 33 | |
|
| |||
| Type | |||
| IgG | 21 (63.6) | NR | 0.243 |
| IgA | 1 (3.0) | 33 | |
| Light chain | 8 (24.2) | 7 | |
| Others | 3 (9.1) | 38 | |
|
| |||
| Bone marrow | |||
| Deauville score ≥4 | 10 (30.3) | 7 | 0.013 |
| Deauville score <4 | 23 (69.7) | 38 | |
|
| |||
| Paramedullary disease | |||
| + | 17 (51.5) | 33 | 0.513 |
| − | 16 (48.5) | 37 | |
|
| |||
| Extramedullary disease | |||
| + | 16 (48.5) | 16 | 0.015 |
| − | 17 (51.5) | NR | |
| Creatinine | |||
| <2 mg/dl | 22 (66.7) | 37 | 0.064 |
| ≥2 mg/dl | 11 (33.3) | 16 | |
|
| |||
| Subgroup of DS Plus | |||
| A | 12 (36.4) | NR | 0.012 |
| B | 21 (63.6) | 16 | |
|
| |||
| Treatment | |||
| None | 11 (33.3) | 31 | 0.146 |
| Chemotherapy | 7 (21.2) | 37 | |
| Chemotherapy + IMIDs | 9 (27.3) | NR | |
| Chemotherapy + BMT | 4 (12.1) | 27 | |
| Others | 2 (6.1) | 7 | |
NR: not reached; BMT: bone marrow transplant.
Multivariate analysis revealed that DS Plus stage III (HR: 11.539, p=0.021) and the Deauville score of bone marrow ≥4 (HR: 3.487, p=0.031) were independent prognostic factors associated with OS (Table 5).
Table 5.
Multivariate analysis of factors associated with OS in univariate analysis, with DS Plus in the model.
| HR | 95% CI | p value | |
|---|---|---|---|
| DS Plus-I (reference) | 1 | — | — |
| DS Plus-II | 4.818 | 0.547–42.40 | 0.156 |
| DS Plus-III | 11.539 | 1.453–91.638 | 0.021 |
| Deauville score of BM ≥4 | 3.487 | 1.121–10.850 | 0.031 |
| Presence of EMD | 1.463 | 0.350–6.107 | 0.602 |
| DS Plus subgroup B | 2.014 | 0.416–9.762 | 0.384 |
4. Discussion
In recent years, new imaging techniques, such as PET or MRI, play an increasingly important role in staging of MM. The impact of MRI on staging patients according to DS Plus has been analyzed in many studies [12–14]. Although 18F-FDG PET/CT has been proved to be prognostically valuable in staging different groups [15–17], only few studies have been reported on DS Plus based on 18F-FDG PET/CT [18]. One of the reasons is that no standard interpretation criteria have been proposed for the evaluation of 18F-FDG PET/CT scans in MM. Therefore, IMPeTUs has been proposed to standardize image interpretation criteria in order to make clinical trial results applicable and reproducible. Since IMPeTUs is only a descriptive criterion according to experience with 18F-FDG PET/CT in lymphoma and MM [17, 19, 20], we decided to adopt the Deauville score ≥4 and diffuse lytic lesions with the Deauville score ≥3 as the cutoff values in this study. Our study confirmed that IMPeTUs was useful to define bone lesions and EMD, which were main criteria of the DS Plus. DS Plus based on IMPeTUs showed fair concordance with the classic RISS, indicating great capability to differentiate patients with good OS from those with a poorer prognosis.
To the best of our knowledge, the present study was the first study designed to compare the DSS, RISS, and DS Plus. In some studies, researchers have compared the DSS with the ISS and found that the ISS has a better reliability, simplicity, and predictability for OS compared with the DSS for MM patients [5, 21, 22]. The RISS, which combines the ISS together with the prognostic power of high-risk CA and LDH, has been proved to give a better differentiation of MM patients into three survival subgroups [6, 7, 23, 24]. Our data also indicated that the RISS provided significant prognostic information.
