Abstract
Small bowel neuroendocrine tumors (NETs) are increasing in incidence and are now the most common primary malignancies of the small intestine. Despite this increase, the vague presentation and slow growth of these tumors lead to long delays in diagnosis, and many patients present with metastases. Patients with metastatic small bowel NETs have a favorable disease prognosis, particularly when contrasted with other GI malignancies, and benefit from aggressive, multimodal therapy. During the past decade, the options for the diagnosis and treatment of small bowel NETs have increased considerably. This review provides a practical framework for the physician who seek to understand the epidemiology, presentation, diagnosis, and management of small bowel NETs.
INTRODUCTION
Neuroendocrine tumors (NETs) are a diverse group of neoplasms that arise from endocrine cells throughout the body. Though they share common histologic and biochemical properties, the natural history, malignant potential, and treatment of these tumors can vary dramatically. The first description of a small bowel NET was made by Langhans,1 in 1867, who described a polypoid tumor of the small intestine. This was followed in 1888 with a report by Lubarsch2 of two patients who, on autopsy, had multiple small tumors of the ileum. In 1890, Ransom3 provided the first description of the carcinoid syndrome in a patient who experienced diarrhea and dyspnea aggravated by food and who, on autopsy, had diffuse hepatic metastases and a distal ileal mass. The term “karzinoide” was first used by Oberndorfer4 to describe a series of six patients who had small bowel tumors. In this initial description, these tumors were considered benign, but their variable malignant potential was soon recognized. Continued study revealed an increasingly broad group of tumors with clinical, histologic, and biochemical similarities to the small bowel carcinoids; in 1963, Williams and Sandler5 proposed an expansion of the term “carcinoid” to encompass these diverse neoplasms. Under this scheme, NETs were classified by embryologic origin into foregut (bronchial, gastric, pancreatic), midgut (bowel from mid-duodenum to midtransverse colon), and hindgut (descending colon and rectum). Although the foregut-midgut-hindgut nomenclature was widely adopted, by the authors' admission, “We have no evidence that this distinction is of fundamental importance, but we consider it a convenient one.”5
The first WHO classification system, published in 1980, continued to refer to most tumors of the neuroendocrine system as carcinoid tumors. Under the WHO classification, carcinoid tumors were divided into enterochromaffin cell (classical) carcinoids, gastrin cell carcinoids, and other carcinoids.6 The increasingly imprecise application of the term “carcinoid,” combined with the fact that the minority of these tumors were actually associated with the carcinoid syndrome, led Capella et al7 to propose the use of the term “neuroendocrine tumor” to refer to all neoplasms of the neuroendocrine system. The WHO classification was revised in 2000 to include benign well-differentiated NET, well-differentiated NET of uncertain behavior, well-differentiated neuroendocrine carcinoma, and poorly differentiated neuroendocrine carcinoma; the presence of local invasion or metastases distinguished NET from neuroendocrine carcinoma (NEC).8 The most recent WHO classification includes NET grade 1, NET grade 2, and NEC; these are distinguished from each other on the basis of proliferative index, which is assessed by the percentage of cells that stain positive for Ki-67, and mitotic rate.9
Here, we review the presentation, diagnosis, and management of NETs that arise in the small bowel from the ligament of Treitz to the ileocecal valve, henceforth referred to as small bowel NETs. This definition does not include duodenal tumors, which are biologically and clinically distinct from tumors that arise in the jejunum or ileum.10 Although tumors of the appendix and proximal colon are also considered midgut NETs, their management is outside the scope of this review.
EPIDEMIOLOGY AND PRESENTATION
The incidence of NETs in general, and small bowel NETs specifically, has been increasing steadily since the 1970s.11-14 Although NETs usually are regarded as rare neoplasms, the increasing incidence combined with the relatively indolent course have resulted in a prevalence that greatly exceeds many other GI malignancies, including esophageal, gastric, and pancreatic cancers.11 In a study of data from the SEER registry, Dasari et al14 reported that the incidence of NETs has increased 6.4 fold since the program’s inception in 1973. This is attributable largely to the increased incidence of NETs of the rectum and small intestine, the two most common primary gastroenteropancreatic NETs.12 According to the most recent SEER data, the incidence of small bowel NETs in the United States is 1.05 per 100,000 persons.14 Data from several large European and Canadian studies suggest a similar increase in incidence.13,15 To what degree this represents an actual increase in incidence versus an apparent increase because of more frequent use of imaging and endoscopy, increased clinician awareness, or improved recognition by pathologists is unclear.13,14
The carcinoid syndrome was first characterized by Thorson et al16 in 1954 and is classically described as flushing, diarrhea, valvular heart disease, and bronchospasm, in order of decreasing frequency.10,17,18 Other clinical signs variably attributed to the carcinoid syndrome include telangiectasias, cyanosis, pellagra-like dermatitis, arthritis, myopathy, edema, and ascites, though it should be noted that these frequently are sequela of hepatic tumor replacement or carcinoid heart disease rather than excess hormone secretion.10,16-18 The syndrome is caused by the secretion of hormones, including serotonin, neurokinin A, histamine, and others.10,19 In tumors confined to the small bowel and regional lymph nodes, most of these hormones enter the portal circulation and are inactivated by the liver; consequently, the classical carcinoid syndrome is rarely seen in the absence of metastatic disease.18,20 Despite the historical use of the term “carcinoid” to refer to both NETs and the carcinoid syndrome, most patients present with nonspecific abdominal pain rather than with symptoms of excess hormone secretion.10,18,21,22 Because of the relatively low incidence, the lack of physician awareness, and the vague presenting symptoms related to small bowel NETs, patients with these small bowel NETs often experience long delays in diagnosis.15,19 The reported duration of symptoms that precede diagnosis varies considerably in the literature from a median of 4.3 months at a large academic institution21 to as long as a median of 9.2 years.19
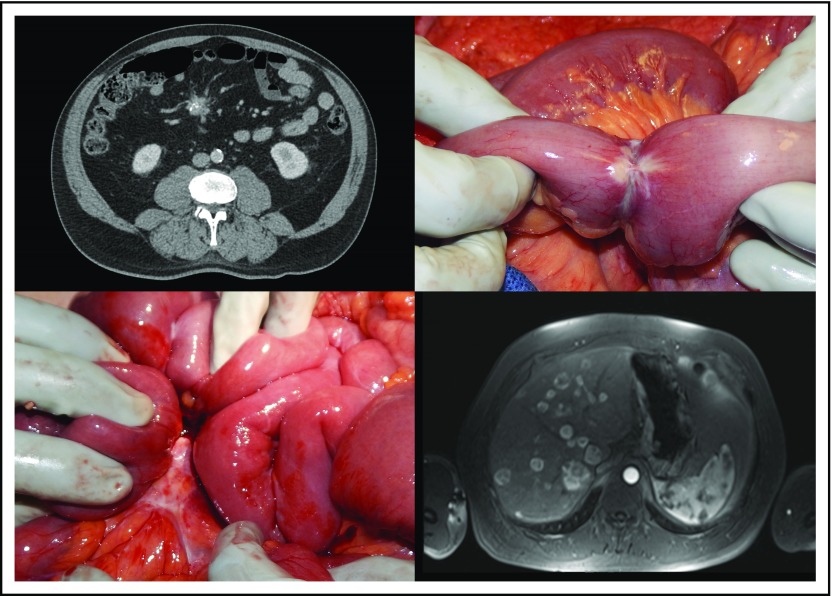
Although small bowel NET primary tumors are typically small, they have a tendency to induce a pronounced fibrotic reaction in the mesentery and often are accompanied by a mesenteric mass that represents enlarged regional lymph nodes (Fig 1).23 Fibrosis associated with small bowel NETs was first described by Moertel et al24 in 1961 and leads to significant morbidity and mortality as a result of intestinal obstruction and ischemia.23-27 Patients may present with episodic, crampy abdominal pain characteristic of recurrent partial bowel obstruction or with complete obstruction that requires emergent surgery; in some reports nearly half of patients with small bowel NETs present with obstructive symptoms.10,25
Fig 1.