Nowadays, it remains largely explored whether the DSS can be used to predict the outcome. Most studies have demonstrated that the DSS cannot show a significant difference in OS among stages I, II, and III [21, 22, 25], while several studies have reported that there are statistical differences in OS among the three groups in the DSS [2, 26]. In the present study, we found that the DSS was not correlated with OS. In most of the studies comparing the frequency distribution of the same patients across the DSS and ISS, the number of patients classified as DSS stage III is larger than that of patients classified as ISS stage III [5, 21, 22, 25, 26]. The percentage of patients with DSS stage III in our study was also higher. The reason might be attributed to that the DSS includes hemoglobin and serum calcium, which can be affected by various factors and advanced lytic bone lesions in X-ray, leading to difficulty in appropriate interpretation due to the lack of standard criteria.
It is believed that DS Plus is a reliable method for both staging and prognostic classification. The prognostic significance of DS Plus has been reported in one recent study using MRI of the spine and pelvis in 85 patients [27]. However, Fechtner et al. have reported that the DS Plus is not better than the DSS for the prediction of OS [28]. Although only few studies have reported the DS Plus in combination with 18F-FDG PET/CT, the prognostic implication of PET/CT has been documented in several articles [16, 29, 30]. Our study confirmed that DS Plus based on 18F-FDG PET/CT has a great potential in predicting OS of MM patients.
DS Plus classification is mainly based on the number of focal bone lesions and the serum creatinine level and/or the presence of EMD. 18F-FDG PET/CT has a superior detection rate of focal bone lesions compared with whole-body X-ray and MRI of the spine and pelvis. Zamagni et al. have shown that 18F-FDG PET/CT has a better sensitivity than whole-body X-ray in 46% of patients, and it enables the detection of myelomatous lesions at sites that are not within the field of view (FOV) of MRI in 35% of patients [31]. Fonti et al. have prospectively evaluated 33 newly diagnosed MM patients by comparing 18F-FDG PET/CT with MRI of the spine and pelvis. They have found that MRI is better in the detection of diffuse diseases, while 18F-FDG PET/CT detects a considerable number of focal bone lesions that are out of the FOV of MRI [29]. The prognostic value of the number of focal bone lesions on 18F-FDG PET/CT has been confirmed in several studies [9, 16]. DS Plus could discriminate patients of different stages in this study, which might be attributed to accurate assessment of the number and site of focal PET positive bone lesions with or without osteolytic characteristics detected by 18F-FDG PET/CT.
In addition, criteria of IMPeTUs include the description of the metabolic state of the bone marrow. In a study of 18 MM patients, clinical course of the disease could be predicted by the associated 18F-FDG bone marrow images [32]. However, in other studies, 18F-FDG uptake of the bone marrow in PET/CT is not significantly associated with OS [29, 33]. The reason might be attributed to that the intensity of accumulation was assessed for 18F-FDG images using different methods. In our study, the univariate and multivariate analyses showed that the metabolic state of the bone marrow was an independent prognostic factor associated with OS.
There were some limitations in the present study: First, the sample size was small. Stage I patients in the DS Plus, RISS, and DSS all survived due to limited patient number. Because PET/CT is not yet a standardized imaging tool in MM, only a few MM patients performed PET/CT based on physicians' advice. In addition, some patients were excluded because of incomplete data. Second, in the present study, positive bone lesions were defined as lesions with a Deauville score ≥4 on PET scan and diffuse lytic lesions ranging between 0.5 and 1 cm in size with a Deauville score ≥3, and such a definition might reduce the specificity and consequently increase the false-positive rate. However, lesions in 51.5% of patients were downstaged according to the DS Plus compared with the DSS in our study. Therefore, we think that this definition is useful to guide therapy. However, a prospective study with a larger population should be adopted to define a cutoff for positivity.
Taken together, our findings indicated that DS Plus based on IMPeTUs was useful for the initial staging of MM. Both DS Plus and RISS possessed a better potential in predicting OS of MM patients compared with the DSS. DS Plus stage III and the Deauville score of bone marrow ≥4 were independent prognostic factors associated with OS.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 81601522), National Key New Drug Creation Special Programs (2017ZX09304-021), Natural Science Foundation of Jiangsu Province (no. BK20160348), Medical Youth Talent Project of Jiangsu Province (no. QNRC2016749), and Science and Technology Project for the Youth of Suzhou (no. kjxw2015004).
Contributor Information
Shibiao Sang, Email: sshibiao@163.com.
Wei Zhang, Email: sdfyyzw336@163.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
The authors Shengming Deng and Bin Zhang contributed equally to this work.
References
- 1.García de Veas Silva J. L., Bermudo Guitarte C., Menéndez Valladares P., Rojas Noboa J. C., Kestler K., Duro Millán R. Prognostic value of serum free light chains measurements in multiple myeloma patients. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166841.e0166841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hari P. N., Zhang M. J., Roy V., et al. Is the international staging system superior to the Durie-Salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia. 2009;23(8):1528–1534. doi: 10.1038/leu.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajkumar S. V. Updated diagnostic criteria and staging system for multiple myeloma. American Society of Clinical Oncology Educational Book. 2016;36:e418–e423. doi: 10.14694/edbk_159009. [DOI] [PubMed] [Google Scholar]
- 4.Durie B. G., Salmon S. E. A clinical staging system for multiple myeloma correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Greipp P. R., San Miguel J., Durie B. G., et al. International staging system for multiple myeloma. Journal of Clinical Oncology. 2005;23(15):3412–3420. doi: 10.1200/jco.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A., Avet-Loiseau H., Oliva S., et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. Journal of Clinical Oncology. 2015;33(26):2863–2869. doi: 10.1200/jco.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandon N., Rajkumar S. V., LaPlant B., et al. Clinical utility of the Revised International Staging System in unselected patients with newly diagnosed and relapsed multiple myeloma. Blood Cancer Journal. 2017;7(2):p. e528. doi: 10.1038/bcj.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durie B. G. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. European Journal of Cancer. 2006;42(11):1539–1543. doi: 10.1016/j.ejca.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Bailly C., Leforestier R., Jamet B., et al. PET imaging for initial staging and therapy assessment in multiple myeloma patients. International Journal of Molecular Sciences. 2017;18(2):p. E445. doi: 10.3390/ijms18020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanni C., Versari A., Chauvie S., et al. Interpretation criteria for FDG PET/CT in multiple myeloma (IMPeTUs): final results. IMPeTUs (Italian myeloma criteria for PET USe) European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(5):712–719. doi: 10.1007/s00259-017-3909-8. [DOI] [PubMed] [Google Scholar]
- 11.Brennan P., Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304(6840):1491–1494. doi: 10.1136/bmj.304.6840.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinter D. J., Neff W. K., Klaus J., et al. Comparison of whole-body MR imaging and conventional X-ray examination in patients with multiple myeloma and implications for therapy. Annals of Hematology. 2009;88(5):457–464. doi: 10.1007/s00277-008-0621-6. [DOI] [PubMed] [Google Scholar]
- 13.Weckbach S., Michaely H. J., Stemmer A., Schoenberg S. O., Dinter D. J. Comparison of a new whole-body continuous-table-movement protocol versus a standard whole-body MR protocol for the assessment of multiple myeloma. European Radiology. 2010;20(12):2907–2916. doi: 10.1007/s00330-010-1865-9. [DOI] [PubMed] [Google Scholar]
- 14.Squillaci E., Bolacchi F., Altobelli S., et al. Pre-treatment staging of multiple myeloma patients: comparison of whole-body diffusion weighted imaging with whole-body T1-weighted contrast-enhanced imaging. Acta Radiologica. 2015;56(6):733–738. doi: 10.1177/0284185114538792. [DOI] [PubMed] [Google Scholar]
- 15.Sager S., Ergül N., Ciftci H., Cetin G., Güner S. I., Cermik T. F. The value of FDG PET/CT in the initial staging and bone marrow involvement of patients with multiple myeloma. Skeletal Radiology. 2011;40(7):843–847. doi: 10.1007/s00256-010-1088-9. [DOI] [PubMed] [Google Scholar]