Top left: A spiculated, partially calcified nodal mesenteric mass seen on computed tomography scan. Top right: Intraoperative image of a primary small bowel neuroendocrine tumor with narrowing of the bowel lumen. Bottom left: Intraoperative image of a mesenteric nodal mass. Bottom right: Contrast-enhanced T1-weighted magnetic resonance imaging in the arterial phase that shows multiple hepatic metastases.
The characteristically indolent growth of NETs has led to their description as cancers in slow motion; however, this slow growth should not be conflated with benign behavior. Metastases at presentation are seen in approximately 30% of patients with small bowel NETs in large, population-based database studies11,15 and in more than 60% of patients at large referral centers.21,27,28 Replacement of the normal hepatic parenchyma with NET metastases contributes to the vague abdominal pain characteristic of small bowel NETs and can eventually lead to liver failure, which is the leading cause of death in these patients.20,29
DIAGNOSIS
Biochemical Testing
Small bowel NETs secrete several biochemical markers that can be measured as part of the diagnostic workup of a patient with symptoms suggestive of the carcinoid syndrome or as biochemical surveillance of those with an established diagnosis. Chromogranin A is an acidic glycoprotein that is secreted by a wide variety of NETs, including nonfunctional tumors (ie, those not associated with a hormonal syndrome).10,19 Chromogranin A is a sensitive and specific marker for NETs that correlates with both tumor volume and prognosis; however, practitioners should be aware that a number of conditions, including proton pump inhibitor therapy, severe hypertension, or renal failure, can cause falsely elevated levels.18,30,31
Another well-studied marker of small bowel NETs is 5-hydroxyindole acetic acid (5-HIAA), which is a breakdown product of serotonin. The 24-hour urinary collection of 5-HIAA is preferred to serum serotonin levels because of fluctuations in serotonin levels throughout the day, and patients should avoid foods and medications known to cause false elevations.18 A list of items to avoid can be found at the Carcinoid Cancer Foundation Web site.32 Serotonin is thought to cause many of the symptoms of the carcinoid syndrome and is implicated in the pathogenesis of carcinoid heart disease.31,33
Numerous other biochemical markers, including pancreastatin, neurokinin A, substance P, serum serotonin, serum 5-HIAA, and neuron-specific enolase, have been proposed for the diagnosis and surveillance of small bowel NETs.10,18,31 An important general concept is that no one biochemical marker will be elevated in all small bowel NETs. A practical approach involves checking a variety of possible biomarkers and then monitoring only those that are elevated.18 Although biochemical markers are commonly used in the diagnosis and surveillance of small bowel NETs, there is little consensus on how they should be used to guide clinical decisions.31
Imaging
Imaging of small bowel NETs can be divided broadly into two categories: anatomic and functional. Anatomic studies include ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), whereas functional imaging includes single-photon emission computed tomography (SPECT) and positron emission tomography (PET). Accurate localization and staging of small bowel NETs often involves a combination of several imaging modalities.
Anatomic imaging
US plays a limited role in the imaging of small bowel NETs. The main disadvantages of US are the inability to visualize the entire abdomen because of body habitus and bowel gas and the time-intensive, operator-dependent nature of the exam. The primary roles for US in the treatment of small bowel NETs are in diagnosis and intraoperative tumor location. Liver metastases associated with small bowel NETs are often first seen on US obtained to evaluate abdominal pain or biliary symptoms, and, in these cases, tissue biopsy can be obtained under US guidance. US also is commonly used in the operating room, where it can be used to locate hepatic metastases and to direct intraoperative ablative therapy.34,35
The most common imaging study obtained for the diagnosis of small bowel NETs is the CT scan (Fig 1).34 CT has many advantages, including its availability, quick acquisition time, excellent anatomic definition, and ability to image the entire abdomen and thorax for staging and operative planning. It is important that a multi-phase CT be obtained for suspected small bowel NETs. The specific timing for image acquisition may vary from one center to another, but it should include an arterial phase, a venous phase, and a delayed phase.35-37 Small bowel NETs and liver metastases are characteristically hypervascular and are demonstrated best on the arterial phase, where they appear bright; however, some can be hypovascular and may be best seen on the venous phase, where they appear dark.35,36 The reported CT sensitivity for primary small bowel NETs ranges from 7% to 38%, but this can be improved to 82% if the presence of mesenteric lymphadenopathy/fibrosis is interpreted as evidence of a small bowel primary tumor.38 CT enteroclysis has also shown better sensitivity, in the range of 50% to 85%, for the detection of small bowel NET primaries.35 Reported CT sensitivities for nodal and liver metastases range from 60% to 70% and 75% to 100%, respectively.35 For surgical planning, CT scans tend to underestimate the degree of liver tumor burden and may miss smaller lesions, particularly compared with MRI and intraoperative US.23,36
MRI is more sensitive than CT for detection of liver metastases and delivers no ionizing radiation, but it is more expensive, is less commonly available, and provides poorer anatomic definition of nodal disease (Fig 1).23 Small bowel NETs and their metastases typically appear as low-intensity lesions on T1-weighted images and as high-intensity lesions on T2- and diffusion-weighted images. Contrast enhancement on MRI is similar to CT. Classic hypervascular metastases will appear bright and are seen best in the arterial phase, whereas hypovascular metastases appear dark and are best demonstrated on the venous phase.35,36 In a prospective study that compared MRI, CT, and somatostatin receptor scintigraphy (SRS), the sensitivities for detection of NET liver metastases was 95.2%, 78%, and 49.3%, respectively, and MRI detected significantly more metastases. In the same study, 77% of patients had hypervascular liver metastases, and 23% had hypovascular metastases.36 Increasingly, MRIs may be obtained with a hepatocyte-specific contrast agent (eg, Eovist, Bayer Pharmaceuticals, Berlin, Germany), which has been shown to provide a better contrast-to-noise ratio and to improve interobserver reliability for the measurement of NET liver metastases compared with an extracellular contrast agent (eg, Gadavist, Bayer Pharmaceuticals, Berlin, Germany).39
Functional imaging
Functional imaging techniques for the diagnosis of small bowel NETs take advantage of the fact that 80% to 100% of these tumors show high expression of somatostatin receptors.40 The first radiolabeled somatostatin analog to gain widespread clinical acceptance was indium-111 (111In)–DTPA-D-Phe-1-octreotide, which was used with SRS (111In-SRS [Octreoscan], Curium, Paris, France; Fig 2). A large series by Krenning et al41 in 1993 found that 111In-SRS was positive in 86% of patients with carcinoids from various primary sites.40 111In-SRS has since evolved by replacing the older planar scintigraphy with SPECT, which provides superior localization.34,35 The overall reported sensitivity of 111In-SRS in recent studies has ranged from 60% to 80% for the detection of any NET disease,35 but only approximately half of primary small bowel NETs will be localized by 111In-SRS.38
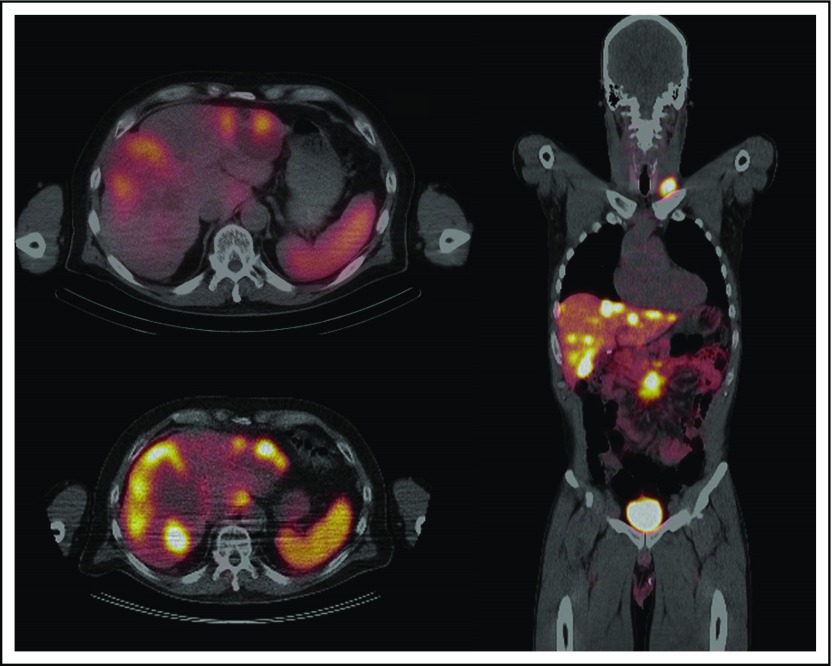
Fig 2.
Top left: Octreoscan showing hepatic metastases of small bowel neuroendocrine tumor. Bottom left: Gallium-68 positron emission tomography/computed tomography (68Ga-PET/CT) of the same patient that demonstrates improved resolution of the metastatic lesions. Right: Coronal section of a 68Ga-PET/CT that demonstrates a mesenteric nodal mass and multiple metastatic lesions to the liver and left supraclavicular node.
In recent years, 111In-SRS has been increasingly supplanted as the functional imaging modality of choice by PET/CT that uses a number of gallium-68 (68Ga)–labeled somatostatin analogs, including 68Ga-DOTATOC, -DOTANOC, and -DOTATATE.34,35 68Ga-PET has several advantages compared with 111In-SRS, including better spatial resolution, faster postinjection image acquisition, less radiation exposure, and improved diagnostic accuracy (Fig 2).34 68Ga-PET/CT has been used clinically since the early 2000s had a 97% sensitivity for any NET and a 99% sensitivity for midgut NETs in a large, single-institution series.42 Four recent meta-analyses have reported mean sensitivities of 68Ga-PET for any NETs from 88% to 93%.35 Until recently, access to a 68Ga radiotracer was limited in the United States.34 However, 68Ga-DOTATATE was approved by the US Food and Drug Administration in June 2016 and is now widely available.
Although [18F]fluorodeoxyglucose ([18F]FDG)–PET is used to detect a variety of other malignancies, well-differentiated small bowel NETs typically do not show avid uptake of radiolabeled glucose, and the reported sensitivity for [18F]FDG-PET ranges from 37% to 72% for NETs.35 [18F]FDG-PET remains useful for the imaging of poorly differentiated tumors, which express the somatostatin receptor less frequently40 and are more metabolically active. In a study that compared 111In-SRS to [18F]-FDG-PET, the sensitivity of PET increased with grade up to 100% in high-grade tumors, whereas 111In-SRS demonstrated significantly worse sensitivity in high-grade versus low-grade tumors.43
Endoscopy
Endoscopic examination of small bowel NETs includes colonoscopy, double-balloon enteroscopy, and capsule endoscopy. The majority of small bowel NETs occur in the distal ileum,10 and, although the terminal ileum is routinely intubated during colonoscopy, the small bowel until recently has been largely inaccessible for additional endoscopic evaluation. Both capsule endoscopy and double-balloon enteroscopy allow for evaluation of the entire small bowel in most patients.44 Data to support the use of both modalities specifically in small bowel NET populations are taken from small retrospective and prospective studies, in which the reported diagnostic yield ranges from 45% to 72% for capsule endoscopy and from 30% to 80% for double balloon enteroscopy.44 The role of these endoscopic techniques in the diagnosis of small bowel NETs continues to be defined, but the techniques are most useful in patients with suspected small bowel NETs when no primary tumor was found on anatomic or functional imaging.