- 16.Tirumani S. H., Sakellis C., Jacene H., et al. Role of FDG-PET/CT in extramedullary multiple myeloma: correlation of FDG-PET/CT findings with clinical outcome. Clinical Nuclear Medicine. 2016;41(1):e7–e13. doi: 10.1097/rlu.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 17.Zamagni E., Patriarca F., Nanni C., et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989–5995. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 18.Cascini G. L., Falcone C., Console D., et al. Whole-body MRI and PET/CT in multiple myeloma patients during staging and after treatment: personal experience in a longitudinal study. La Radiologia Medica. 2013;118(6):930–948. doi: 10.1007/s11547-013-0946-7. [DOI] [PubMed] [Google Scholar]
- 19.Awan U. E., Siddiqui N., SaadUllah M., et al. FDG-PET scan in assessing lymphomas and the application of Deauville criteria. Journal of Pakistan Medical Association. 2013;63:725–730. [PubMed] [Google Scholar]
- 20.Moon S. H., Lee A. Y., Kim W. S., et al. Value of interim FDG PET/CT for predicting outcome of patients with angioimmunoblastic T-cell lymphoma. Leukemia & Lymphoma. 2017;58(6):1341–1348. doi: 10.1080/10428194.2016.1236380. [DOI] [PubMed] [Google Scholar]
- 21.Choi J. H., Yoon J. H., Yang S. K. Clinical value of new staging systems for multiple myeloma. Cancer Research and Treatment. 2007;39(4):171–174. doi: 10.4143/crt.2007.39.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihou D., Katodritou I., Zervas K. Evaluation of five staging systems in 470 patients with multiple myeloma. Haematologica. 2006;91:1149–1150. [PubMed] [Google Scholar]
- 23.Jimenez-Zepeda V. H., Duggan P., Neri P., Rashid-Kolvear F., Tay J., Bahlis N. J. Revised international staging system applied to real world multiple myeloma patients. Clinical Lymphoma Myeloma and Leukemia. 2016;16(9):511–518. doi: 10.1016/j.clml.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Kastritis E., Terpos E., Roussou M., et al. Evaluation of the Revised International Staging System in an independent cohort of unselected patients with multiple myeloma. Haematologica. 2017;102(3):593–599. doi: 10.3324/haematol.2016.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Sohn H. J., Kim S., et al. New staging systems can predict prognosis of multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation as first-line therapy. Biology of Blood and Marrow Transplantation. 2006;12(8):837–844. doi: 10.1016/j.bbmt.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Tao Z. F., Fu W. J., Yuan Z. G., Wang D. X., Chen Y. B., Hou J. Prognostic factors and staging systems of multiple myeloma. Chinese Medical Journal. 2007;120:1655–1658. [PubMed] [Google Scholar]
- 27.Filonzi G., Mancuso K., Zamagni E., et al. A comparison of different staging systems for multiple myeloma: can the MRI pattern play a prognostic role? American Journal of Roentgenology. 2017;209(1):152–158. doi: 10.2214/ajr.16.17219. [DOI] [PubMed] [Google Scholar]
- 28.Fechtner K., Hillengass J., Delorme S., et al. Staging monoclonal plasma cell disease: comparison of the Durie-Salmon and the Durie-Salmon PLUS staging systems. Radiology. 2010;257(1):195–204. doi: 10.1148/radiol.10091809. [DOI] [PubMed] [Google Scholar]
- 29.Fonti R., Salvatore B., Quarantelli M., et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in evaluation of patients with multiple myeloma. Journal of Nuclear Medicine. 2008;49(2):195–200. doi: 10.2967/jnumed.107.045641. [DOI] [PubMed] [Google Scholar]
- 30.Fonti R., Larobina M., Del Vecchio S., et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. Journal of Nuclear Medicine. 2012;53(12):1829–1835. doi: 10.2967/jnumed.112.106500. [DOI] [PubMed] [Google Scholar]
- 31.Zamagni E., Nanni C., Patriarca F., et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92(1):50–55. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 32.Castellani M., Carletto M., Baldini L., et al. The prognostic value of F-18 fluorodeoxyglucose bone marrow uptake in patients with recent diagnosis of multiple myeloma: a comparative study with Tc-99m sestamibi. Clinical Nuclear Medicine. 2010;35(1):1–5. doi: 10.1097/rlu.0b013e3181c3619c. [DOI] [PubMed] [Google Scholar]
- 33.Haznedar R., Akı S. Z., Akdemir O. U., et al. Value of 18F-fluorodeoxyglucose uptake in positron emission tomography/computed tomography in predicting survival in multiple myeloma. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38(6):1046–1053. doi: 10.1007/s00259-011-1738-8. [DOI] [PubMed] [Google Scholar]