Pathologic Exam
Although imaging and biochemical findings can suggest the diagnosis of small bowel NET, pathologic exam is required for confirmation. Tissue may be obtained from a surgical specimen or biopsy, and core needle biopsy is preferable to fine-needle aspiration.20,31 In patients with clinical or histologic suggestion of NET, immunohistochemistry should be performed to confirm the diagnosis. Both chromogranin and synaptophysin are considered reliable general markers for NETs.20,31,45 However, it is not sufficient to provide only the diagnosis of NET, particularly in patients who present with liver metastases and an unknown primary tumor. Additional stains will help identify the primary site: diffuse staining for CDX2 (caudal type homeobox 2) suggests a small bowel primary; PAX6 (paired box 6), PAX8 (paired box 8), or ISL1 (Islet 1) positivity suggests a pancreatic primary; and TTF-1 (thyroid transcription factor-1) positivity suggests a bronchial primary.45,46 One caveat is that these immunohistochemical results may not apply to poorly differentiated tumors.45
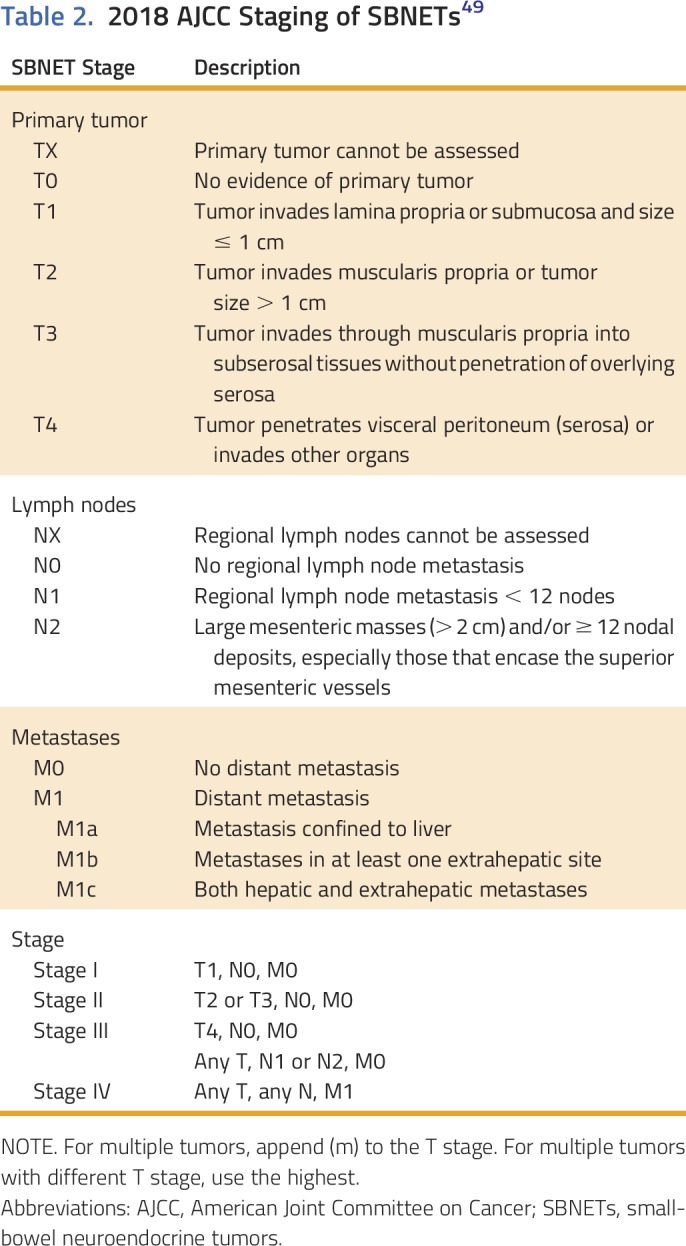
GI NETs are graded according to the 2010 WHO Classification of Tumors of the Digestive System based on the proliferative index, which is assessed by the percentage of cells that stain positively for Ki-67, and mitotic rate (Table 1).9 A more recent WHO classification for pancreatic NETs was published in 2017. This system clarifies the grading of tumors with a proliferative index between 2% and 3% (left ambiguous in the 2010 guidelines) and divides high-grade tumors into well-differentiated grade 3 NET and poorly differentiated grade 3 NEC.47 Although this classification technically is applicable only to pancreatic NETs, the upcoming fifth edition of the WHO Classification of Tumors of the Digestive System is anticipated to be similar. Importantly, the Ki-67 index should be assessed both for primary tumors and for lymph or liver metastases from surgical specimens, because patient survival is most accurately predicted from the highest grade seen at any site.48 Small bowel NETs are staged according to the American Joint Committee on Cancer staging system (Tables 1 and 2).49
Table 1.
Comparison of the 2010 WHO Classification of Tumors of the Digestive System and the 2017 WHO Classification of Tumors of Endocrine Organs
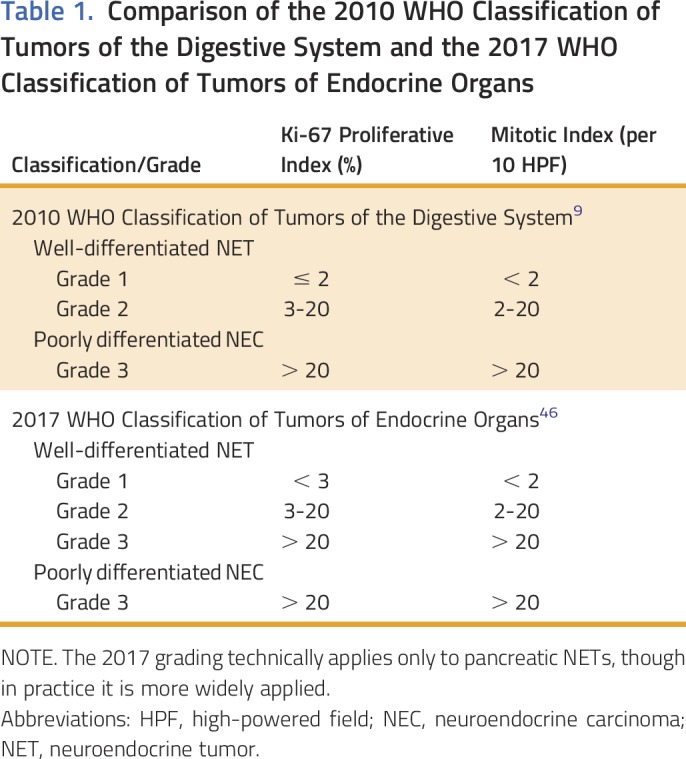
Table 2.
2018 AJCC Staging of SBNETs49
TREATMENT OF SMALL BOWEL NETS
Locoregional Disease
In patients with locoregional small bowel NETs, the standard of care is surgical resection.23,27,31,50 These patients often present with bowel obstruction or abdominal pain, and a mass is discovered on imaging; as a result, many will undergo resection for these signs or symptoms before diagnosis.25,50 The optimal surgical treatment of small bowel NETs is segmental small bowel resection or ileocecectomy (for distal ileal tumors) with resection of the regional lymph nodes up to the segmental branches of the superior mesenteric artery and vein.23 The abdomen should be diligently inspected for evidence of peritoneal and liver metastases, which are present in up to 20% and 60% of patients, respectively, who undergo surgery for small bowel NETs.27 Careful palpation of the entire small bowel is critical to detect multifocal tumors, which often are subcentimeter in size and are present in roughly half of patients.51 The use of somatostatin analogs is associated with an elevated risk of gallstone development. For patients who are likely to require future somatostatin analog therapy (eg, those with extensive lymph node involvement), prophylactic cholecystectomy should be offered at the time of surgery.23 Although surgery is potentially curative, recurrence rates of 42% have been reported after resection, and the liver is the most common site of recurrence.50 Recurrence may occur many years after the initial surgery because of the slow growth of small bowel NETs. Current recommendations are for 6-month surveillance visits for 1 year followed by yearly radiographic, clinical, and biochemical surveillance for 10 years.31
Metastatic Disease
Small bowel NETs are metastatic at presentation in roughly 30% of patients in population-based studies and in 60% of patients seen at large referral centers.11,15,21,27 The presentation in these patients can vary significantly from asymptomatic, incidentally discovered liver metastases to debilitating flushing and diarrhea from the carcinoid syndrome. Compared with many other GI malignancies, patients with metastatic small bowel NETs have a favorable prognosis: median overall survival was 103 months for patients diagnosed with well-differentiated tumors from 2000 to 2012.14 During the past decade, results from a number of phase III randomized trials have improved the treatment options for patients with metastatic small bowel NETs.
Somatostatin analogs
Somatostatin analogs are the first-line treatment of functional and nonfunctional metastatic small bowel NETs, both for their antiproliferative effects and for control of carcinoid symptoms.20,31 Patients often are treated with injection of a long-acting somatostatin analog (octreotide LAR [long-acting repeatable] or lanreotide) every 4 weeks, and short-acting octreotide injections are used as needed for rescue therapy to improve symptomatic control. The PROMID trial examined the effect of octreotide LAR versus placebo in patients with well-differentiated, mostly grade 1 (81 of 85 patients had Ki-67 index < 2%), metastatic, midgut NETs and found significantly improved median progression-free survival (PFS) in the treatment group (14.3 v 6.0 months).52 A somewhat different population was examined in the CLARINET trial, which included patients with metastatic grade 1 or 2 (Ki-67 index < 10%) NETs of the pancreas, midgut, hindgut, or unknown origin—96% of whom had stable disease at baseline. This study confirmed the antiproliferative effects of lanreotide by demonstrating an improved median PFS in the treatment group compared with placebo (not reached v 18 months).53 Octreotide LAR and lanreotide currently have different approved indications in the United States (palliation of carcinoid syndrome and control of tumor growth, respectively). However given their overall similarity, they are often used interchangeably.31
Everolimus
Everolimus is a mammalian target of rapamycin inhibitor that has been studied in patients with advanced NETs with carcinoid syndrome (RADIANT-2) and advanced nonfunctional NETs (RADIANT-4). In the RADIANT-2 trial, a trend was seen toward improved PFS for treatment with everolimus and octreotide LAR compared with octreotide LAR alone; however, this trend did not reach statistical significance.54 In contrast, the results of the RADIANT-4 trial showed improved median PFS when everolimus monotherapy was compared with placebo (11.0 v 3.9 months).55 On the basis of these results, everolimus was approved for use only in progressive nonfunctional NETs, but it is often used in patients with progressive disease irrespective of tumor functionality.31 It is important to note that, in the RADIANT-2 trial, 52% of patients had small bowel NETs and the next most common primary sites were the lung (10%) and colon (7%), whereas 31% of patients in the RADIANT-4 trial had small bowel NETs and the next most common primary sites were the lung (30%) and rectum (13%).
Peptide receptor radionuclide therapy
Peptide receptor radionuclide therapy (PRRT) has been used since 1992 for the treatment of NETs.56 In PRRT, a radiolabeled somatostatin analog is used to deliver radionuclides directly to the tumor. The two most widely used isotopes are yttrium-90 and leutetium-177 (90Y and 177Lu, respectively), and 177Lu emits beta and gamma rays that have a maximum range of 2 mm.56 In the NETTER-1 trial, 229 patients with metastatic, well-differentiated, midgut NETs were randomly assigned to treatment with 177Lu-DOTATATE and 30 mg/month of octreotide LAR or to 60 mg/month octreotide LAR alone. The study found a significantly improved median PFS (not reached v 8.4 months) as well as improved overall survival on interim analysis as well as an improved response rate in the treatment group (18% v 3%).56 Peptide receptor radionuclide therapy was previously unavailable in the United States outside of the research setting. However, on January 26, 2018, the US Food and Drug Administration approved 177Lu-DOTATATE for the treatment of gastroenteropancreatic NETs. For patients with small bowel NETs who experience disease progression while receiving somatostatin analog treatment, PRRT will likely become the preferred second-line treatment.
Chemotherapy
Cytotoxic chemotherapy is well established in the treatment of pancreatic NETs but plays a limited role in the treatment of well-differentiated small bowel NETs.57 Streptozocin, often used in combination with fluorouracil or doxorubicin, is approved for use in pancreatic NETs, but its efficacy against small bowel NETs is not established, and it is associated with significant toxicity.57,58 Other chemotherapeutic options include dacarbazine, oxaliplatin plus capecitabine or fluorouracil, and irinotecan-based therapy.58 Strong evidence to favor any one chemotherapeutic regimen compared with another for small bowel NETs is lacking, and most studies identified in a 2016 meta-analysis were nonrandomized and included NETs from a variety of primary sites.57 Interferon alfa, which was used as a control arm in three randomized trials in the aforementioned meta-analysis, is thought to inhibit tumor growth and improve symptom control on the basis of a number of small prospective and retrospective studies, but it is not widely used at this time because of the lack of high-quality evidence and an unfavorable adverse effect profile.31,57 In recent years, the combination of capecitabine and temozolomide has been demonstrated to yield response rates from 30% to 70% in pancreatic NETs.59 Data to support the use of capecitabine and temozolomide in small bowel NETs is based on small numbers of patients in retrospective studies, and objective response rates in nonpancreatic NETs are significantly lower—ranging from 14% in a heavily pretreated population to 42%.60,61 Despite this, the convenient oral route of administration and favorable adverse effect profile make capecitabine and temozolomide a reasonable second- or third-line option in patients with progressive small bowel NETs.
NECs that arise from the small bowel are exceedingly rare, and, because of their dismal prognosis, patients with NECs are not generally considered surgical candidates.23 The first-line treatment of patients with NECs, regardless of primary site, is cisplatin or carboplatin and etoposide.58,62 Patients with Ki-67 indices toward the low end of high-grade disease (20% to 55%) have shown lower response rates to platinum-based chemotherapy, but there is neither a standard approach to these patients nor a standard second-line regimen for NECs.58,62
Carcinoid heart disease
Carcinoid heart disease, which affects up to 20% of patients with the carcinoid syndrome, is characterized by fibrosis that primarily affects the right-sided heart valves and is thought to be due to high circulating levels of serotonin.26,33 This valvular fibrosis eventually leads to heart failure and is associated with significantly worse prognosis.27,31,33 The presentation of carcinoid heart disease can be subtle, and it is most reliably detected with echocardiography, although N-terminal pro b-type natriuretic peptide (NT-proBNP) is a serum marker with high negative predictive value.31,33 Data to support screening in asymptomatic patients are limited; however, given the association between elevated serotonin levels and carcinoid heart disease, annual echocardiography is recommended for patients with significantly elevated serum serotonin or urinary 5-HIAA, and screening of all patients with metastatic small bowel NETs should be considered.31 Definitive treatment of carcinoid heart disease for patients with symptomatic, severe valvular disease is surgical valve replacement. However, cardiac surgery for carcinoid heart disease is associated with mortality rates of 10% to 20%, and the benefits of surgery only begin to outweigh medical management at approximately 6 months.33 Because of the association of carcinoid heart disease with elevated serotonin levels, the use of telotristat has been proposed in patients with both carcinoid heart disease and high serotonin levels; however, the benefit is purely theoretical at this point.31
Surgery for metastatic small bowel NETs
Unlike many other GI malignancies, patients with metastatic small bowel NETs are not precluded from surgery.23 Numerous retrospective studies have demonstrated improved survival and symptomatic control with resection of nodal25,27 and hepatic22,63,64 metastases: 5-year overall survival rates varied from 65% to 88%. These procedures are rarely curative, and 5- and 10-year recurrence rates are 95% and 99%, respectively.64 This high recurrence rate shifts the emphasis from cure to debulking, a notion which also is supported by the lack of survival benefit associated with R0 versus R1 or R2 resection.63,64 Historically, hepatic debulking has been attempted only when resection of 90% of the metastases was deemed feasible, but recent studies have found equivalent survival with a threshold of 70% cytoreduction.22,63 Adoption of this lower debulking threshold—along with the use of parenchyma-sparing surgical techniques, which include wedge resection, enucleation, and intraoperative radiofrequency or microwave ablation—allows for as many as 76% of patients to undergo hepatic debulking.22 In contrast, when patients are selected on the basis of the feasibility of achieving 90% cytoreduction, less than 25% will undergo surgery.22,23 Contraindications to surgery are debated, but, in general, patients with greater than 50% liver replacement, numerous small metastases, poor performance status, liver dysfunction, or high-grade disease should not be considered for hepatic debulking.23 Resection of the primary tumor, which can be accomplished at the same time as hepatic cytoreduction, should be performed when feasible to avoid potential future complications from an obstructing small bowel lesion or mesenteric mass and to prevent carcinomatosis or the development of additional liver metastases.23 Even if a patient’s hepatic metastases are unresectable, resection of the primary appears to improve survival.25,29 In patients who are ineligible for hepatic debulking, liver transplantation may offer the potential for curative resection and appears to improve survival.65,66 Eligibility for transplantation is determined by the Milan-NET criteria,66 but the potential benefits of this extensive procedure must be weighed against the national shortage of grafts.65 Finally, cholecystectomy should be performed at the time of surgery for metastatic small bowel NETs because of the high incidence of gallstones in patients who receive somatostatin analogs.23
Liver-directed therapy
In addition to surgical debulking, less invasive methods, such as hepatic artery embolization and percutaneous liver ablation, can be used to treat small bowel NET liver metastases. Embolization involves the injection of particles into the hepatic artery or its branches to occlude blood flow to the liver metastases, which are supplied primarily by the hepatic arteries.67 It can be performed with inert particles (bland), beads along with a chemotherapeutic agent (chemoembolization), or 90Y-conjugated beads (radioembolization). To date, no single therapy has shown clear superiority.20,67 Symptomatic response rates to hepatic artery embolization range from 39% to 95%.67 Percutaneous liver ablation involves the insertion of a microwave or radiofrequency probe into hepatic metastases under image guidance and subsequent heating of the lesions to induce necrosis. High-quality evidence to support the use of percutaneous ablation is lacking. Although reported complication rates are low and symptomatic response rates are favorable, many series pool the results from operative and percutaneous ablative procedures, which makes interpretation difficult.68 For patients with liver-dominant disease who are not surgical candidates, both hepatic artery embolization and percutaneous ablation may be considered for disease control.20,31
Telotristat
Diarrhea and flushing associated with the carcinoid syndrome can be debilitating, especially in patients with significant hepatic disease. Telotristat ethyl is an inhibitor of tryptophan hydroxylase that acts to reduce serotonin levels and has emerged as a promising agent for control of refractory carcinoid syndrome diarrhea. The phase III TELESTAR and TELECAST trials demonstrated that treatment with telotristat ethyl was associated with significant reduction in both urinary 5-HIAA and bowel movement frequency in patients with carcinoid syndrome.69,70 Telotristat is now approved for the treatment of carcinoid syndrome diarrhea inadequately controlled by somatostatin analogs alone. It is critical to differentiate diarrhea from the carcinoid syndrome from that caused by reduced intestinal length after surgery or from bile salt malabsorption. Diarrhea caused by pancreatic insufficiency secondary to somatostatin analog use is often oily and malodorous and should be treated with pancreatic enzyme replacement.20
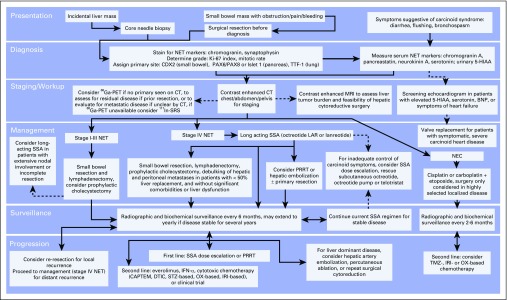
In conclusion, the diagnosis and treatment of small bowel NETs is a multidisciplinary effort. As the incidence of these tumors increases, so too does the importance of understanding this rapidly evolving field. The presentation of small bowel NETs is characterized by vague GI complaints, which often leads to long delays from symptom onset to diagnosis. Once a small bowel NET is suspected, every effort should be made to confirm the diagnosis with a combination of biochemical testing, anatomic and functional imaging, and tissue biopsy. Thorough pathologic examination performed by a pathologist familiar with NETs will help guide clinical decision making and prognostication. The provided algorithm describes our approach to the diagnosis and treatment of small bowel NETs (Fig 3). In brief, patients with localized disease are treated with surgery and then undergo radiographic and biochemical surveillance every 6 to 12 months for at least 10 years. Patients with metastatic disease are uniformly treated with somatostatin analogs and are considered for primary tumor resection and cytoreductive surgery. Cytoreductive surgery is offered to roughly 75% of those evaluated by surgical oncology at our institution. After surgery, patients are maintained on somatostatin analog treatment, and the disease is surveilled every 6 months. Increasingly, PRRT is the therapy of choice for progression during somatostatin analog treatment. Other options for the treatment of progressive disease include somatostatin analog dose escalation, chemotherapy, targeted therapy, and hepatic artery embolization or percutaneous ablation for liver-dominant disease. It is imperative that physicians who care for these patients maintain familiarity with the ever-expanding armamentarium available for the treatment of small bowel NETs to provide optimal care.
Fig 3.
An algorithmic approach to the diagnosis and treatment of small bowel neuroendocrine tumors (NETs). BNP, brain natriuretic peptide; CAPTEM, capecitabine and temozolomide; CT, computed tomography; DTIC, dacarbazine; Ga-PET, gallium positron emission tomography; IFN, interferon; In-SRS, indium somatostatin receptor scintigraphy; IRI, irinotecan; LAR, long-acting repeatable; NEC, neuroendocrine carcinoma; OX, oxaliplatin; PRRT, peptide receptor radionuclide therapy; SSA, somatostatin analog; STZ, streptozocin; TMZ, temozolomide.
ACKNOWLEDGMENT
Supported by T32 Grant No. CA148062-0 (to A.T.S.) and Specialized Programs of Research Excellence Grant No. P50 CA174521-01 (to J.R.H.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Management of Small Bowel Neuroendocrine Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Aaron T. Scott
No relationship to disclose
James R. Howe
No relationship to disclose
REFERENCES
- 1.Langhans T. Ueber einen drüsenpolyp im ileum. Virchows Arch. 1867;38:559–560. [Google Scholar]
- 2.Lubarsch O. Ueber den primären krebs des ileum nebst bemerkungen über das gleichzeitige vorkommen von krebs und tuberculose. Virchows Arch. 1888;111:280–317. [Google Scholar]
- 3.Ransom WB. A case of primary carcinoma of the ileum. Lancet. 1890;136:1020–1023. [Google Scholar]
- 4.Oberndorfer S. Karzinoide tumoren des dünndarms. Frankf Z Pathol. 1907;1:425–432. [Google Scholar]
- 5.Williams ED, Sandler M. The classification of carcinoid tumours. Lancet. 1963;1:238–239. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 6.Williams ED, Siebenmann RE, Sobin LH. Histological Typing Of Endocrine Tumours (ed 2) Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 7.Capella C, Heitz PU, Höfler H, et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425:547–560. doi: 10.1007/BF00199342. [DOI] [PubMed] [Google Scholar]
- 8.Solcia E, Klöppel GN, Sobin LH. Histological Typing of Endocrine Tumours (ed 2) Berlin, Germany: Springer-Verlag; 2000. [Google Scholar]
- 9.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System (ed 4) Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 10.Modlin IM, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18,vii. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Fraenkel M, Kim M, Faggiano A, et al. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr Relat Cancer. 2014;21:R153–R163. doi: 10.1530/ERC-13-0125. [DOI] [PubMed] [Google Scholar]
- 14.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 16.Thorson A, Biorck G, Bjorkman G, et al. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis: A clinical and pathologic syndrome. Am Heart J. 1954;47:795–817. doi: 10.1016/0002-8703(54)90152-0. [DOI] [PubMed] [Google Scholar]
- 17.Creutzfeldt W, Stöckmann F. Carcinoids and carcinoid syndrome. Am J Med. 1987;82:4–16. doi: 10.1016/0002-9343(87)90422-0. [DOI] [PubMed] [Google Scholar]
- 18.Vinik AI, Chaya C. Clinical presentation and diagnosis of neuroendocrine tumors. Hematol Oncol Clin North Am. 2016;30:21–48. doi: 10.1016/j.hoc.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Vinik AI, Silva MP, Woltering EA, et al. Biochemical testing for neuroendocrine tumors. Pancreas. 2009;38:876–889. doi: 10.1097/MPA.0b013e3181bc0e77. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Asa SL, Dey C, et al. Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence-based Canadian consensus. Cancer Treat Rev. 2016;47:32–45. doi: 10.1016/j.ctrv.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Ter-Minassian M, Chan JA, Hooshmand SM, et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: Results from a prospective institutional database. Endocr Relat Cancer. 2013;20:187–196. doi: 10.1530/ERC-12-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell JE, Sherman SK, O’Dorisio TM, et al. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery. 2016;159:320–333. doi: 10.1016/j.surg.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howe JR, Cardona K, Fraker DL, et al. The surgical management of small bowel neuroendocrine tumors: Consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46:715–731. doi: 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moertel CG, Sauer WG, Dockerty MB, et al. Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901–912. doi: 10.1002/1097-0142(196109/10)14:5<901::aid-cncr2820140502>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg. 2002;26:991–997. doi: 10.1007/s00268-002-6630-z. [DOI] [PubMed] [Google Scholar]
- 26.Modlin IM, Shapiro MD, Kidd M. Carcinoid tumors and fibrosis: An association with no explanation. Am J Gastroenterol. 2004;99:2466–2478. doi: 10.1111/j.1572-0241.2004.40507.x. [DOI] [PubMed] [Google Scholar]
- 27.Norlén O, Stålberg P, Öberg K, et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. 2012;36:1419–1431. doi: 10.1007/s00268-011-1296-z. [DOI] [PubMed] [Google Scholar]
- 28.Dahdaleh FS, Calva-Cerqueira D, Carr JC, et al. Comparison of clinicopathologic factors in 122 patients with resected pancreatic and ileal neuroendocrine tumors from a single institution. Ann Surg Oncol. 2012;19:966–972. doi: 10.1245/s10434-011-1997-4. [DOI] [PubMed] [Google Scholar]
- 29.Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140:891–897. doi: 10.1016/j.surg.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Yang Y, Li Z, et al. Diagnostic value of circulating chromogranin a for neuroendocrine tumors: A systematic review and meta-analysis. PLoS One. 2015;10:e0124884. doi: 10.1371/journal.pone.0124884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707–714. doi: 10.1097/MPA.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carcinoid Cancer Foundation Preparing for the 24-hour uring 5HIAA test. https://www.carcinoid.org/for-doctors/diagnosis-and-surveillance/preparing-for-the-24-hour-urine-5hiaa-test/
- 33.Luis SA, Pellikka PA. Carcinoid heart disease: Diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2016;30:149–158. doi: 10.1016/j.beem.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: An update for the clinician. Int J Endocr Oncol. 2015;2:159–168. doi: 10.2217/ije.14.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundin A, Arnold R, Baudin E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Radiological, nuclear medicine & hybrid imaging. Neuroendocrinology. 2017;105:212–244. doi: 10.1159/000471879. [DOI] [PubMed] [Google Scholar]
- 36.Dromain C, de Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumors: A prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70–78. doi: 10.1200/JCO.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Ruf J, Schiefer J, Furth C, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: Spotlight on the CT phases of a triple-phase protocol. J Nucl Med. 2011;52:697–704. doi: 10.2967/jnumed.110.083741. [DOI] [PubMed] [Google Scholar]
- 38.Keck KJ, Maxwell JE, Menda Y, et al. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. 2017;161:272–279. doi: 10.1016/j.surg.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirumani SH, Jagannathan JP, Braschi-Amirfarzan M, et al. Value of hepatocellular phase imaging after intravenous gadoxetate disodium for assessing hepatic metastases from gastroenteropancreatic neuroendocrine tumors: Comparison with other MRI pulse sequences and with extracellular agent. Abdom Radiol (NY) doi: 10.1007/s00261-018-1496-1. . [Epub ahead of print on February 22, 2018] [DOI] [PubMed] [Google Scholar]
- 40.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80:51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 41.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: The Rotterdam experience with more than 1,000 patients. Eur J Nucl Med. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 42.Skoura E, Michopoulou S, Mohmaduvesh M, et al. The impact of 68Ga-DOTATATE PET/CT imaging on management of patients with neuroendocrine tumors: Experience from a national referral center in the United Kingdom. J Nucl Med. 2016;57:34–40. doi: 10.2967/jnumed.115.166017. [DOI] [PubMed] [Google Scholar]
- 43.Squires MH, III, Volkan Adsay N, Schuster DM, et al. Octreoscan versus FDG-PET for neuroendocrine tumor staging: A biological approach. Ann Surg Oncol. 2015;22:2295–2301. doi: 10.1245/s10434-015-4471-x. [DOI] [PubMed] [Google Scholar]
- 44.Rossi RE, Conte D, Elli L, et al. Endoscopic techniques to detect small-bowel neuroendocrine tumors: A literature review. United European Gastroenterol J. 2017;5:5–12. doi: 10.1177/2050640616658220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: A clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol. 2013;20:285–314. doi: 10.1097/PAP.0b013e3182a2dc67. [DOI] [PubMed] [Google Scholar]
- 46.Maxwell JE, Sherman SK, Stashek KM, et al. A practical method to determine the site of unknown primary in metastatic neuroendocrine tumors. Surgery. 2014;156:1359–1365. doi: 10.1016/j.surg.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lloyd RV, Osamura RY, Klöppel G, et al. WHO Classification of Tumours of Endocrine Organs (ed 4) Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 48.Keck KJ, Choi A, Maxwell JE, et al. Increased grade in neuroendocrine tumor metastases negatively impacts survival. Ann Surg Oncol. 2017;24:2206–2212. doi: 10.1245/s10434-017-5899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin MB, Edge SB, Greene F, et al. AJCC Cancer Staging Manual (ed 8). New York, NY: Springer2017 [Google Scholar]
- 50.Le Roux C, Lombard-Bohas C, Delmas C, et al. Relapse factors for ileal neuroendocrine tumours after curative surgery: A retrospective French multicentre study. Dig Liver Dis. 2011;43:828–833. doi: 10.1016/j.dld.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Choi AB, Maxwell JE, Keck KJ, et al. Is multifocality an indicator of aggressive behavior in small bowel neuroendocrine tumors? Pancreas. 2017;46:1115–1120. doi: 10.1097/MPA.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 53.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 54.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 55.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strosberg J, El-Haddad G, Wolin E, et al. NETTER-1 Trial Investigators Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamarca A, Elliott E, Barriuso J, et al. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract: A systematic review and meta-analysis—A lost cause? Cancer Treat Rev. 2016;44:26–41. doi: 10.1016/j.ctrv.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Carbonero R, Rinke A, Valle JW, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms: Systemic therapy 2—Chemotherapy. Neuroendocrinology. 2017;105:281–294. doi: 10.1159/000473892. [DOI] [PubMed] [Google Scholar]
- 59.Cives M, Ghayouri M, Morse B, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23:759–767. doi: 10.1530/ERC-16-0147. [DOI] [PubMed] [Google Scholar]
- 60.Ramirez RA, Beyer DT, Chauhan A, et al. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. Oncologist. 2016;21:671–675. doi: 10.1634/theoncologist.2015-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotteas EA, Syrigos KN, Saif MW. Profile of capecitabine/temozolomide combination in the treatment of well-differentiated neuroendocrine tumors. OncoTargets Ther. 2016;9:699–704. doi: 10.2147/OTT.S72155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ilett EE, Langer SW, Olsen IH, et al. Neuroendocrine carcinomas of the gastroenteropancreatic system: A comprehensive review. Diagnostics (Basel) 2015;5:119–176. doi: 10.3390/diagnostics5020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graff-Baker AN, Sauer DA, Pommier SJ, et al. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery. 2014;156:1369–1376. doi: 10.1016/j.surg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 65.Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery. 2017;162:525–536. doi: 10.1016/j.surg.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: How to select patients for liver transplantation? J Hepatol. 2007;47:460–466. doi: 10.1016/j.jhep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Kennedy AS. Hepatic-directed therapies in patients with neuroendocrine tumors. Hematol Oncol Clin North Am. 2016;30:193–207. doi: 10.1016/j.hoc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Mohan H, Nicholson P, Winter DC, et al. Radiofrequency ablation for neuroendocrine liver metastases: A systematic review. J Vasc Interv Radiol. 2015;26:935–942 e1. doi: 10.1016/j.jvir.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Kulke MH, Hörsch D, Caplin ME, et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35:14–23. doi: 10.1200/JCO.2016.69.2780. [DOI] [PubMed] [Google Scholar]
- 70.Pavel M, Gross DJ, Benavent M, et al. Telotristat ethyl in carcinoid syndrome: Safety and efficacy in the TELECAST phase 3 trial. Endocr Relat Cancer. 2018;25:309–322. doi: 10.1530/ERC-17-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]